Abstract
Recent genome-wide association studies (GWAS) indicated that polymorphisms in ADAMTS7 were associated with artery disease caused by atherosclerosis. However, the correlation between the ADAMTS7 polymorphism and plaque stability remains unclear. The objective of this study was to evaluate the association between 2 ADAMTS7 variants rs3825807 and rs7173743 and ischemic stroke or atherosclerotic plaque vulnerability.
This research is an observational study. Patients with ischemic stroke and normal control individuals admitted to Beijing Tiantan Hospital from May 2014 to October 2017 were enrolled. High-resolution magnetic resonance imaging was used to distinguish vulnerable and stable carotid plaques. The ADAMTS7 SNPs were genotyped using TaqMan assays on real-time PCR system. The multivariate logistic regression analyses were used to adjust for multiple risk factors between groups.
Three hundred twenty-six patients with ischemic stroke (189 patients with vulnerable plaque and 81 patients with stable plaque) and 432 normal controls were included. ADAMTS7 polymorphisms of both rs7173743 and rs3825807 were associated with carotid plaque vulnerability but not the prevalence of ischemic stroke. The T/T genotype of rs7173743 [odds ratio (OR) = 1.885, 95% confidence interval (CI) = 1.067–3.328, P = .028] and A/A genotype of rs3825807 (OR = 2.146, 95% CI = 1.163–3.961, P = .013) were considered as risk genotypes for vulnerable plaque susceptibility.
In conclusion, ADAMTS7 variants rs3825807 and rs7173743 are associated with the risk for carotid plaque vulnerability.
Keywords: ADAMTS7, atherosclerosis, single-nucleotide polymorphism, vulnerable plaque
1. Introduction
Ischemic stroke is a serious health condition that impacts human life. Thromboembolism caused by rupture of atherosclerotic plaques is the main pathogenic factor of ischemic stroke.[1,2] Recent genome-wide association studies (GWAS) indicated that artery disease caused by atherosclerosis is associated with multiple single-nucleotide polymorphisms (SNPs).[3,4] According to these studies, 2 variants of ADAMTS7 (a disintegrin and metalloprotease with thrombospondin motif 7), rs3825807[5–7] and rs7173743,[8] were closely related to atherosclerosis and coronary artery disease (CAD). ADAMTS7, which was first identified in 1999,[9] belongs to a class of zinc metalloproteinases. The ADAMTS family consists of 19 proteases with proteolytic activity against the extracellular matrix. These proteases play important roles in a variety of physiological processes, including coagulation, inflammation, angiogenesis, and organ development.[10] However, there are few studies on the function and mechanism of ADAMTS7 polymorphism in plaque vulnerability.
In this study, we enrolled 326 patients who had experienced ischemic stroke and 432 healthy people as controls, and examined carotid plaque vulnerability using magnetic resonance imaging (MRI). We aimed to determine whether the ADAMTS7 rs7173743 C/T or rs3825807 A/G polymorphism played a role in ischemic stroke or atherosclerotic plaque vulnerability.
2. Materials and methods
2.1. Study population
Three hundred seventy-one patients with ischemic stroke were recruited from Beijing Tiantan Hospital between May 2014 and October 2017, in which 326 patients finished the clinical examination. Patients aged from 40 to 85 years and diagnosed with anterior circulation and noncardiogenic ischemic stroke were included. The presence and severity of ischemic stroke were determined by staff from the neurology and radiology department following “Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2014[11]”. Patients with cardiogenic stroke, those who have undergone carotid surgery, stent implantation or carotid radiation therapy, and those with previous history of liver or renal disease were excluded from this study. Four hundred thirty-two volunteers were recruited from the physical examination center of Beijing Tiantan Hospital served as the normal controls. All controls had no family history of stroke and no personal history of symptomatic transient ischemic attack (TIA) or ischemic stroke. This study has been approved by the ethics committee of Beijing Tiantan Hospital (Approval code: ky2014-004-04). Informed consent has been obtained from all participants.
