Supplemental Digital Content is available in the text
Keywords: meta-analysis, neonate, retinopathy of prematurity, sepsis, systematic review
Abstract
Background:
Retinopathy of prematurity (ROP) is a retinal vasoproliferative disease affected by multiple factors such as infection and preterm birth. The role of sepsis in the development of ROP remains controversial. This systematic review and meta-analysis aimed to identify the impact of sepsis on ROP.
Methods:
The PubMed, Embase, and Cochrane Library databases were searched using terms related to sepsis and ROP. Cohort or case–control studies that reported the association of sepsis and ROP were eligible. The odds ratios (ORs) together with the 95% confidence interval (CI) were extracted from the studies or computed by authors if not provided.
Results:
Thirty-four studies were ultimately included in this meta-analysis. The pooled results showed that sepsis increased the risk for the development of any stage ROP (OR = 2.16; 95% CI: 1.65–2.82). Both early onset (OR = 2.50; 95% CI: 1.97–3.18) and late-onset (OR = 1.37; 95% CI: 1.22–1.55) sepsis were associated with severe ROP. Furthermore, both bacterial sepsis (OR = 1.74; 95% CI: 1.21–2.50) and fungal sepsis (OR = 2.96; 95% CI: 2.05–4.28) were also found to be associated with severe ROP.
Conclusion:
Sepsis increased the risk of any stage ROP, especially for the severe ROP. Further high-quality clinical studies are needed to eliminate heterogeneity and publication bias to validate these findings.
1. Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disease that affects the developing retinal vascular system in premature infants.[1] Blindness and visual disability are the main long-term visual outcomes of ROP.[2] It has been reported that ROP blinds approximately 20,000 infants annually.[3] With increased survival of premature infants due to advanced neonatal care, the number of children affected by ROP is rising in low- and middle-income countries.[3] Increasing evidence indicates that infants with ROP are at increased risk of dysfunctions associated with nonvisual neural defects.[4,5] Therefore, retinopathy and brain injury in premature infants may share an etiology with similar risk factors.
Infection is an important risk factor for neonatal brain damage in premature infants.[6] Sepsis is a key cause of neonatal inflammation, which contributes to neonatal morbidity.[6,7] Recently, neonatal inflammation has been reported to be associated with ROP.[8,9] Although a meta-analysis has reported that systemic fungal infection is associated with the development of any stage ROP, including severe ROP, in very low birth weight infants,[10] there have been conflicting findings regarding the role of fungal sepsis as an independent risk factor for severe ROP.[11,12] In addition, bacterial sepsis has been identified as an independent risk factor in a study of extremely low gestational age newborns.[13]
Thus, the objective of this systematic review and meta-analysis was to investigate the impact of sepsis on the development of any stage of ROP, as well as of severe ROP, in particular.
2. Materials and methods
This study was conducted according to PRISMA and MOOSE Guidelines for meta-analysis.
2.1. Study identification and selection
The PubMed, Embase, and Cochrane Library databases were searched. The search keywords and Medical Subject Headings (MeSH) terms were the following: “Sepsis” OR “Bacteremia” OR “Fungemia” OR “Bacterial sepsis” OR “Fungal sepsis” AND “Retinopathy of Prematurity” OR “ROP” OR “Retrolental Fibroplasia.” The last search was updated on October 24, 2018. The search was limited to human studies and was restricted to English language reports. Potential studies were identified by initially screening the titles and abstracts of all studies. If the title and abstract suggested that the study discussed risk factors for ROP, the full text of the report was read independently by 2 of the authors (YL and HC) to determine its eligibility according the following inclusion criteria: studies investigated the association between sepsis and ROP; with the diagnosis of ROP based on the results of ophthalmoscopy; classification of the ROP stage from 1 to 5 according to the International Classification of ROP,[14] with ROP defined as any stage ROP and severe ROP (stages 3 to 5, plus disease, surgical, and threshold ROP); sepsis was diagnosed as culture-proven sepsis (fungal sepsis was diagnosed by the positive fungal culture but not the indirect mycological tests) and clinical sepsis without culture evidence; case–control or cohort studies; and studies that reported risk ratios or odds ratios (ORs) and corresponding 95% confidence intervals (CIs) or with other data available for calculating the CIs. The following exclusion criteria were also applied: studies with overlapping populations; those that did not include a control group; and those for which the original data were not available. Therefore, to accurately evaluate the association between sepsis and ROP, we only included the studies of culture-positive sepsis in the meta-analysis. Any disagreements were reconciled by a third author (YT), who independently reviewed the studies, and then discussed disagreements with the initial 2 reviewers until a consensus was reached.
