Abstract
Serious bacterial infection (SBI) is a major cause of morbidity and mortality in children. Distinguishing SBI from self-limiting viral infections is a very important task in the emergency department (ED), especially in the children with fever without source (FWS). The aim of this study was to analyze whether parents’ statements about clinical manifestations, which were categorized according to grades, are related to the actual diagnosis of SBI in children with FWS.
Retrospective analysis was conducted using prospectively acquired cohort data for all febrile children in the pediatric ED of Seoul National University Hospital from August 2016 to August 2017. The association of clinical manifestations and SBI was the main outcome of this study. The SBIs included diagnoses such as bacteremia, bacterial meningitis, urinary tract infection, and pneumonia. Clinical manifestations including activity, urination, and feeding were categorized into 3 or 4 grades according to the parents’ statements. The linear-by-linear association test was used to examine linear associations between the severity of clinical manifestations and SBI. Receiver operating characteristic curves for clinical manifestations were constructed for patients with SBI. Area under the curve (AUC) statistics and 95% confidence intervals (CIs) were obtained to evaluate the predictive performance of clinical manifestations.
There was no linear association between SBI and non-SBI when compared by severity of the clinical manifestations, such as duration of fever (P = .299), activity (P = .781), feeding (P = .161), and urination (P = .834). The AUC was 0.54 (95% CI 0.41–0.67) for duration of fever, 0.52 for activity (95% CI 0.40–0.64), 0.42 for feeding (95% CI 0.32–0.53), and 0.51 for urination (95% CI 0.39–0.62).
There was no evidence that the test performance of the clinical manifestations is valid for predicting SBIs, even considering the severity of manifestations. For optimal evaluation of the children with FWS, more comprehensive approach including laboratory tests, are needed.
Keywords: bacterial infections, fever, symptom assessment
1. Introduction
The most common reason why children visit the emergency room is fever[1,2] Most fevers in children are caused by viral infections, but about 5% to 20% of febrile children may have a serious bacterial infection (SBI) such as pneumonia, urinary tract infection (UTI), bacterial meningitis, or bacteremia. Because SBIs in children have a relatively high mortality rate, rapid diagnosis and treatment are essential for better outcomes.[3] However, even after clinical assessment including physician's history taking and careful physical examination, the exact cause of fever is uncertain especially in children with fever without source (FWS). Considering the difficulty of pediatric laboratory tests in emergency room settings, it is important to find other evidence suggesting SBI in children with FWS.[4,5]
Studies on predictors of SBI have been actively conducted. In particular, the results of laboratory tests such as white blood cell count (WBC), absolute neutrophil count (ANC), C-reactive protein (CRP), and procalcitonin have been widely regarded as helpful.[3,6–8] However, it is practically impossible to perform laboratory tests for all children with fever. Therefore, there have been continuous attempts to evaluate the relationship between clinical manifestations and SBI. Recently, the UK National Institute for Health and Clinical Excellence (NICE) published a guideline for the initial assessment of febrile children, but actual clinical application is limited due to insufficient accuracy.[9,10] In most previous studies, physicians’ and/or nurses’ findings were evaluated rather than parents’ statements for predicting SBI in febrile children.
The aim of this study was to analyze whether parents’ statements about clinical manifestations, which were categorized according to grades, are related to the actual diagnosis of SBI in children with FWS. We hypothesized that the greater the severity of symptoms in children with FWS, the greater the likelihood of diagnosis of SBIs.
2. Methods
2.1. Study setting and design
This retrospective analysis was conducted using prospectively acquired cohort data for all febrile children (“fever registry”) in the pediatric emergency department (ED) of Seoul National University Hospital from August 2016 to August 2017 (13 months).
2.2. Data source
The fever registry was a consecutive collection of data on all children under 5 years of age who visited the pediatric ED within 24 hours of fever (body temperature of 38 or above). Initially, resident physicians were required to fill out the template with age, gender, recent antibiotic usage, severity of clinical manifestations, degree and duration of fever, vaccination history, mental status, and whether the patients were determined to have FWS. In particular, clinical manifestations including activity, urination, and feeding were categorized into 3 or 4 grades.
In the case of feeding, we asked parents to express in percentage how much the child's diet had decreased. In the case of activity and urination, we asked the parents to choose the grade of severity by subjective assessment. Because parents may have difficulty in recognizing changes in urination of their child, we classified the grade about urination more simply. All the grade of severity were presented to the parents in easy term
Next, research assistants reviewed the medical records and registered additional data including initial vital signs, clinical diagnosis, disposition, and the results of blood, urine, and cerebrospinal fluid tests if conducted. The institutional review board (IRB) at the Seoul National University Hospital approved the study protocol for children (IRB No. 1809-111-974).
