Abstract
Perineural invasion (PNI) is a prognostic factor in patients with colorectal cancer. Neurotrophic factors, molecular determinants of PNI, are altered in their expression levels in patients with ulcerative colitis. In this study, we evaluated the frequency of PNI in colitis-associated cancer (CAC) and sporadic cancer.
We retrospectively reviewed 778 colorectal cancers with pathological T3-T4 in 761 patients all of whom were surgically resected without preoperative treatment. The lesions were classified into either CAC or sporadic cancer based on the clinical information. Clinicopathological findings including PNI were compared between CACs and sporadic cancers. Moreover, we analyzed the risk factors for positive PNI by multivariate analysis using a logistic regression model.
Ten of the cancers (1.3%) were diagnosed as CACs, and the remaining 768 as sporadic cancers. CACs were characterized by being nonobstructive and predominantly located in the rectum. The CACs had a larger size and more frequent undifferentiated histology than sporadic cancers. PNI was observed more frequently in CACs (90%) than in sporadic cancers without obstruction (45%, P = .007). On multivariate analysis, CAC was one of the significant factors associated with PNI (odds ratio: 9.05, P = .040).
Our results suggest that CAC was more likely to exhibit PNI than sporadic colorectal cancer.
Keywords: colitis-associated cancer, perineural invasion, sporadic colorectal cancer
1. Introduction
In addition to lymphatic and vascular invasion, malignant cells can invade nervous structures and spread along nerve sheaths as a metastatic route.[1] In several malignancies such as head and neck and prostate cancers, it has been well documented that perineural invasion (PNI) plays a critical role in tumor growth and progression by mutual signaling interactions among tumors, nerves, and stroma.[2–4] In colorectal cancer (CRC), it was recently revealed that PNI is associated with local and distant organ recurrence and a worse postoperative survival rate.[5–10]
As clinical factors, the placement of a metallic colon stent as a bridge to surgery and obstruction has been shown to potentially increase the frequency of PNI in colon cancers, as reported by our group and others.[11–13] In addition, several neurotrophic factors such as nerve growth factor (NGF), glial cell-derived neurotrophic factor (GCNF), and brain-derived neurotrophic factor (BDNF), have been identified as determinants of PNI in rodent tumor models as well as various human neoplasms.[14] These molecules have been suggested to be involved in the crosstalk between tumor cells and nerves.[14]
Ulcerative colitis (UC) is one of the prevalent inflammatory bowel disease (IBD) entities, and CRC is a well-recognized complication especially when patients have risk factors such as a longstanding extensive disease, a young age at diagnosis, and persistent inflammation.[15–18] There is accumulating evidence suggesting that chronic intestinal inflammation is accompanied by alterations of the enteric nervous system. Neurotrophins such as NGF, GDNF, BDGF, and neurotrophin-3 as well as their receptors are highly expressed in the intestine of human IBD patients and several animal models of IBD,[19,20] whereas regulatory roles for several neurotrophins are recognized in experimental inflammation of the gut.[21] We considered that the frequency of PNI in colitis-associated cancer (CAC) may be different from that in sporadic CRC due to altered expressions in neurotrophins and their receptors.
In the present study, we investigated the frequency of PNI in CACs and sporadic cancers and addressed the association of CACs with PNI.
2. Methods
Using our database, we searched for consecutive patients with pathological T3-T4 primary colorectal cancer who underwent tumor resection in the Department of Surgical Oncology, University of Tokyo Hospital between July 2013 and January 2019. Cancers arising in IBD patients were included whereas patients with anal fistula-associated cancer were excluded from the study. Patients who underwent surgery for perforation, or received preoperative chemotherapy and/or radiotherapy, stent placement for malignant obstruction were also excluded.
We retrieved demographic data and tumor-related parameters including age, sex, body mass index, preoperative levels of tumor markers, that is, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, tumor location, and obstruction from medical charts. Histopathological diagnoses of the primary tumor using such factors as tumor type (sporadic cancer or CAC), size, histological grade, depth, lymphatic and venous invasion, and PNI were made by experienced gastrointestinal pathologists according to the eighth edition of the American Joint Committee on Cancer Staging System.[22] In our hospital, the upper normal limits of serum CEA and CA 19-9 are 5.0 ng/mL and 37 U/mL, respectively. Recurrence-free survival (RFS) was defined as time between surgery and first recurrence in curatively operated CRC patients without distant metastases.