2.2. Data extraction
Two authors (YL and HC) independently collected data from each study and then compared results. Any disagreement was resolved by discussions with a 3rd author (TZ). The extracted data included the 1st author, publication year, country, study design, included population, diagnosis of ROP, sample size, birth weight, gestational age, impact of sepsis on ROP as primary outcome, risk of bias, and adjustment of confounding factors. The ORs together with the 95% CI were extracted from the studies or computed by authors if not provided.
2.3. Quality evaluation
To assess the methodological quality of each included study, 2 authors (YT and TZ) independently screened the study design, the representativeness of the study population, the quality of the statistical analysis, and the validity of outcomes by using the Newcastle–Ottawa scale (NOS).[15] The NOS was widely applied to evaluate cohort or case–control studies, based on group selection (4 items); comparability between groups (1 item); and outcome and exposure assessment (3 items). The maximum score was 9 stars. Studies with at least 5 stars were considered to be high-quality studies.[16] Disagreements between the 2 reviewing authors were examined by a 3rd author (JH) and resolved by discussion.
2.4. Statistical analysis
The ORs of each included study were pooled to assess the association between sepsis and any stage ROP or severe ROP in particular. The pooled OR was calculated by using a random-effects model if statistical heterogeneity was found among studies.[17,18] Otherwise, a fixed-effects model was used. Q statistics and I2 tests were applied to estimate heterogeneity among studies. Data were considered statistically heterogeneous if P < .1 and I2 > 50%. Forest plots were used to show the ORs and 95% CIs of each individual study, as well as the pooled ORs and 95% CIs. To find the source of the heterogeneity, we performed subgroup analysis combined with meta-regression analysis according to the variance in the studies. Publication bias was evaluated by funnel plots and Begg test,[19] where a value of P < .05 was considered to be statistically significant. The nonparametric “trim and fill” procedure was also performed to assess the possible effect of publication bias.[20,21] All statistical tests were performed using Review Manager 5.3 or STATA 12.0 software.
3. Results
3.1. Study selection process and characteristics
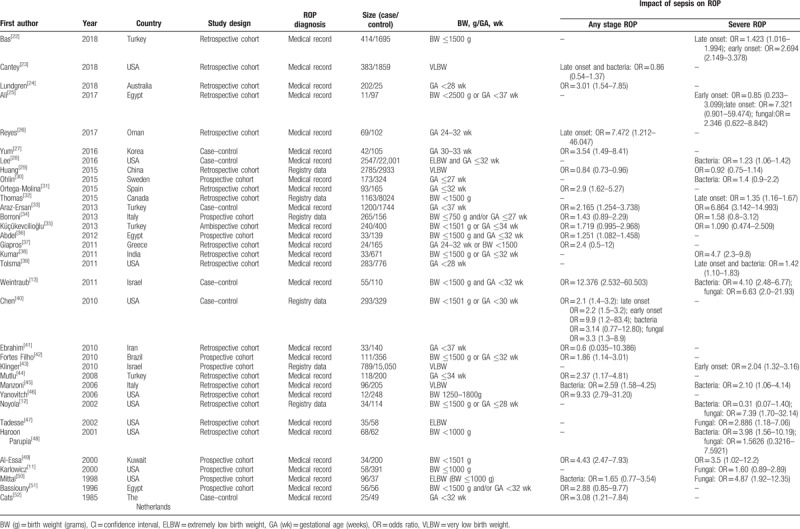
The literature search identified 639 studies based on our search strategy. After careful screening, we excluded the studies with culture-negative sepsis (31 studies) because the diagnosis criteria of clinical sepsis in different studies were inconsistent and the confounders were commonly existed in those studies. Finally, 34 studies were ultimately selected for the meta-analysis. A flow diagram detailing the selection process is shown in Figure 1. Characteristics of the included studies are summarized in Table 1. These included studies were published between 1985 and 2018. The sample sizes (case/control) varied from a maximum of 2547/22,001 to a minimum of 25/49. All the studies involved preterm neonates (gestational age before 37 weeks, and birth weight <2500 g). For the assessment of ROP, the included studies measured or used data from medical records, based on ophthalmoscopy. According to the onset time and etiology of sepsis, 6 studies reported late-onset sepsis, 4 reported early onset sepsis, 10 reported bacterial sepsis, and 7 reported fungal sepsis. Outcomes were categorized into 2 broad categories: “any stage ROP” and “severe ROP.”
Figure 1.