2.2. Data collection
Demographic information (gender, age, and enrollment time) and stroke risk factors (hypertension, diabetes, hyperlipidemia, previous history of cardiovascular and cerebrovascular diseases, smoking history) were documented. Physical examination included height, weight, heart rate, and blood pressure (systolic blood pressure and diastolic blood pressure). Laboratory examination included triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), and glycosylated hemoglobin (GHb). Imaging examination included carotid high-resolution MRI, and carotid ultrasound.
2.3. Study size
Frequency of T allele of rs7173743 and A allele of rs3825807 is 53% and 75% respectively according to the data on dbSNP website. Precision (δ) = 0.1× proportion, confidence level (1-α) = 0.95. This study needs 341 participants for cases and controls respectively.
2.4. Grouping
Magnetom Trio Tim 3.0T (Siemens Medical Systems, Erlangen, Germany) was used to perform carotid high-resolution MRI examination of enrolled patients. Patients were divided into vulnerable and stable plaque group according to the characteristics of plaque. Vulnerable plaques are defined as those with intraplaque hemorrhage and/or plaque surface defects, such as disrupted luminal surface, ulceration, or mural thrombosis.
2.5. Blinding
Clinicians were blinded to the genotype of patients when they made judgments about ischemic stroke and plaque characteristics. Researchers were blinded to the group allocation when they tested the genotype. In this way, the potential bias in the experiment can be reduced.
2.6. Genotype analyses
Genomic DNA was extracted from 2 mL of peripheral blood using the RelaxGene Blood DNA System (Tiangen Biotech, Beijing, China). The ADAMTS7 rs7173743 C/T (Assay ID: C_31837971_10) and rs3825807 A/G (Assay ID: C_25982426_10) SNPs were genotyped using TaqMan SNP Genotyping Assays (Applied Biosystems, CA) on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems). The cycling conditions for the PCR were as follows: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minutes. Undetected samples and samples of different genotypes were confirmed by sequencing.
2.7. Statistical analyses