Comparisons of parameters among groups were performed using the Wilcoxon rank-sum, Fisher exact, and Chi-square tests, applying Yates’ correction as appropriate. Predictive factors for PNI were estimated by univariate and multivariate analyses using a logistic regression model. RFS curves were drawn using the Kaplan–Meier method and differences were evaluated by the log-rank test. Statistical analysis was performed using JMP 14.2.0 (SAS Institute Inc, Cary, NC). P-values of <.05 were considered significant.
This study was conducted following approval by the ethics committee of the University of Tokyo Hospital (No. 3252-(8)).
3. Results
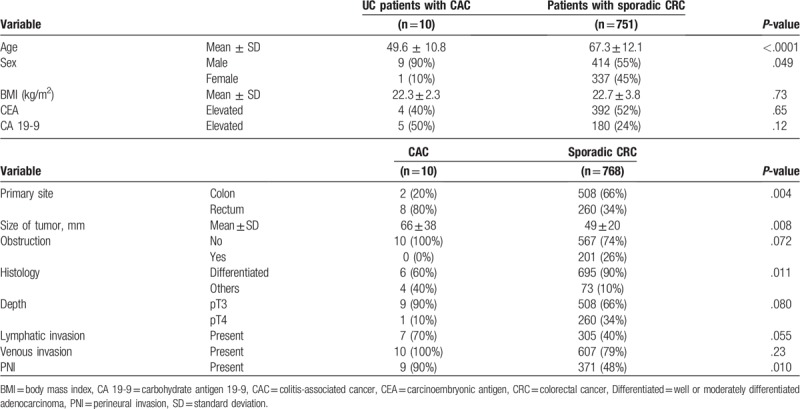
During the study period, 924 patients underwent surgery for pathological T3-T4 carcinoma of the colorectum. As shown in Figure 1, 761 patients were enrolled in the present study (Fig. 1). Among 778 CRCs in these patients, 10 cancers (1.3%) were CACs that had developed in 10 UC patients. Two sporadic CRCs were simultaneously resected in 15 patients and 3 in 1 patient. First, the clinicopathological findings were compared between CAC and sporadic CRC. As shown in Table 1, UC patients were younger than patients with sporadic CRC, and predominantly male. CACs were more frequently found in the rectum than sporadic cancers (80% vs 34%, P = .004). In addition, tumor size was larger in CACs by 17 mm (P = .008). Despite this finding, obstructive cancers were found only in sporadic cases (26%, P = .072). Histological analysis showed that cancer types other than differentiated adenocarcinoma were more often found in CACs than in sporadic CRCs (40% vs 10%, P = .011). PNI was more frequently observed in CACs (90%) than in sporadic cancers (48%, P = .010). There was no significant difference in lymphatic and venous invasion, or pT stage distribution.
Figure 1.
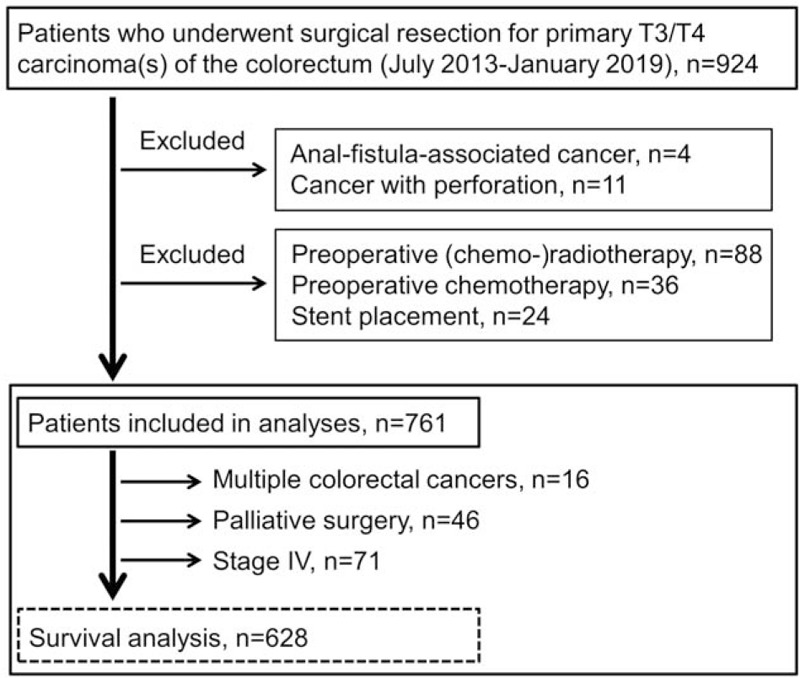
Study flow graph.