2.3. Study population
All children classified as having FWS were enrolled. FWS was defined as a case in which the child has no localizing source of fever after clinical assessment including physicians’ history taking and physical examination. We excluded cases who were suspected to be at high-risk for SBI due to underlying medical conditions such as malignancy or other immunocompromised conditions, vesicoureteral reflux, or congenital disease. Those with a history of antibiotic use within the 3 days before the ED visit were also excluded.
2.4. Study outcome
The association of clinical manifestations and SBI was the main outcome of this study. The SBIs included diagnoses such as bacteremia, bacterial meningitis, UTI, and pneumonia. Bacteremia was defined as growth of a single pathogenic microorganism on blood culture. Bacterial meningitis was defined as positive cerebrospinal fluid culture. UTI was defined as growth of a single urinary tract pathogen at 104–5 CFU/mL on a catheterized urine specimen. Pneumonia was defined as the presence of consolidation on chest radiography as interpreted by radiologists. The secondary outcome measure was predictive performance of the graded clinical manifestations to predict SBI in children with FWS.
2.5. Statistical analysis
Study subjects were divided into 2 groups based on whether the diagnosis was SBI. Categorical variables were reported as percentages and were compared with the χ2 test or Fisher exact test as appropriate. Continuous variables that were not distributed normally are presented as medians with interquartile ranges (IQRs). The linear-by-linear association test was used to examine linear associations between the severity (grades) of clinical manifestations and SBI. Receiver operating characteristic (ROC) curves for clinical manifestations were constructed for patients with SBI. Area under the curve (AUC) statistics and 95% confidence intervals (CIs) were obtained to evaluate the predictive performance of clinical manifestations. All statistical analyses were performed using SPSS version 20.0.
3. Results
From August 2016 to August 2017, there were 6026 visits by febrile children under 5 years of age who were enrolled in the fever registry. A flow chart of the patient disposition is shown in Figure 1. Six hundred sixteen patients met the FWS definition (10.2%), and 29 patients were excluded because of an uncompleted template. Therefore, 587 febrile children (9.7%) were included in the study. The initial cohort included 34 patients diagnosed with SBIs. We excluded 5 patients with potentially confounding comorbidity, 4 patients with antibiotics therapy in the 48 hours before diagnosis. Finally, SBIs were found in 25 (4.30%) of the 587 febrile children with FWS. These included pneumonia in 6 (1.02%), UTIs in 17 (2.90%), bacteremia in 2 (0.34%), and meningitis in 0 (0.00%).
Figure 1.
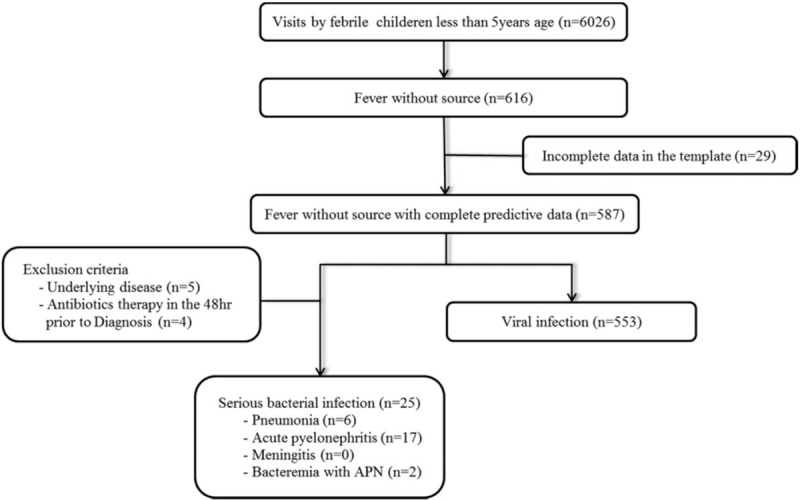
Patient flow chart of the study.
