Supplemental Digital Content is available in the text
Keywords: body composition, gestational diabetes mellitus, pregnant women, risk factor
Abstract
Studies have found that the measurement of body composition can be used to identify the gestational diabetes mellitus (GDM) risk in pregnant women. However, few studies focused on the relationship between body composition and GDM development in low GDM risk population. Thus, the objective of this study was to examine the association between body composition and the development of GDM in pregnant women with low risk of gestational diabetes.
A retrospective case-control study was conducted. We reviewed the medical records of 3965 pregnant women who had body composition measurement from March, 2016 to May, 2018 in our hospital. Their sociodemographic, clinical data, and body composition information were collected from medical record. Multiple logistic regression analyses were used.
A total of 2698 subjects were eligible for the study. The mean age of the gravidas was 30.95 ± 4.01 years old. Of all gravidas, 462 had gestational diabetes. Percentage body fat was the strongest risk factor for gestational diabetes after adjusting pre-pregnancy body mass index (BMI) (odds ratio = 1.786, 95% confidence interval = 1.112–2.866, P = .02). The age and extracellular water/intracellular water ratio were independently associated with gestational diabetes.
Percentage body fat was the strongest risk factor for gestational diabetes after adjusting pre-pregnancy BMI. Assessment of body composition may provide important guidance to identify gestational diabetes in pregnant women with low gestational diabetes risk.
1. Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of impaired glucose tolerance developed during pregnancy, which is the main cause of maternal and neonatal complications.[1] The report from Liao shows that the prevalence of GDM in southwest China is 24.5%,[2] with an increasing trend in recent years. GDM can increase the mortality of the pregnant and neonatal and the healthcare expenditure.[3] The incidence of GDM was influenced by several risk factors. Several studies have found that previous GDM, a family history of diabetes and polycystic ovary syndrome can increase the risk of GDM in pregnant women.[4–6] In other words, pregnant women with these factors were in a high risk of GDM. However, pregnant women without these risk factors may also develop GDM. Kalok et al[7] conducted a prospective cross-sectional study among low GDM risk pregnant women above the age of 25 years in Malaysia and found the incidence of GDM was 14% in this population. Unfortunately, there is few methods to screen potential GDM in pregnant women with low GDM risk.
Body composition measurement seems to be a feasible direction for screening potential GDM. Body composition is a known risk factor for a number of conditions such as diabetes,[8] pregnancy-induced hypertension,[9] and preeclampsia.[10] Body composition, such as waist circumference[11] is considered to be closely related to glucose metabolism in humans. A long-term follow-up survey conducted by Yoshimi in Japanese revealed that the percentage of leg fat was negatively associated with the development of diabetes in women (odds ratio [OR] = 0.68, 95% confidence interval [CI] 0.65–0.85).[8] Bolognani et al[11] made a cross-sectional study included 240 pregnant women in Brazil and found that the waist circumference at 20 to 24 weeks of gestation was correlated with GDM risk (OR = 4.02, 95% CI 1.12–13.78). Nevertheless, whether body composition is associated with GDM morbidity in pregnant women without risk factors of GDM have remained unknown. Therefore, the aim of this study was to examine the relationship between body composition and the development of GDM in pregnant women at low risk of GDM in early weeks. The bioelectrical impedance analysis (BIA) technology was used in our research, and the time of BIA measurement was usually earlier than 20 weeks of gestations. To our knowledge, this study is the first of its kind and will; therefore, fill a gap in the literature. The information obtained may be crucial in reducing morbidity of GDM in pregnancy without identified risk factors, which affects millions worldwide. Additionally, the main method of diagnosing GDM is oral glucose tolerance test (OGTT) at 24 to 28 weeks at present.[12] If we can identify the pregnant women having a GDM risk earlier than 24 to 28 gestational weeks, we can early implement the intervention to prevent GDM.
2. Materials and methods
2.1. Setting and subjects
A retrospective case-control study was conducted at the West China Second University Hospital, Sichuan University, a hospital receiving approximately 10,600 pregnant women in southwest China each year. We studied data on pregnant women who registered and whose body composition was measured at the Department of Obstetrics from March, 2016 to May, 2018. Pregnant women of 18 years and older and with single pregnancy were included in the study. The exclusion criteria were:
-
(1)
previous GDM or any type of pre-pregnancy diabetes;
-
(2)
disease whose medical treatment may affect glucose metabolism, such as chronic hypertension and thyroid disease;
-
(3)
abortion or induced labor because of deformity;
-
(4)
a family history of diabetes;
-
(5)
incomplete data; and
-
(6)
2 or more pregnancies.