Statistical analyses were performed using SPSS 17.0 (SPSS Inc, IL). Continuous variables were evaluated for the normal distributions using Shapiro–Wilk test. Normally distributed variables were reported by their mean and standard deviation (SD), and non-normally distributed variables were reported by their median and interquartile range (IQR). Continuous variables were compared using independent sample t test or Mann–Whitney U test. Categorical variables were described as frequency and percentage. Categorical variables were compared using χ2 test. The polymorphism was tested for Hardy–Weinberg equilibrium using χ2 test. The multivariate logistic regression analyses were used to adjust for multiple risk factors between both groups. The association between ADAMTS7 genotype and risk of ischemic stroke or vulnerable plaque was estimated by computing odds ratio (OR) and 95% confidence interval (CI).
3. Results
3.1. Characteristics of the study population
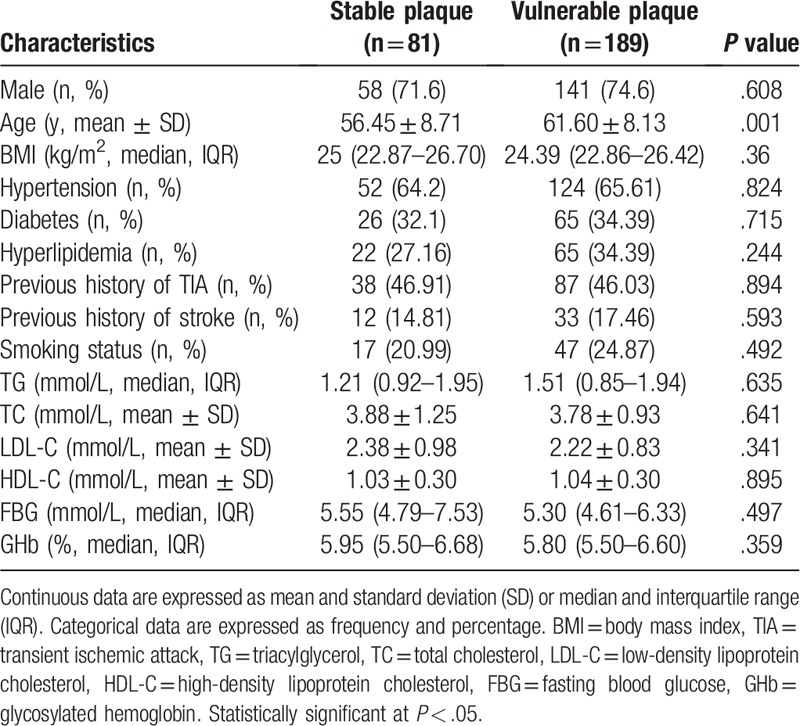
We recruited 371 patients who had experienced ischemic stroke, in whom 326 patients (240 were males and the average age was 57.97 years) finished the clinical examinations and 45 patients were unwilling or unable to perform carotid MRI. Those who did not finish the clinical examinations were excluded from the study. Among the enrolled patients, 270 patients had carotid plaques determined by carotid MRI (189 patients had vulnerable plaques and 81 patients had stable plaques). We recruited 432 volunteers (295 were males and the average age was 51.23 years) as normal controls. Baseline characteristics of ischemic stroke patients and controls included in the study are shown in Table 1. The laboratory parameters of TG, TC, LDL-C, HDL-C, FBG, and GHb were significantly different between 2 groups. Baseline clinical characteristics of patients with vulnerable and stable plaques are shown in Table 2. The traditional risk parameters of ischemic stroke were not significantly different between patients with vulnerable and stable plaques.
Table 1.
Baseline clinical characteristic in controls and patients with ischemic stroke.
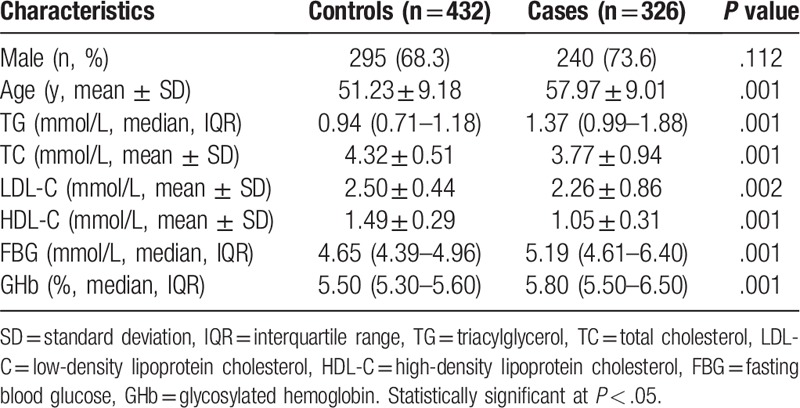
Table 2.
Baseline clinical characteristic in patients with stable and vulnerable plaques.
3.2. Relationship between ADAMTS7 polymorphism and ischemic stroke
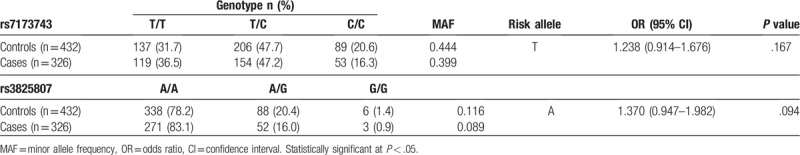
Genotype and allele frequencies of ADAMTS7 polymorphisms in patients of ischemic stroke and controls are shown in Table 3. The distribution of alleles was in Hardy–Weinberg equilibrium (P > .05). There was no significant different genotype distribution of the rs7173743 and rs3825807 polymorphism in patients of ischemic stroke and normal controls (P > .05).
Table 3.
Association between ADAMTS7 polymorphisms and ischemic stroke in Chinese samples.