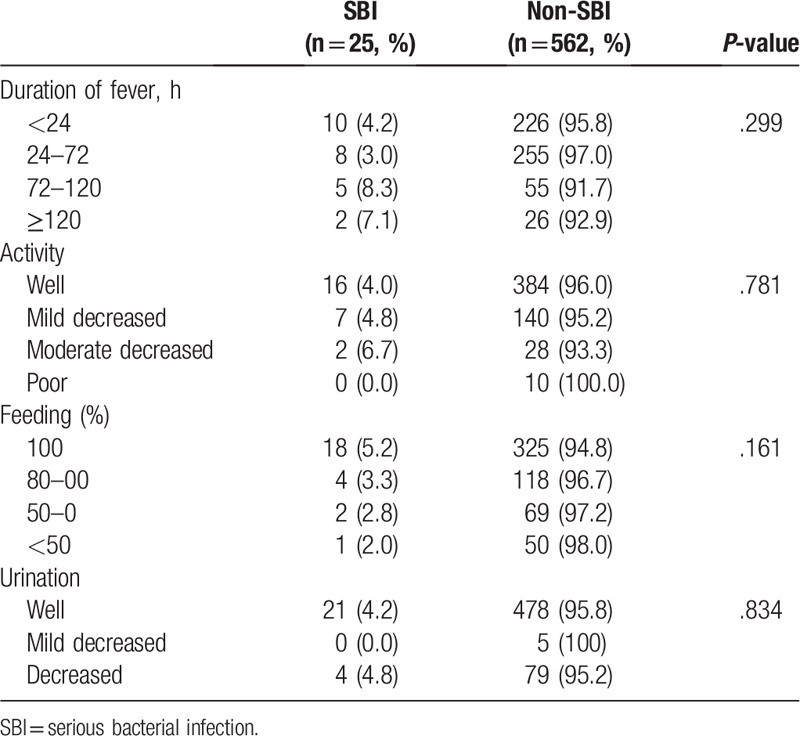
Demographics are shown in Table 1. The median patient age was 0.42 years (IQR 0.25–0.96) in SBIs and 1.50 years (IQR 0.92–2.94) in non-SBIs. The SBI group was significantly younger than the non-SBI group (P ≤ .001). A comparison of the 2 groups is shown in Table 2. There was no linear association between SBI and non-SBI when compared by severity of the clinical manifestations, such as duration of fever (P = .299), activity (P = .781), feeding (P = .161), and urination (P = .834).
Table 1.
Demographics.
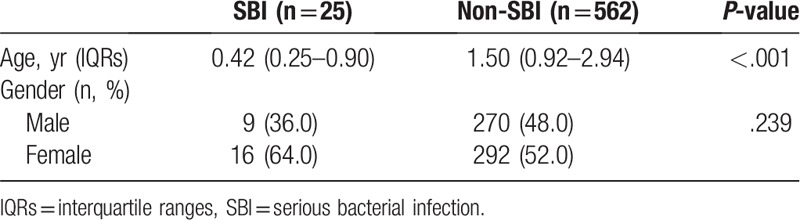
Table 2.
Comparison of severity of clinical manifestations between serious bacterial infection versus nonserious bacterial infection group.
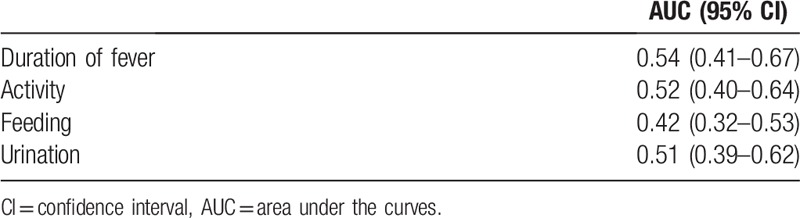
The ROC curves of clinical manifestations are shown in Figure 2. The AUC was 0.54 (95% CI 0.41–0.67) for duration of fever, 0.52 for activity (95% CI 0.40–0.64), 0.42 for feeding (95% CI 0.32–0.53), and 0.51 for urination (95% CI 0.39–0.62) (Table 3). There was no evidence that the test performance of the clinical manifestations is valid for predicting SBIs.
Figure 2.
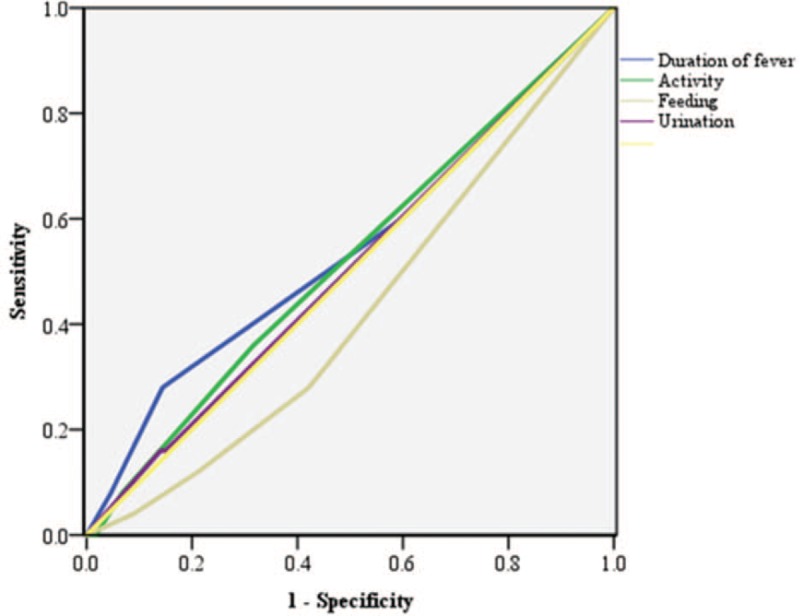
Receiver operating characteristic curves for clinical manifestations.