Screening for GDM was performed on all subjects at 24 to 28 gestational weeks using a 1-step 75 g oral glucose tolerance test (OGTT), and GDM was diagnosed according to the International Association of Diabetes Pregnancy Study Groups criteria.[12]
2.2. Data collection
The sociodemographic and clinical information such as age, education level, pre-pregnancy weight, height, ethnicity, parity, history of disease, the value of OGTT, family history of diabetes were collected from medical records by 1 trained researcher using the hospital database. The total body water (TBW), intracellular water (ICW), extracellular water (ECW), protein, minerals, fat free mass (FFM), fat mass (FM), and other body composition information, which were tested between 13 and 20 weeks of the pregnant women by the InBody 770 system, were also collected.
2.3. Measurement instrument
Measurement of body composition was made using multifrequency BIA with 8-point tactile electrodes (InBody 770; Biospace, Seoul, Korea). This analyzer estimated 5 segments composition, using an alternating current of 250 mA at variable frequencies of 1, 5, 50, 250, 500, and 1000 kHz. Evaluation was performed between the time of registration and 20 gestational weeks.
2.4. Ethics consideration
This study was approved by the Ethics Committee of West China Second University Hospital, Sichuan University.
2.5. Statistical analysis
Data were analyzed using SPSS Statistics V23.0. Qualitative data were reported as counts and percentages. Quantitative variables were recorded as median, interquartile range or mean ± standard deviation according to the data distribution. Student t test was used for quantitative data, and Pearson χ2 test was used for qualitative data. Body composition percentage was equal to body composition (kg) divided by body weight (kg). And then we divided the data into quartiles (25%, 50%, and 75%). To identify the risk quartile of body composition associated with the development of GDM, a univariate logistic regression analysis were first be used (variables with P-value < .1 went to next step) and then a stepwise multiple logistic regression model was composed through a purposeful selection process of variables. Hosmer–Lemeshow statistic were calculated as measures of model fit. The significant level was set at α < .05.
3. Results
3.1. Characteristics and body composition of the subjects
A total of 3965 pregnant women who registered in our hospital and completed the body composition and OGTT were assessed for eligibility. Based on the inclusion and exclusion criteria, 1267 women were excluded. Finally, 2698 pregnant women entered the final analysis, of whom 462 developed GDM (17.1%). A flow diagram was presented in Figure 1.
Figure 1.
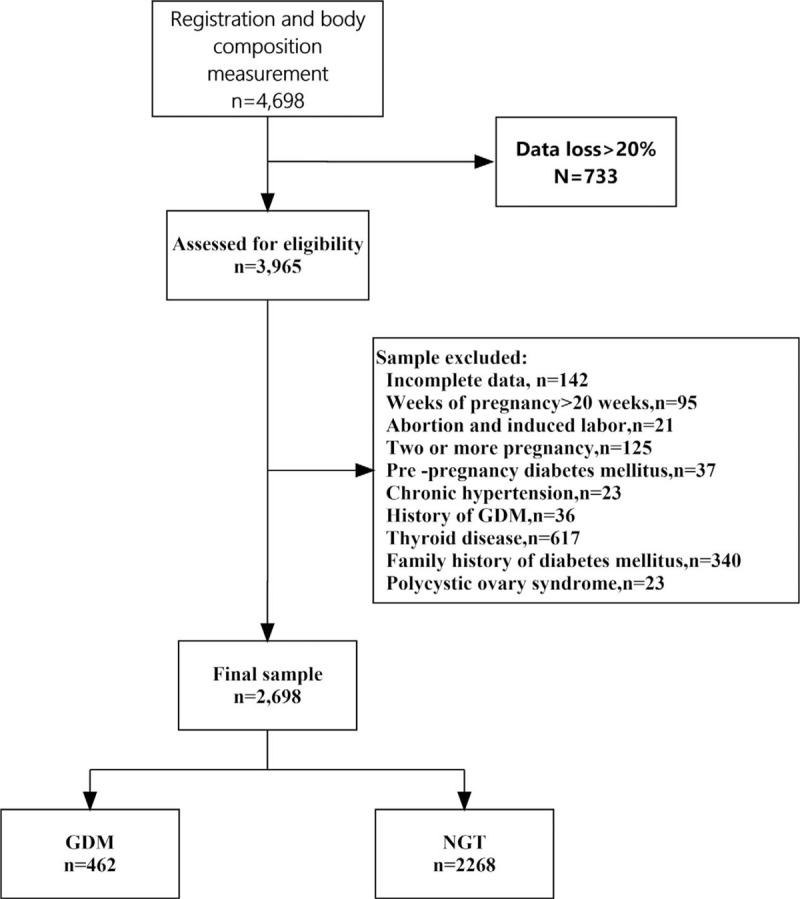
Flow chart of the population study. GDM = gestational diabetes mellitus, NGT = normal glucose tolerance.