Table 1.
Comparison of patient and tumor characteristics between colitis-associated and sporadic colorectal cancers.
The frequency of PNI in CACs and sporadic CRCs was further analyzed according to obstruction and type of CRC. As shown in Figure 2, the positive rate of PNI (90%) was notably higher in CACs with pT3/T4 than in nonobstructive sporadic pT3/T4 tumors (45%, P = .007). In sporadic CRCs, PNI was observed in 59% of obstructive cancers, a significantly higher rate than the nonobstructive cancers (P = .0006).
Figure 2.
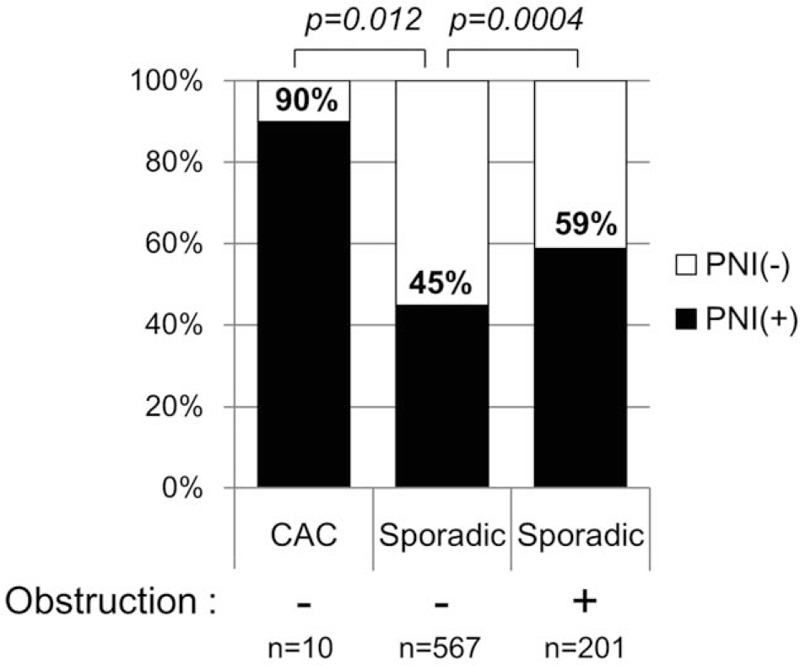
Frequency of perineural invasion in pathological T3/T4 colorectal cancers according to colitis and obstruction. CAC = colitis-associated cancer, PNI = perineural invasion.
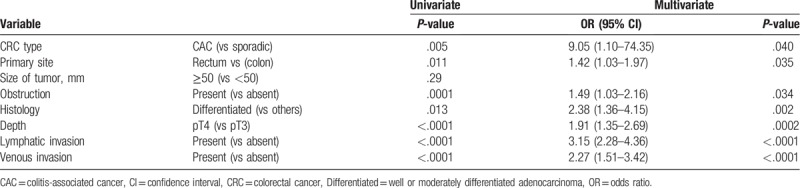
We attempted to identify the factors associated with PNI in CRCs. Univariate analysis revealed that CAC, rectal cancer, differentiated histology, pT4, lymphatic and venous invasions were potential factors related to PNI, which were further entered into a multivariate model. As summarized in Table 2, all the above factors were independently associated with PNI; CAC showed an odds ratio of 9.05 for PNI against sporadic cancer (P = .040).
Table 2.
Univariate and multivariate analysis of factors associated with perineural invasion.
Finally, RFS curves were compared in patients with and without PNI. Here we analyzed 628 patients who underwent curative-intent surgery for a pathological T3/T4 CRC without distant metastases, as shown in Figure 1. The median follow-up period was 36.5 months. The RFS of patients with PNI-negative cancer was significantly better than that of patients with PNI-positive cancer (3-year rate: 84% vs 65%, P < .0001; Fig. 3).
Figure 3.