Table 3.
Area under the curves of the receiver operating characteristic for clinical manifestations predicting serious bacterial infection.
4. Discussion
In this retrospective cohort study, we found that the severity of clinical manifestations failed to have a linear association with SBIs in febrile children with FWS. Although the severity of each clinical manifestation was increased, there was no correlation with the increase in the detection rate of SBI. According to our study, clinical manifestations that are widely considered to be indicators of SBI seem to be unreliable screening tools.
The introduction of Haemophilus influenzae type B and pneumococcal vaccines has dramatically reduced the incidence of SBI, but the importance of rapid diagnosis and early antibiotics remains unchanged.[11] With the development of laboratory tests, studies of acute phase reactants such as WBC, CRP, ANC, and procalcitonin are actively being carried out.[12,13] Recent studies reported that CRP and procalcitonin were both strong predictors of SBI.[8,12] However, clinical manifestations are a basic and easy-to-assess tool in medical practice. Many clinical tools are still widely used in the evaluation of febrile children, such as the NICE traffic system and the Yale observation scale score (YOS).[14]
Several recent studies looked into the efficacy of these clinical tools for predicting SBI. Except for studies in developing countries, the results are disappointing. In a large prospective cohort study, neither the YOS nor unstructured clinician suspicion reliably identified those with SBI.[15] The NICE traffic system has low specificity for detecting SBI.[10] Additionally, there was no significant relationship between a single clinical symptom and SBI. Erell et al,[16] in a prospective case-control study, reported that shivering, presumed to be more common in children with SBI, was not associated with increased risk of SBI.
In previous studies, physicians assessed clinical symptoms and performed the physical examination to predict SBI. This study differs from other studies in that the parents directly assessed the severity of clinical manifestations. It was assumed that evaluation by parents, who had been caring for their child all day during the course of the child's illness, would be more accurate. Contrary to expectations, the results were not significantly different. Parents tend to over-report because of the involvement of subjective emotions rather than a medical approach. Based on our findings, parents’ reports of clinical symptoms are not suitable for use as predictors of SBI.
In our results, UTI was the most common SBI, similar to other reports.
Bacteremia was not independently detected except in 2 cases with UTI. There were no cases of meningitis. When UTI accounts for the majority of SBIs because of vaccination, it is more reasonable to perform urine tests if there is no clear cause of fever on the first examination.
5. Limitations
This study has several limitations. First, the data were collected from a single large ED. Therefore our results might suffer from selection bias, and generalizability to other EDs is questionable. However, because our ED also serves as a primary care center in the local area, this data collection from various patient groups within the possible range can be helpful to evaluate the SBI issue.
Second, we used well-known clinical symptoms as SBI predictors to analyze trends according to severity. When analyzed by univariate logistic regression analysis using our registry data, there was no significant association between the presence of each symptom and SBI. Furthermore, there were no different results when 2 or more symptoms were present at the same time. The data was not enough and the proper sample size was not calculated, so it seemed that we could not get meaningful results.
Third, the SBI group was significantly younger. This may also reflect selection bias because it is known that the risk of SBI increases with younger age. If enough data can be gathered, it will be necessary to analyze them according to age group.
Another limitation of the study is that the number of cases confirmed SBI was small, and there were no cases of meningitis. Because only febrile children without an apparent infection source were included in the study, the number of children who met the criteria was relatively small. However, the percentage of children with FWS among febrile children was consistent with previously published data, which supports the validity of the fever registry.
Finally, parental education could not be analyzed in this study because whether the parents educated in related medical sciences were not included in the “fever registry.” The extent of the parent's medical knowledge could influence the assessment of clinical manifestations.
6. Conclusion
There was no evidence that the test performance of the clinical manifestations is valid for predicting SBI, even considering the severity of manifestations. For optimal evaluation of the children with FWS, more comprehensive approach including laboratory tests, are needed.