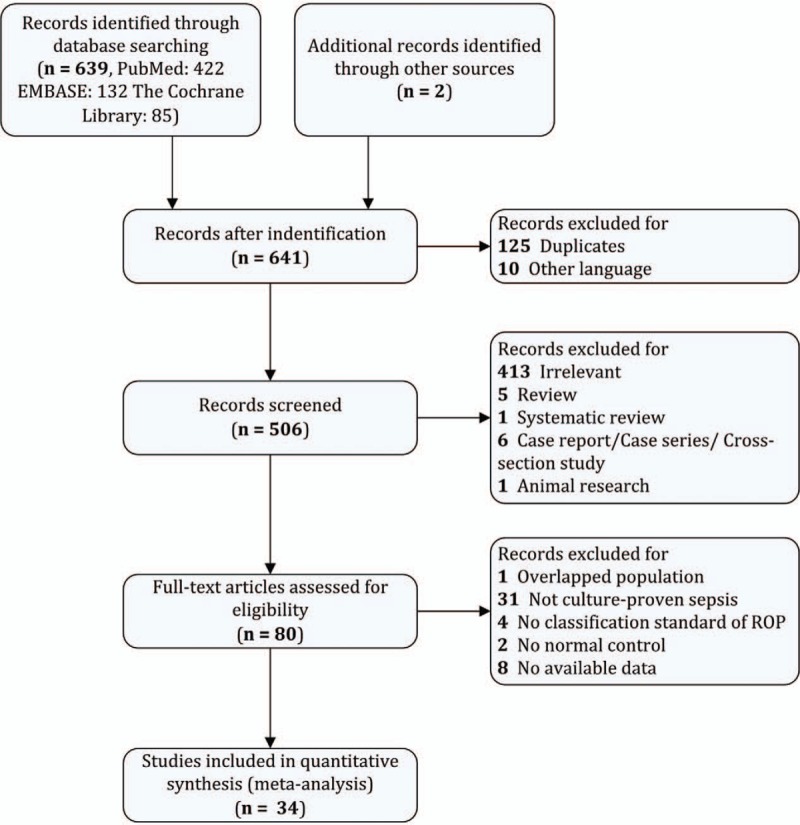
Flow chart of study selection process.
Table 1.
Characteristics of included studies.
3.2. Quality of included studies
A total of 28 cohort studies and 6 case–control studies were selected in this meta-analysis. The methodologic quality scores ranged from 4 to 8 stars. Most studies were deemed to be high quality, except for 1 study.[38] Three studies[25,33,41] included all preterm neonates without restriction of birth weight or gestation age, but the selection of cases in the other studies may have lacked representativeness. Most studies adjusted for potential confounding factors, but 8 did not.[25,27,38,42,45,46,50,51] Most ORs were evaluated by multiple logistic regression analysis, adjusted for gestational age (24 studies), birth weight (16 studies), oxygen use (14 studies), mechanical ventilation (11 studies), and sex (8 studies) (Supplemental Digital Content, Table S1). Thirteen cohort studies and 1 case–control study provided a no-response rate at the end of follow-up (Supplemental Digital Content, Tables S2 and S3). All included publications were assessed using the NOS (Supplemental Digital Content, Tables S2 and S3).
3.3. Sepsis and any stage ROP
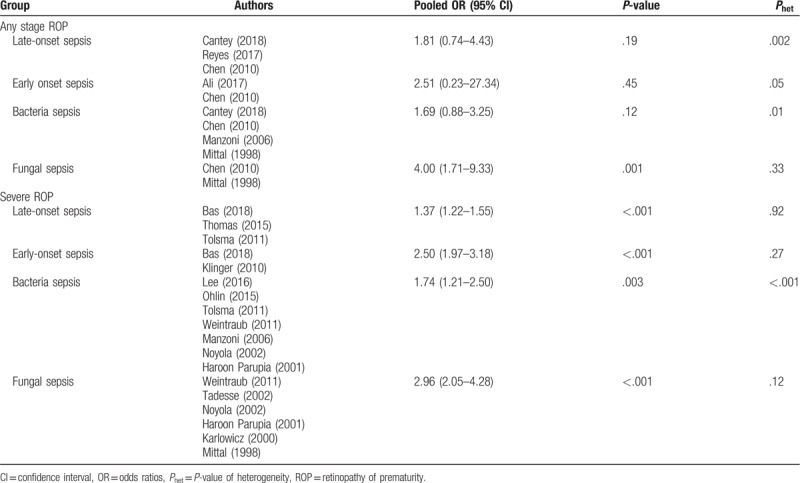
Twenty-two ORs of any stage ROP were pooled in the meta-analysis. The pooled OR from the random-effects model was 2.16 (95% CI: 1.65–2.82) (Fig. 2). Substantial heterogeneity was observed (P < .001; I2 = 84%). According to the onset time and etiology of sepsis, we estimated the respective impacts of early onset sepsis, late-onset sepsis, bacterial sepsis, and fungal sepsis on any stage ROP. The pooled ORs are shown in Table 2. Fungal sepsis had a significant impact on any stage ROP (OR = 4.00; 95% CI: 1.71–9.33; P = .001).