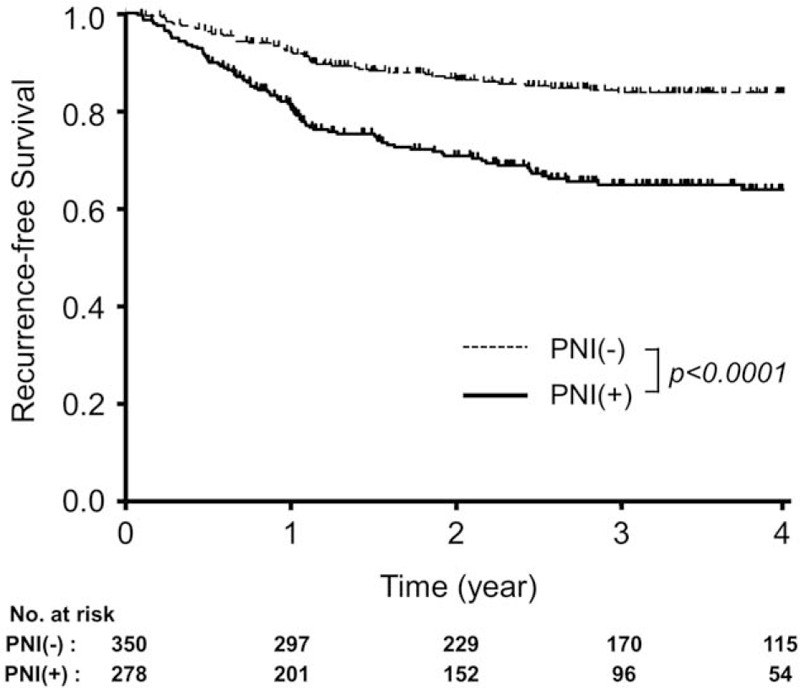
RFS curves of T3/T4 colorectal cancer patients without distant metastases who underwent curative-intent surgery stratified by PNI. The bold line indicates the RFS curve of patients with PNI-positive CRC, and the dashed line that of patients with PNI-negative CRC. CRC = colorectal cancer, PNI = perineural invasion, RFS = recurrence-free survival.
4. Discussion
In previous studies, tumor depth, regional lymph node metastases, and lymphatic and venous invasions were found to be factors associated with PNI[5,10,23,24]; some of their associations were also confirmed in our study. The relationship between histological grades and PNI remains controversial.[5,8,10,12] Neoadjuvant treatments such as chemotherapy and radiotherapy were reported to reduce PNI in rectal cancer.[10] Recently, we demonstrated obstruction as an independent factor of positive PNI.[13] In the present study, we demonstrated that CACs carry a high-risk for PNI independently of the aforementioned risk factors.
Not only prominent histological features such as frequent undifferentiated and invasive histology,[25,26] but also specific genetic alterations characterize CACs. Sporadic CRCs develop by genetic alterations in the adenomatous polyposis coli, RAS, and p53, in the adenoma-carcinoma sequence, whereas alterations in other genes are also implicated in the dysplasia-carcinoma sequences in CACs.[25,27] We previously found that DNA copy numbers and in situ expression of runt-related transcription factor (RUNX)3 in the rectal mucosa of CAC patients were lower than those in UC patients without neoplasms.[28,29] RUNX3 downregulates the expression of tropomyosin receptor kinase B, a high-affinity receptor for BDNF and neurotrophin-4, and controls the neurotrophin receptor phenotype in vivo.[30] Based on our results, we speculate that the interaction of neurotrophins and their receptors may be enhanced by reduced RUNX3 expression, which subsequently contributes to the accelerated PNI in CACs. Further studies are required to address the possible underlying mechanism.
Nationwide population-based cohort studies in 2 different countries reported that CAC patients exhibited an increased mortality.[26,31] A meta-analysis showed poorer overall survival in CAC patients than in patients with sporadic CRC.[32] On the other hand, several groups including us demonstrated that PNI was correlated with increased local recurrence as well as decreased RFS and overall survival in CRC.[10,13] Based on these findings, there is a possibility that the poor survival rate can be attributable to innately frequent PNI in CACs.
We previously demonstrated that obstruction was significantly associated with a higher rate of PNI in sporadic T3/T4 colon cancer.[13] In the present study, PNI was analyzed in sporadic cancers of both the colon and rectum as counterparts of CACs. The same findings were confirmed in pathological T3-T4 CRCs by multivariate analysis in the present study.
Our study contains several limitations. In our series, the expressions of neurotrophins and their receptors were not compared between non-neoplastic mucosa and neoplasia in IBD patients. Second, we cannot exclude the possibility that sporadic-type cancers developed in the IBD patients; however, pathologists confirmed that all of the 10 CACs had the pathological and immunohistochemical features of cancers arising from a colitis background such as p53 overexpression. Moreover, the small number of CACs made it difficult to perform survival analysis to address the prognostic significance of PNI in the UC patient group alone. Lastly, we did not encounter CACs arising in patients with the Crohn disease.