Author contributions
Conceptualization: Ha Ni Lee, Young Ho Kwak.
Data curation: Ha Ni Lee, Young Ho Kwak, Se Uk Lee, Do Kyun Kim.
Formal analysis: Young Ho Kwak, Joong Wan Park.
Methodology: Young Ho Kwak, Jae Yun Jung, Se Uk Lee, Joong Wan Park, Do Kyun Kim.
Resources: Do Kyun Kim.
Software: Jae Yun Jung, Joong Wan Park, Do Kyun Kim.
Supervision: Young Ho Kwak.
Writing – original draft: Ha Ni Lee.
Writing – review and editing: Young Ho Kwak.
Young Ho Kwak orcid: 0000-0003-2062-7575.
Footnotes
Abbreviations: ANC = absolute neutrophil count, AUC = area under the curve, CRP = C-reactive protein, ED = emergency department, FWS = fever without source, NICE = the UK National Institute for Health and Clinical Excellence, ROC = receiver operating characteristic, SBI = serious bacterial infection, UTI = urinary tract infection, WBC = white blood cell count, YOS = Yale observation scale score.
How to cite this article: Lee HN, Kwak YH, Jung JY, Lee SU, Park JW, Kim DK. Are parents’ statements reliable for diagnosis of serious bacterial infection among children with fever without an apparent source? Medicine. 2019;98:42(e17530).
The authors disclose they had no prior publication of study data.
The data that support the findings of this present study may be available from the corresponding author upon reasonable request.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31–5.. [DOI] [PubMed] [Google Scholar]
- [2].Trautner BW, Caviness AC, Gerlacher GR, et al. Prospective evaluation of the risk of serious bacterial infection in children who present to the emergency department with hyperpyrexia (temperature of 106 F or higher). Pediatrics 2006;118:34–40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thayyil S, Shenoy M, Hamaluba M, et al. Is procalcitonin useful in early diagnosis of serious bacterial infections in children? Acta Paediatr 2005;94:155–8.. [DOI] [PubMed] [Google Scholar]
- [4].Bleeker SE, Moons KG, Derksen-Lubsen G, et al. Predicting serious bacterial infection in young children with fever without apparent source. Acta Paediatr 2001;90:1226–32.. [DOI] [PubMed] [Google Scholar]
- [5].Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med 2015;22:240–3.. [DOI] [PubMed] [Google Scholar]
- [6].De S, Williams GJ, Hayen A, et al. Value of white cell count in predicting serious bacterial infection in febrile children under 5 years of age. Arch Dis Child 2014;99:493–9.. [DOI] [PubMed] [Google Scholar]
- [7].Manzano S, Bailey B, Girodias JB, et al. Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. Am J Emerg Med 2010;28:647–53.. [DOI] [PubMed] [Google Scholar]
- [8].Nijman RG, Moll HA, Smit FJ, et al. C-reactive protein, procalcitonin and the lab-score for detecting serious bacterial infections in febrile children at the emergency department: a prospective observational study. Pediatr Infect Dis J 2014;33:e273–9.. [DOI] [PubMed] [Google Scholar]
- [9].National Collaborating Centre for Women's and Children's Health. Feverish Illness in Children: Assessment and Initial Management in Children Younger than 5 Years. London: National Collaborating Centre for Women's and Children's Health; 2013. [Google Scholar]
- [10].De S, Williams GJ, Hayen A, et al. Accuracy of the “traffic light” clinical decision rule for serious bacterial infections in young children with fever: a retrospective cohort study. BMJ 2013;346:f866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J 2002;21:584–8.. [DOI] [PubMed] [Google Scholar]
- [12].Galetto-Lacour A, Zamora SA, Gervaix A. Bedside procalcitonin and C-reactive protein tests in children with fever without localizing signs of infection seen in a referral center. Pediatrics 2003;112:1054–60.. [DOI] [PubMed] [Google Scholar]
- [13].Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275–9.. [DOI] [PubMed] [Google Scholar]
- [14].McCarthy PL, Sharpe MR, Spiesel SZ, et al. Observation scales to identify serious illness in febrile children. Pediatrics 1982;70:802–9.. [PubMed] [Google Scholar]
- [15].Nigrovic LE, Mahajan PV, Blumberg SM, et al. The Yale observation scale score and the risk of serious bacterial infections in febrile infants. Pediatrics 2017;140:e20170695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Erell Y, Youngster I, Abu-Kishk I, et al. Shivering in Febrile children: frequency and usefulness in predicting serious bacterial infections - a prospective case-control study. J Pediatr 2017;190:258–60.e1.. [DOI] [PubMed] [Google Scholar]