3.3. Relationship between ADAMTS7 polymorphism and plaque vulnerability
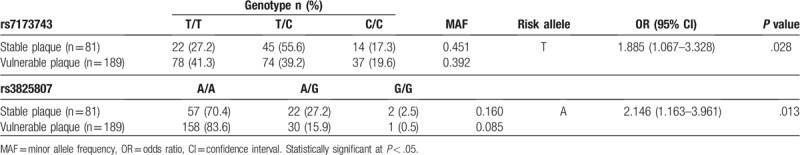
There were different genotype and allele distributions of gene polymorphisms of both rs7173743 and rs3825807 in patients with stable and vulnerable plaques (Table 4). Specifically, 60.8% of patients with vulnerable plaques and 54.9% of patients with stable plaques possessed the risk allele (T) of rs7173743. Furthermore, 91.5% of patients with vulnerable plaques and 84.0% of patients with stable plaques possessed risk allele (A) of rs3825807. The OR was 1.885 (95% CI = 1.067–3.328, P = .028) for the risk genotype T/T of rs7173743 and 2.146 (95% CI = 1.163–3.961, P = .013) for the risk genotype A/A of rs3825807.
Table 4.
Association between ADAMTS7 polymorphisms and vulnerable plaques in Chinese samples.
4. Discussion
Recent progress has focused on the genetics of complex diseases, such as atherosclerosis and CAD, using GWAS analysis. Through high-throughput sequencing and DNA microarray technology, researchers have discovered thousands of DNA markers consisting of SNPs.[7,12] Some were highly correlated with atherosclerosis in multiple independent experiments, such as CDKN2A/2B, ADAMTS7, PHACTR1, SH2B3, and COL4A1/2.[5,6,8,13,14] However, many of these GWAS studies were based on European populations.[4] Owing to differences in the underlying genomic compositions between ethnicities, some atherosclerosis susceptibility loci, which were previously identified in Europeans, cannot be directly identified in the Chinese population. For example, the OR values of 9p21.3, SORT1, and PDGFD in East Asians were smaller than that reported in Europeans.[8] Therefore, when using GWAS data from the European cohort, we need to first validate them in the Chinese population.
Many genes located at atherosclerosis-associated loci participated in glycerolipid and steroid metabolic processes, blood coagulation, cell proliferation, migration, and adhesion.[3] The function of other genes may not be immediately obvious. Further, majority of SNPs were located in the noncoding regions of genome, such as in intergenic spacer or intron.[15] The mechanisms of these SNPs affecting atherosclerosis require further study. According to previous GWAS studies, 2 ADAMTS7 variants rs3825807 and rs7173743 were closely related to atherosclerosis and CAD.[16] The SNP rs7173743 is located in an intergenic spacer, about 40 kb upstream of ADAMTS7. It is involved in the substitution of cytosine (C) to thymine (T). The possible mechanism of rs7173743 polymorphism is not clear. Considering its physical location in relation to ADAMTS7, it may influence ADAMTS7 expression or it may be a genetic locus, which is coexpressed with the disease. The SNP rs3825807 is a missense polymorphism, where adenine (A) is replaced by guanine (G), resulting in a serine (Ser)-to-proline (Pro) substitution in the pro-domain of ADAMTS7. The mechanism of rs3825807 polymorphism has been reported in some studies. Pro-domain of ADAMTS7 is cleaved off during it maturation and activation.[17] Activated ADAMTS7 has proteolytic activity, and it cleaves cartilage oligomeric matrix protein (COMP), which inhibits vascular smooth muscle cell (VSMC) migration.[18,19] VSMC migration is an important process in atherogenesis and vulnerable plaque formation. The G/G genotype of rs3825807 has been shown to affect ADAMTS7 pro-domain cleavage, reduce the cleaved form of COMP, and furthermore inhibit the migratory ability of VSMCs both in vivo and in vitro.[20] These studies demonstrated that the G/G genotype of rs3825807 plays protective roles in atherosclerosis and CAD through reduced ADAMTS7 function.[21]
Atherosclerosis, which is considered a systemic disease, is influenced by multiple factors. Thromboembolism (caused by rupture of an atherosclerotic plaque) is the main pathogenic factor of ischemic stroke. Atherosclerotic plaques in the carotid arteries or intracranial arteries can lead to arterial stenosis, thrombosis, and even embolization, which can trigger to ischemic stroke.[2] In the clinic, vulnerable atherosclerotic plaques are closely related to ischemic stroke. It is important to identify and analyze vulnerable plaques for the secondary prevention of stroke.[1] Histopathological studies have shown that the characteristics of vulnerable plaques included active inflammation, thin or ruptured fibrous cap, large lipid core, disrupted luminal surface, intraplaque hemorrhage, mural thrombosis.[22,23] Therefore, research on the pathophysiological mechanism of vulnerable plaques has a broad prospect with regard to clinical application. There is an urgent need for biomarkers to identify patients with atherosclerotic plaque destabilization.[24] In this study, we aimed to identify molecular markers of vulnerable plaques at the level of genetic polymorphism. We found that the rs7173743 and rs3825807 of ADAMTS7 were closely related to plaque stability. T/T genotype (T allele) of rs7173743 and A/A genotype (A allele) of rs3825807 significantly increased the risk of plaque vulnerability.