Figure 2.
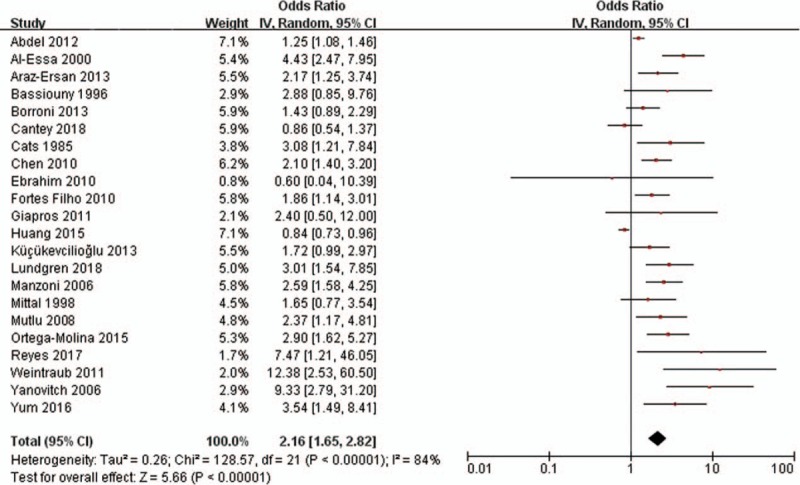
Forest plot showing the association between sepsis and any stage of retinopathy of prematurity. The pooled odds ratios (ORs) of association between sepsis and any stage of retinopathy of prematurity were calculated. Diamond marker indicates pooled effect sizes. Some ORs could slightly differ (centesimal) from published values owing to the rounding of primary values. CI = confidence interval.
Table 2.
The pooled ORs of association between retinopathy of prematurity and sepsis according to the onset time and etiology.
3.4. Sepsis and severe ROP
Twenty ORs of severe ROP were pooled in the meta-analysis. The pooled OR from the random-effects model was 1.87 (95% CI: 1.53–2.29) (Fig. 3). Substantial heterogeneity was observed (P < .001; I2 = 78%). Based on the onset time and etiology of sepsis, we estimated the respective impacts of early onset sepsis, late-onset sepsis, bacteria sepsis, and fungal sepsis on the development of severe ROP. The pooled ORs are shown in Table 2. We found that early onset sepsis (OR = 2.50; 95% CI: 1.97–3.18; P < .001), late-onset sepsis (OR = 1.37; 95% CI: 1.22–1.55; P < .001), bacterial sepsis (OR = 1.74; 95% CI: 1.21–2.50; P = .003), and fungal sepsis (OR = 2.96; 95% CI: 2.05–4.28; P < .001) each had a significant impact on the development of severe ROP.
Figure 3.
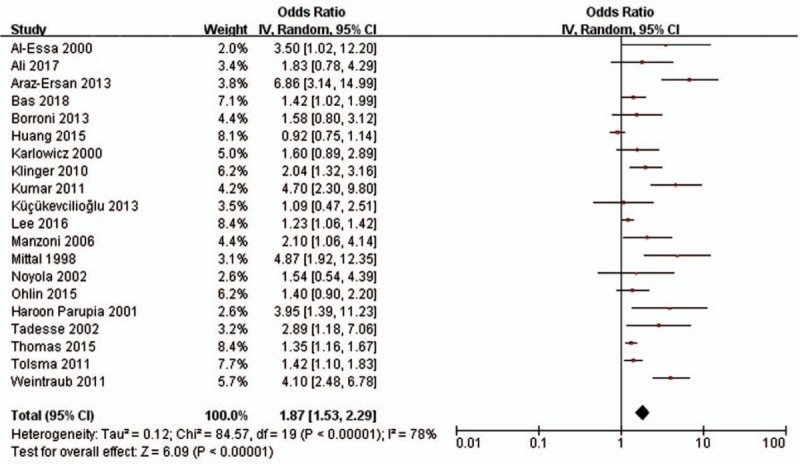
Forest plot showing the association between sepsis and severe retinopathy of prematurity. The pooled odds ratios (ORs) of association between sepsis and severe retinopathy of prematurity were calculated. Diamond marker indicates pooled effect sizes. Some ORs could slightly differ (centesimal) from published values owing to the rounding of primary values. CI = confidence interval.