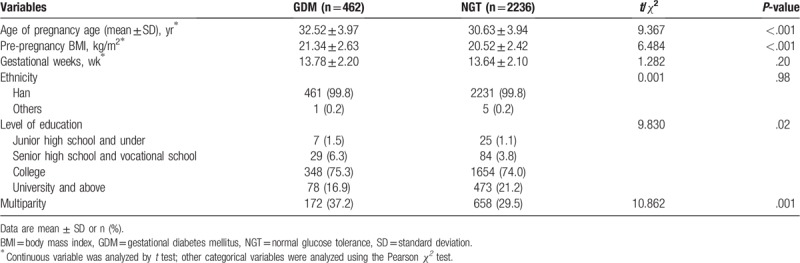
Detailed sociodemographic and clinical characteristics of the subjects were summarized in Table 1. There were no significant differences in ethnicity, and gestational weeks. Age of pregnancy, pre-pregnancy body mass index (BMI), the rate of and multiparity were significantly higher in the GDM than in the normal glucose tolerance (NGT) group.
Table 1.
Characteristics of pregnant women (n = 2698) who entered the final analysis.
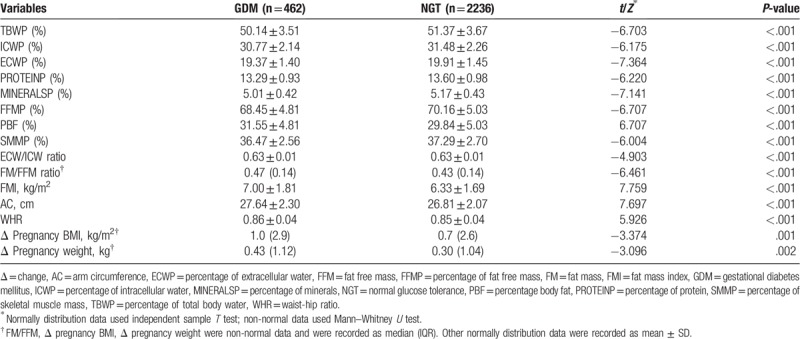
There were significant differences in body composition between the 2 groups (Table 2). The percentage of TBW, percentage of ICW, percentage of ECW, percentage of minerals, percentage of FFM, and so on were higher in NGT group compare with GDM group (P < .001).
Table 2.
The comparisons of body composition percentage between case group and control group.
3.2. Risk factors associated with the development of GDM
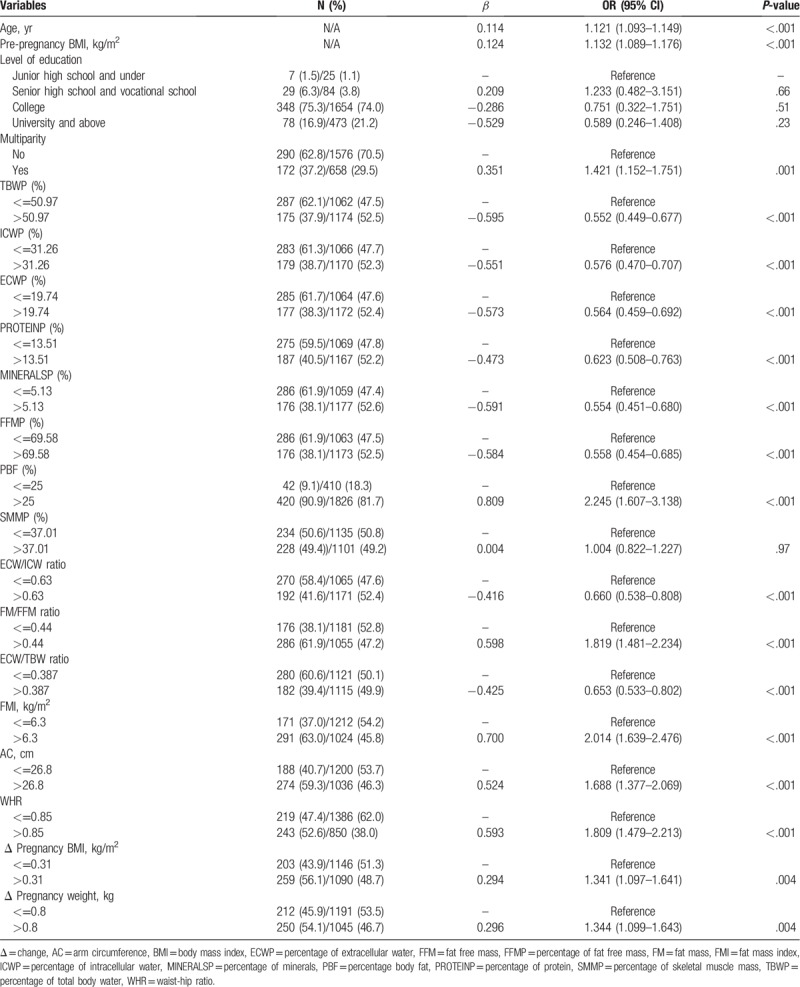
The potential risk factors for GDM included age, pre-pregnancy BMI, percentage body fat, fat mass index (FMI), waist-hip ratio, and ECW/ICW ratio, and so on (Supplementary Table 1). For ease to read, we simplified the Supplementary Table 1, as shown in Table 3.