We searched the Chinese-specific allele frequency of these 2 SNPs in the Chinese Millionome Database from China National GeneBank. Allele frequencies of rs7173743 and rs3825807 were 0.3965 (3767/9488) and 0.1264 (1813/14202) respectively. The allele frequencies in the database were close to our study. It indicated that the population under study was representative of the Chinese population.
The genotype of ADAMTS7 variants rs3825807 and rs7173743 were not significantly different between patients of ischemic stroke and controls. Meanwhile, the traditional risk parameters (TG, TC, LDL-C, HDL-C, FBG, and GHb) of ischemic stroke were significantly different between the 2 groups. We speculated that these risk factors might reduce the contribution of ADAMTS7 polymorphism. For the study on correlation between plaque vulnerability and SNP, the difference of traditional risk parameters of ischemic stroke were not observed between vulnerable and stable plaque groups. It indicated that risk factors for stroke might not affect plaque vulnerability.
One limitation of our study is that the number of patients included is relatively small. A larger sample size would enable us to identify associations of very small effect sizes; however, these associations frequently have no real clinical importance. In this study, we aimed to search for an association of strong effect size and real clinical impact, which is more effective. The other limitation of the study was that the controls were apparently asymptomatic population, without MRI or ultrasound evidence of the absence of ischemic stroke. Although we administered a questionnaire to exclude individuals with family or personal history of stroke to reduce the bias in our study, the high prevalence of stroke in the Chinese population raised the possibility that some of the controls might have had potential TIA or ischemic stroke. Therefore, this limitation may cause potential bias in the study. Besides, as atherosclerosis is a systemic disease, patients may also have stenosis and vulnerable plaques in cerebral vessel, coronary artery, or aorta. Plaque status in these vessels was not evaluated. Therefore, the relationship between ADAMTS7 polymorphism and atherosclerotic plaque vulnerability in other vessels still needs further study.
In conclusion, our study provides new evidence implicating ADAMTS7 genetic variants with ischemic stroke and plaque vulnerability in the Chinese population. Specifically, the rs7173743 and rs3825807 polymorphisms of ADAMTS7 were associated with the risk of carotid plaque vulnerability but not the prevalence of ischemic stroke. However, the function and mechanism of these polymorphisms in plaque require further study.
Author contributions
Data curation: Zirui Li.
Formal analysis: Mi Shen.
Funding acquisition: Peiyi Gao, Yajie Wang.
Investigation: Haowen Li, Mi Shen, Binbin Sui.
Project administration: Haowen Li.
Resources: Peiyi Gao, Yuxin Wang.
Validation: Jingli Cao, Wenli Zhang.
Writing – original draft: Haowen Li.
Writing – review & editing: Peiyi Gao, Yajie Wang.
Footnotes
Abbreviations: BMI = body mass index, CAD = coronary artery disease, CI = confidence interval, FBG = fasting blood glucose, GHb = glycosylated hemoglobin, GWAS = genome-wide association studies, HDL-C = high-density lipoprotein cholesterol, IQR = interquartile range, LDL-C = low-density lipoprotein cholesterol, MAF = minor allele frequency, MRI = magnetic resonance imaging, OR = odds ratio, SD = standard deviation, SNP = single-nucleotide polymorphism, TC = total cholesterol, TG = triacylglycerol, TIA = transient ischemic attack.
How to cite this article: Li Hw, Shen M, Gao Py, Li Zr, Cao Jl, Zhang Wl, Sui Bb, Wang Yx, Wang Yj. Association between ADAMTS7 polymorphism and carotid artery plaque vulnerability. Medicine. 2019;98:43(e17438).