3.5. Subgroup analysis
With adjustment for confounding factors, study design, classification of birth weight or gestation age, and sample size, a stratified meta-analysis, using subgroups, was performed to explore study heterogeneity. Control for confounding factors (adjusted R2 = 26.32%, P = .059 for any stage ROP; adjusted R2 = 32.32%, P = .068 for severe ROP) and sample size (adjusted R2 = 19.23%, P = .031 for any stage ROP; adjusted R2 = 30.45%, P = .053 for severe ROP) were significant factors in study heterogeneity (Supplemental Digital Content, Table S4).
3.6. Publication bias
Asymmetries were shown in funnel plots (Fig. 4) and publication biases were found by Begg test in both any stage ROP (P < .001) and severe ROP (P = .001). Therefore, we performed a sensitivity analysis and used the “trim and fill” method to produce symmetrical funnel plots by imputing hypothetical negative unpublished studies (Fig. 4). When we incorporated these hypothetical studies, the pooled analysis continued to show a statistically significant association between sepsis and severe ROP (OR = 1.296; 95% CI: 1.047–1.605; P = .017). However, the pooled analysis showed no statistically significant association between sepsis and any stage ROP (OR = 1.276; 95% CI: 0.980–1.691; P = .07).
Figure 4.
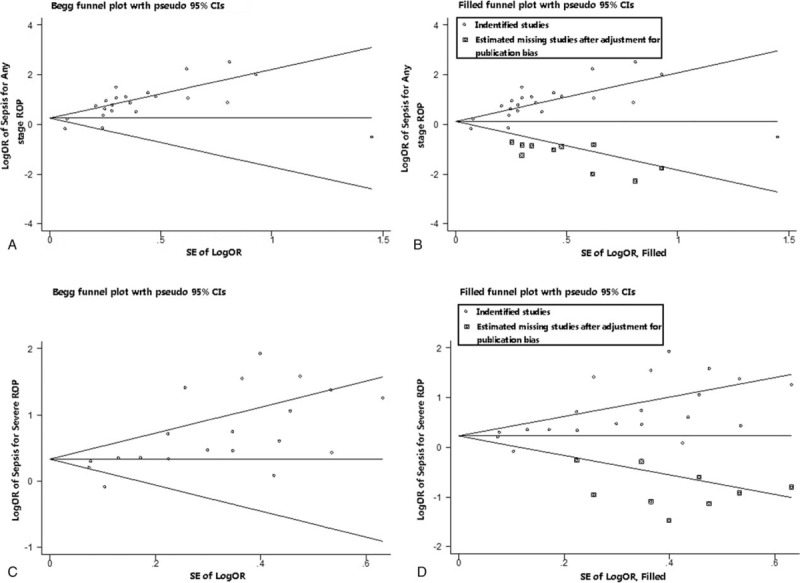
Funnel plots without and with trim and fill. The pseudo 95% confidence interval (CI) is computed as part of the analysis that produces the funnel plot, and corresponds to the expected 95% CI for a given standard error (SE). OR = odds ratio.
4. Discussion
To our knowledge, no previous meta-analysis had assessed the association between sepsis and the development of ROP based on currently available studies. The meta-analysis showed that sepsis was significantly associated with the development of any stage ROP as well as of severe ROP in particular. Based on the onset time and etiology of sepsis, fungal sepsis increased the risk for the development of any stage ROP. Moreover, early onset sepsis, late-onset sepsis, bacterial sepsis, and fungal sepsis each associated with the development of severe ROP.
The internal association underlying the sepsis and development of ROP may summarized by the relationship between inflammation and angiogenesis.[53] Some proinflammatory proteins and angiopoietins have been reported to play multiple roles in vascular processes,[53–55] which may promote the abnormal angiogenesis of retina. In this meta-analysis, a stronger association with severe ROP was revealed for early onset sepsis than for late-onset sepsis. Neonatal early onset sepsis is secondary to intrauterine infection in most cases, which may increase the risk for the development ROP.[56,57] Moreover, infants with intrauterine infection after birth always have a worse respiratory condition, which is typically treated with a higher concentration of oxygen and more advanced mechanical ventilation (such as invasive mechanical ventilation), which also increases the likelihood of ROP occurrence.[58,59] On the contrary, we found that the association of fungal sepsis with ROP was stronger than that of bacterial sepsis. In terms of the etiology of fungal sepsis, of the 7 studies included, 5 studies involved Candida sepsis.[11,12,45,47,50] As far as the type of bacteria resulted in the sepsis, 4 studies identified the specific bacteria. Three of them[13,23,30] investigated the relationship of coagulase-negative Staphylococcus (CoNS) sepsis and ROP. However, there was no study to identify the different impact factors on ROP between bacterial and fungal sepsis. Therefore, further studies should be carried out to explain this difference.