In conclusion, we demonstrated that CAC was an independent factor associated with PNI in pathological T3 and T4 CRC. The molecular mechanisms responsible for the relationship between colitis-associated carcinogenesis and PNI need to be clarified.
Acknowledgment
The authors thank the Medical English Service (Kyoto, Japan) for editing the manuscript.
Author contributions
Conceptualization: Hiroaki Nozawa, Keisuke Hata.
Data curation: Hiroaki Nozawa, Keisuke Hata, Tetsuo Ushiku, Kazushige Kawai, Toshiaki Tanaka, Yasutaka Shuno, Takeshi Nishikawa, Kazuhito Sasaki, Shigenobu Emoto, Manabu Kaneko, Koji Murono, Hirofumi Sonoda.
Formal analysis: Hiroaki Nozawa, Soichiro Ishihara.
Funding acquisition: Hiroaki Nozawa, Keisuke Hata, Kazushige Kawai, Hirofumi Sonoda.
Investigation: Hiroaki Nozawa.
Methodology: Hiroaki Nozawa, Keisuke Hata, Tetsuo Ushiku.
Supervision: Soichiro Ishihara.
Writing – original draft: Hiroaki Nozawa, Keisuke Hata.
Writing – review and editing: Tetsuo Ushiku, Kazushige Kawai, Toshiaki Tanaka, Yasutaka Shuno, Takeshi Nishikawa, Kazuhito Sasaki, Shigenobu Emoto, Manabu Kaneko, Koji Murono, Hirofumi Sonoda, Soichiro Ishihara.
Footnotes
Abbreviations: APC = adenomatous polyposis coli, BDNF = brain-derived neurotrophic factor, CA = carbohydrate antigen, CAC = colitis-associated cancer, CEA = carcinoembryonic antigen, CRC = colorectal cancer, GCNF = glial cell-derived neurotrophic factor, IBD = inflammatory bowel disease, NGF = nerve growth factor, PNI = perineural invasion, RFS = recurrence-free survival, RUNX = Runt-related transcription factor, Trk = tropomyosin receptor kinase, UC = ulcerative colitis.
How to cite this article: Nozawa H, Hata K, Ushiku T, Kawai K, Tanaka T, Shuno Y, Nishikawa T, Sasaki K, Emoto S, Kaneko M, Murono K, Sonoda H, Ishihara S. Accelerated perineural invasion in colitis-associated cancer. Medicine. 2019;98:42(e17570).
This work was supported in part by Grants-in-Aid for Scientific Research (C: grant number; 17K10620, C: grant number; 17K10621, C: grant number; 17K10622, and C: grant number; 18K07194) from Japan Society for the Promotion of Science, and by the Project for Cancer Research and Therapeutic Evolution (P-CREATE, grant number: JP19cm0106502) from the Japan Agency for Medical Research and Development (AMED).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 1985;94:426–7.. [PubMed] [Google Scholar]
- [2].Villers A, McNeal JE, Redwine EA, et al. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol 1989;142:763–8.. [DOI] [PubMed] [Google Scholar]
- [3].Mendenhall WM, Amdur RJ, Hinerman RW, et al. Skin cancer of the head and neck with perineural invasion. Am J Clin Oncol 2007;30:93–6.. [DOI] [PubMed] [Google Scholar]
- [4].Lee IH, Roberts R, Shah RB, et al. Perineural invasion is a marker for pathologically advanced disease in localized prostate cancer. Int J Radiat Oncol Biol Phys 2007;68:1059–64.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fujita S, Nakanisi Y, Taniguchi H, et al. Cancer invasion to Auerbach's plexus is an important prognostic factor in patients with pT3-pT4 colorectal cancer. Dis Colon Rectum 2007;50:1860–6.. [DOI] [PubMed] [Google Scholar]
- [6].Liebig C, Ayala G, Wilks J, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol 2009;27:5131–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peng J, Sheng W, Huang D, et al. Perineural invasion in pT3N0 rectal cancer: the incidence and its prognostic effect. Cancer 2011;117:1415–21.. [DOI] [PubMed] [Google Scholar]
- [8].Ueno H, Shirouzu K, Eishi Y, et al. Characterization of perineural invasion as a component of colorectal cancer staging. Am J Surg Pathol 2013;37:1542–9.. [DOI] [PubMed] [Google Scholar]
- [9].Yang Y, Huang X, Sun J, et al. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointest Surg 2015;19:1113–22.. [DOI] [PubMed] [Google Scholar]