All authors have read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (81361120402 and 81572474).
The authors have no conflicts of interest to disclose.
References
- [1].Spacek M, Zemanek D, Hutyra M, et al. Vulnerable atherosclerotic plaque—a review of current concepts and advanced imaging. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018;162:10–7.. [DOI] [PubMed] [Google Scholar]
- [2].Yurdagul A, Jr, Doran AC, Cai B, et al. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med 2018;4:86–95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schunkert H, von Scheidt M, Kessler T, et al. Genetics of coronary artery disease in the light of genome-wide association studies. Clin Res Cardiol 2018;107Suppl 2:2–9.. [DOI] [PubMed] [Google Scholar]
- [4].Clarke SL, Assimes TL. Genome-wide association studies of coronary artery disease: recent progress and challenges ahead. Curr Atheroscler Rep 2018;20:47. [DOI] [PubMed] [Google Scholar]
- [5].Ozaki K, Tanaka T. Molecular genetics of coronary artery disease. J Hum Genet 2016;61:71–7.. [DOI] [PubMed] [Google Scholar]
- [6].van Setten J, Isgum I, Smolonska J, et al. Genome-wide association study of coronary and aortic calcification implicates risk loci for coronary artery disease and myocardial infarction. Atherosclerosis 2013;228:400–5.. [DOI] [PubMed] [Google Scholar]
- [7].Prins BP, Lagou V, Asselbergs FW, et al. Genetics of coronary artery disease: genome-wide association studies and beyond. Atherosclerosis 2012;225:1–0.. [DOI] [PubMed] [Google Scholar]
- [8].McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res 2016;118:564–78.. [DOI] [PubMed] [Google Scholar]
- [9].Hurskainen TL, Hirohata S, Seldin MF, et al. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem 1999;274:25555–63.. [DOI] [PubMed] [Google Scholar]
- [10].Patel RS, Ye S. ADAMTS7: a promising new therapeutic target in coronary heart disease. Expert Opin Ther Targets 2013;17:863–7.. [DOI] [PubMed] [Google Scholar]
- [11].Chinese_Society_of_Neurology. Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2014. Chin J Neurol 2015;48:246–57.. [Google Scholar]
- [12].Howson JMM, Zhao W, Barnes DR, et al. Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat Genet 2017;49:1113–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dichgans M, Malik R, Konig IR, et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 2014;45:24–36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee JY, Lee BS, Shin DJ, et al. A genome-wide association study of a coronary artery disease risk variant. J Hum Genet 2013;58:120–6.. [DOI] [PubMed] [Google Scholar]
- [15].Davydov EV, Goode DL, Sirota M, et al. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput Biol 2010;6:e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chan K, Pu X, Sandesara P, et al. Genetic variation at the ADAMTS7 locus is associated with reduced severity of coronary artery disease. J Am Heart Assoc 2017;6:1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hanby HA, Zheng XL. Biochemistry and physiological functions of ADAMTS7 metalloprotease. Adv Biochem 2013;1: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bauer RC, Tohyama J, Cui J, et al. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation 2015;131:1202–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang L, Zheng J, Bai X, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res 2009;104:688–98.. [DOI] [PubMed] [Google Scholar]
- [20].Pu X, Xiao Q, Kiechl S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet 2013;92:366–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pereira A, Palma Dos Reis R, Rodrigues R, et al. Association of ADAMTS7 gene polymorphism with cardiovascular survival in coronary artery disease. Physiol Genomics 2016;48:810–5.. [DOI] [PubMed] [Google Scholar]
- [22].Sun R, Wang L, Guan C, et al. Carotid atherosclerotic plaque features in patients with acute ischemic stroke. World Neurosurg 2018;112:e223–8.. [DOI] [PubMed] [Google Scholar]
- [23].Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108:1664–72.. [DOI] [PubMed] [Google Scholar]
- [24].Hellings WE, Peeters W, Moll FL, et al. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med 2007;17:162–71.. [DOI] [PubMed] [Google Scholar]