Subgroup analysis indicated that control for confounding factors is one of the significant factors in study heterogeneity. The various possible confounders and the control for different confounders in studies may affect the final pooled results. Gestational age and birth weight were the main adjusted confounders in the included studies, which are 2 well-recognized risk factors for ROP.[59] Moreover, recent studies have identified the impact of the use of oxygen and mechanical ventilation on the development of ROP.[58,60] Thus, it is important to adjust for the influence of oxygen and mechanical ventilation on the relationship of sepsis and ROP. However, of the 34 studies included, only 7 studies[22,26,33,35,36,40,44] had concurrently adjusted for this 2 factors. In addition, although there are 14 studies which adjusted the confounder of oxygen use, the bias may still exist because it is difficult to adjust appropriately for oxygenation to normalizing the oxygenation among studies.
This study has several limitations. First, significant heterogeneity was found in the meta-analysis, caused by adjustment for confounding factors and sample size, according to a subgroup analysis and meta-regression analysis. The various possible confounders mentioned above might be the main source of the heterogeneity and skewing of results. Second, significant publication bias was also found in this meta-analysis. After the “trim and fill” procedure, the association between sepsis and severe ROP was significant and stable, while that of sepsis and any stage ROP was not. This instability of association of sepsis and any stage ROP may come from the strict inclusion of studies and some unpublished negative results. Our review only included English publications, some results in non-English studies may be missed.
5. Conclusion
Our systematic review and meta-analysis showed that neonatal sepsis increased the risk of any stage ROP as well as that of severe ROP, in particular. To eliminate the heterogeneity and publication bias of our results, more high-quality clinical studies are needed to provide convincing evidence for the impact of sepsis on ROP occurrence.
Acknowledgment
The authors appreciate the English editors from Editage Company for proof reading this manuscript.
Author contributions
Conceptualization: Jichong Huang, Dezhi Mu.
Funding acquisition: Dezhi Mu.
Methodology: Jichong Huang, Ying Tang, Tingting Zhu, Yafei Li, Hua Chun.
Software: Jichong Huang.
Validation: Dezhi Mu.
Writing – original draft: Jichong Huang.
Writing – review & editing: Ying Tang, Yi Qu, Dezhi Mu.
Dezhi Mu orcid: 0000-0002-2599-7041.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, NOS = Newcastle–Ottawa scale, ORs = odds ratios, ROP = retinopathy of prematurity.
How to cite this article: Huang J, Tang Y, Zhu T, Li Y, Chun H, Qu Y, Mu D. Cumulative evidence for association of sepsis and retinopathy of prematurity. Medicine. 2019;98:42(e17512).
JH and YT contributed equally to the work.
This work was supported by the National Science Foundation of China (nos: 81630038, 81971433, 81971428, 81842011, 81330016, 81771634, 81300524), the National Key R&D Program of China (2017YFA0104200), the Grants from Ministry of Education of China (IRT0935), the Grants from Science and Technology Bureau of Sichuan Province (2016TD0002), and the grant of clinical discipline program (Neonatology) from the Ministry of Health of China (1311200003303).
This is a systematic review and meta-analysis, thus the ethical approval was not necessary.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].McGregor ML, Bremer DL, Cole C, et al. Retinopathy of prematurity outcome in infants with prethreshold retinopathy of prematurity and oxygen saturation >94% in room air: the high oxygen percentage in retinopathy of prematurity study. Pediatrics 2002;110:540–4.. [DOI] [PubMed] [Google Scholar]
- [2].Paysse EA, Lindsey JL, Coats DK, et al. Therapeutic outcomes of cryotherapy versus transpupillary diode laser photocoagulation for threshold retinopathy of prematurity. J AAPOS 1999;3:234–40.. [DOI] [PubMed] [Google Scholar]
- [3].Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013;74Suppl 1:35–49.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allred EN, Capone A, Jr, Fraioli A, et al. Retinopathy of prematurity and brain damage in the very preterm newborn. J AAPOS 2014;18:241–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schmidt B, Davis PG, Asztalos EV, et al. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA 2014;311:523–5.. [DOI] [PubMed] [Google Scholar]