- [10].Knijn N, Mogk SC, Teerenstra S, et al. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol 2016;40:103–12.. [DOI] [PubMed] [Google Scholar]
- [11].Kim HJ, Choi GS, Park JS, et al. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis 2013;28:407–14.. [DOI] [PubMed] [Google Scholar]
- [12].Sabbagh C, Chatelain D, Trouillet N, et al. Does use of a metallic colon stent as a bridge to surgery modify the pathology data in patients with colonic obstruction? A case-matched study. Surg Endosc 2013;27:3622–31.. [DOI] [PubMed] [Google Scholar]
- [13].Nozawa H, Morikawa T, Kawai K, et al. Obstruction is associated with perineural invasion in T3/T4 colon cancer. Colorectal Dis 2019;21:917–24.. [DOI] [PubMed] [Google Scholar]
- [14].Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature. Cancer 2009;115:3379–91.. [DOI] [PubMed] [Google Scholar]
- [15].Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baars JE, Kuipers EJ, van Haastert M, et al. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol 2012;47:1308–22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol 2013;11:1601–8.e1-e4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kishikawa J, Hata K, Kazama S, et al. Results of a 36-year surveillance program for ulcerative colitis-associated neoplasia in the Japanese population. Dig Endosc 2018;30:236–44.. [DOI] [PubMed] [Google Scholar]
- [19].Reinshagen M, von Boyen G, Adler G, et al. Role of neurotrophins in inflammation of the gut. Curr Opin Investig Drugs 2002;3:565–8.. [PubMed] [Google Scholar]
- [20].di Mola FF, Friess H, Zhu ZW, et al. Nerve growth factor and Trk high affinity receptor (TrkA) gene expression in inflammatory bowel disease. Gut 2000;46:670–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reinshagen M, Rohm H, Steinkamp M, et al. Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology 2000;119:368–76.. [DOI] [PubMed] [Google Scholar]
- [22].Amin MB, Edge S, Greene F. AJCC Cancer Staging Manual. Springer, 8th edNew York, NY: 2017. [Google Scholar]
- [23].Huh JW, Kim HR, Kim YJ. Lymphovascular or perineural invasion may predict lymph node metastasis in patients with T1 and T2 colorectal cancer. J Gastrointest Surg 2010;14:1074–80.. [DOI] [PubMed] [Google Scholar]
- [24].Suzuki T, Suwa K, Ogawa M, et al. Adjuvant chemotherapy for the perineural invasion of colorectal cancer. J Surg Res 2015;199:84–9.. [DOI] [PubMed] [Google Scholar]
- [25].Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. Surg Oncol 2010;101:706–12.. [DOI] [PubMed] [Google Scholar]
- [26].Watanabe T, Konishi T, Kishimoto J, et al. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis 2011;17:802–8.. [DOI] [PubMed] [Google Scholar]
- [27].Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807–16.. [DOI] [PubMed] [Google Scholar]
- [28].Watanabe T, Kobunai T, Ikeuchi H, et al. RUNX3 copy number predicts the development of UC-associated colorectal cancer. Int J Oncol 2011;38:201–7.. [PubMed] [Google Scholar]
- [29].Shinagawa T, Hata K, Morikawa T, et al. Loss of RUNX3 Immunoreactivity in non-neoplastic rectal mucosa may predict the occurrence of ulcerative colitis-associated colorectal cancer. Digestion. doi: 10.1159/000497272. [DOI] [PubMed] [Google Scholar]
- [30].Inoue K, Shiga T, Ito Y. Runx transcription factors in neuronal development. Neural Dev 2008;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ording AG, Horváth-Puhó E, Erichsen R, et al. Five-year mortality in colorectal cancer patients with ulcerative colitis or Crohn's disease: a nationwide population-based cohort study. Inflamm Bowel Dis 2013;19:800–5.. [DOI] [PubMed] [Google Scholar]
- [32].Ou B, Zhao J, Guan S, et al. Survival of colorectal cancer in patients with or without inflammatory bowel disease: a meta-analysis. Dig Dis Sci 2016;61:881–9.. [DOI] [PubMed] [Google Scholar]