- [6].Strunk T, Inder T, Wang X, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014;14:751–62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wynn JL, Neu J, Moldawer LL, et al. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol 2009;29:79–88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee J, Dammann O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med 2012;17:26–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim SJ, Port AD, Swan R, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol 2018;63:618–37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bharwani SK, Dhanireddy R. Systemic fungal infection is associated with the development of retinopathy of prematurity in very low birth weight infants: a meta-review. J Perinatol 2008;28:61–6.. [DOI] [PubMed] [Google Scholar]
- [11].Karlowicz MG, Giannone PJ, Pestian J, et al. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (</=1000 g) neonates? Pediatrics 2000;105:1036–40.. [DOI] [PubMed] [Google Scholar]
- [12].Noyola DE, Bohra L, Paysse EA, et al. Association of candidemia and retinopathy of prematurity in very low birthweight infants. Ophthalmology 2002;109:80–4.. [DOI] [PubMed] [Google Scholar]
- [13].Weintraub Z, Carmi N, Elouti H, et al. The association between stage 3 or higher retinopathy of prematurity and other disorders of prematurity. Can J Ophthalmol 2011;46:419–24.. [DOI] [PubMed] [Google Scholar]
- [14].International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–9.. [DOI] [PubMed] [Google Scholar]
- [15].GA Wells BS OCD PJ, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa score for nonrandomized studies. Available at: http://www.ohri.ca. Accessed October 2018. [Google Scholar]
- [16].Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.. [DOI] [PubMed] [Google Scholar]
- [18].Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 1999;150:206–15.. [DOI] [PubMed] [Google Scholar]
- [19].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101.. [PubMed] [Google Scholar]
- [20].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63.. [DOI] [PubMed] [Google Scholar]
- [21].Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–64.. [DOI] [PubMed] [Google Scholar]
- [22].Bas AY, Demirel N, Koc E, et al. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol 2018;102:1711–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cantey JB, Anderson KR, Kalagiri RR, et al. Morbidity and mortality of coagulase-negative staphylococcal sepsis in very-low-birth-weight infants. World J Pediatr 2018;14:269–73.. [DOI] [PubMed] [Google Scholar]
- [24].Lundgren P, Athikarisamy SE, Patole S, et al. Duration of anaemia during the first week of life is an independent risk factor for retinopathy of prematurity. Acta Paediatr 2018;107:759–66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ali AA, Gomaa NAS, Awadein AR, et al. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr 2017;106:1919–27.. [DOI] [PubMed] [Google Scholar]
- [26].Reyes ZS, Al-Mulaabed SW, Bataclan F, et al. Retinopathy of prematurity: revisiting incidence and risk factors from Oman compared to other countries. Oman J Ophthalmol 2017;10:26–32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yum SK, Moon CJ, Youn YA, et al. Expanded criteria for retinopathy of prematurity screening in moderately preterm infants: Single-center pilot study. Pediatr Int 2016;58:1158–62.. [DOI] [PubMed] [Google Scholar]
- [28].Lee JH, Hornik CP, Testoni D, et al. Insulin, hyperglycemia, and severe retinopathy of prematurity in extremely low-birth-weight infants. Am J Perinatol 2016;33:393–400.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang HC, Yang HI, Chou HC, et al. Preeclampsia and retinopathy of prematurity in very-low-birth-weight infants: a population-based study. PLoS One 2015;10:e0143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ohlin A, Bjorkman L, Serenius F, et al. Sepsis as a risk factor for neonatal morbidity in extremely preterm infants. Acta Paediatr 2015;104:1070–6.. [DOI] [PubMed] [Google Scholar]
- [31].Ortega-Molina JM, Anaya-Alaminos R, Uberos-Fernandez J, et al. Genetic and environmental influences on retinopathy of prematurity. Mediators Inflamm 2015;2015:764159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thomas K, Shah PS, Canning R, et al. Retinopathy of prematurity: risk factors and variability in Canadian neonatal intensive care units. J Neonatal Perinatal Med 2015;8:207–14.. [DOI] [PubMed] [Google Scholar]
- [33].Araz-Ersan B, Kir N, Akarcay K, et al. Epidemiological analysis of retinopathy of prematurity in a referral centre in Turkey. Br J Ophthalmol 2013;97:15–7.. [DOI] [PubMed] [Google Scholar]
- [34].Borroni C, Carlevaro C, Morzenti S, et al. Survey on retinopathy of prematurity (ROP) in Italy. Ital J Pediatr 2013;39:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Küçükevcilioğlu M, Mutlu FM, Sarici SU, et al. Frequency, risk factors and outcomes of retinopathy of prematurity in a tertiary care hospital in Turkey. Turkish J Pediatr 2013;55:467–74.. [PubMed] [Google Scholar]
- [36].Abdel HA, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of incidence and risk factors in NICU of Al-Minya University Hospital in Egypt. J Clin Neonatol 2012;1:76–81.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Giapros V, Drougia A, Asproudis I, et al. Low gestational age and chronic lung disease are synergistic risk factors for retinopathy of prematurity. Early Hum Dev 2011;87:653–7.. [DOI] [PubMed] [Google Scholar]
- [38].Kumar P, Sankar MJ, Deorari A, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr 2011;78:812–6.. [DOI] [PubMed] [Google Scholar]
- [39].Tolsma KW, Allred EN, Chen ML, et al. Neonatal bacteremia and retinopathy of prematurity: the ELGAN study. Arch Ophthalmol 2011;129:1555–63.. [DOI] [PubMed] [Google Scholar]
- [40].Chen M, Citil A, McCabe F, et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology 2011;99:125–32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ebrahim M, Ahmad RS, Mohammad M. Incidence and risk factors of retinopathy of prematurity in Babol, North of Iran. Ophthalmic Epidemiol 2010;17:166–70.. [DOI] [PubMed] [Google Scholar]
- [42].Fortes Filho JB, Eckert GU, Valiatti FB, et al. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP). Graefes Arch Clin Exp Ophthalmol 2010;248:893–900.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Klinger G, Levy I, Sirota L, et al. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics 2010;125:e736–40.. [DOI] [PubMed] [Google Scholar]
- [44].Mutlu FM, Altinsoy HI, Mumcuoglu T, et al. Screening for retinopathy of prematurity in a tertiary care newborn unit in Turkey: frequency, outcomes, and risk factor analysis. J Pediatr Ophthalmol Strabismus 2008;45:291–8.. [DOI] [PubMed] [Google Scholar]
- [45].Manzoni P, Maestri A, Leonessa M, et al. Fungal and bacterial sepsis and threshold ROP in preterm very low birth weight neonates. J Perinatol 2006;26:23–30.. [DOI] [PubMed] [Google Scholar]
- [46].Yanovitch TL, Siatkowski RM, McCaffree M, et al. Retinopathy of prematurity in infants with birth weight ≥1250 grams-incidence, severity, and screening guideline cost-analysis. J AAPOS 2006;10:128–34.. [DOI] [PubMed] [Google Scholar]
- [47].Tadesse M, Dhanireddy R, Mittal M, et al. Race, Candida sepsis, and retinopathy of prematurity. Biol Neonate 2002;81:86–90.. [DOI] [PubMed] [Google Scholar]
- [48].Haroon Parupia MF, Dhanireddy R. Association of postnatal dexamethasone use and fungal sepsis in the development of severe retinopathy of prematurity and progression to laser therapy in extremely low-birth-weight infants. J Perinatol 2001;21:242–7.. [DOI] [PubMed] [Google Scholar]
- [49].Al-Essa M, Azad RV, Rashwan N. Threshold stage of retinopathy of prematurity: maternal and neonatal risk factors. Ann Saudi Med 2000;20:129–31.. [DOI] [PubMed] [Google Scholar]
- [50].Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics 1998;101:654–7.. [DOI] [PubMed] [Google Scholar]
- [51].Bassiouny MR. Risk factors associated with retinopathy of prematurity: a study from Oman. J Trop Pediatr 1996;42:355–8.. [DOI] [PubMed] [Google Scholar]
- [52].Cats BP, Tan KE. Retinopathy of prematurity: review of a four-year period. Br J Ophthalmol 1985;69:500–3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hong HK, Lee HJ, Ko JH, et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J Neuroinflammation 2014;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tremblay S, Miloudi K, Chaychi S, et al. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest Ophthalmol Vis Sci 2013;54:8125–39.. [DOI] [PubMed] [Google Scholar]
- [55].Stalmans I. Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome. Verh K Acad Geneeskund Belg 2005;67:229–76.. [PubMed] [Google Scholar]
- [56].Mitra S, Aune D, Speer CP, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: a systematic review and meta-analysis. Neonatology 2014;105:189–99.. [DOI] [PubMed] [Google Scholar]
- [57].Kim CY, Jung E, Kim EN, et al. Chronic placental inflammation as a risk factor of severe retinopathy of prematurity. J Pathol Transl Med 2018;52:290–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Podraza W, Michalczuk B, Jezierska K, et al. Correlation of retinopathy of prematurity with bronchopulmonary dysplasia. Open Med 2018;13:67–73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Phelps DL. Martin RJ, Fanaroff AA, Walsh MC. Retinopathy of prematurity. Fanaroff and Martin's Neonatal Perinatal Medicine: Diseases of the Fetus and Infant. Philadelphia, PA: Elsevier Saunders; 2015. 1767–74.. [Google Scholar]
- [60].Chen ML, Guo L, Smith LE, et al. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics 2010;125:e1483–92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