Table 3.
Univariate logistic regression analysis for the association between maternal body composition and gestational diabetes (n = 2698).
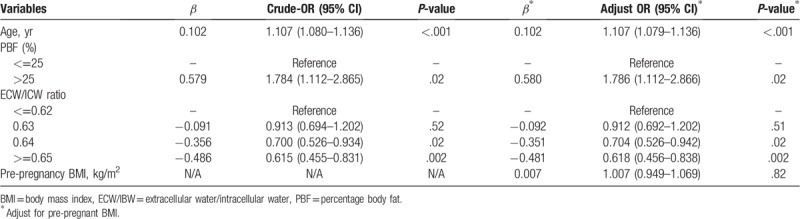
After removing the less clinically relevant and no statistically significant variables, 18 variables entered into the multivariate logistic regression model. Three of these 18 variables remained in the model (Table 4). After adjusting pre-pregnancy BMI and age, the risk for GDM increased in women whose percentage body fat higher than 25% compared with percentage body fat lower than 25% (OR = 1.786, 95% CI = 1.112–2.866, P = .02). The risk for GDM decreased in women who had higher values in ECW/ICW ratio. The Hosmer–Lemeshow goodness of fit test indicated no significant difference between the observed and the expected values (χ2 = 9.838, degrees of freedom = 8, P = .277)
Table 4.
Stepwise multivariate logistic regression analysis for the association between body composition and gestational diabetes.
4. Discussion
In this study, we found that the age, percentage body fat, and ECW/ICW ratio were independently associated with the development of GDM.
Not surprisingly, the age was a risk factor for GDM after adjusting pre-pregnancy BMI. This result was consistent with previous studies.[4,13] A research revealed that older age as a risk factor of developing GDM was evident among Asian women.[14] The reason may be that aging can lead to insulin insensitivity, impaired lipid metabolism, and glucose tolerance. After entering middle age, pregnant women are prone to obesity. About 15% of adult women aged 18 years and older have obesity worldwide.[15] Obesity itself is an independent risk factor for diabetes. Therefore, the older age of pregnant women may experience a higher incidence of GDM, which has greater impacts on maternal and child health.[16] Besides, since the universal 2-child policy was implemented in China from the end of 2015, the number of pregnant women at an advanced age (>=35 years) has been increasing. Data showed that the pregnant women at advanced maternal age took 19.9% in all pregnant women only in the first 6 months of 2016 in China,[17] which is similar to 19.16% in this study. Therefore, we should strengthen the health education about GDM risk management to pregnant women at advanced age.
As expected, this study found that pregnant women in the group with higher percentage body fat (>25%) had significantly higher risk of GDM than those with normal percentage body fat (<=25%) after adjusting age and pre-pregnancy BMI. Obviously, this result was supported by Iqbal's report. Iqbal conducted a nest case-control study in South Asian women and found the higher percentage body fat increased the risk of GDM (OR = 1.07, 95% CI 1.03–1.13). Percentage body fat was determined FM (kg) by weight (kg). FM, the sum mass of subcutaneous fat, visceral fat, and intramuscular fat, is related to the serum adiponectin level and insulin resistance.[18] Visceral fat can reportedly damage islet function,[19,20] and lead to hepatic insulin resistance through its high degree of lipolytic activity and high release of free fatty acids into portal circulation.[21] Subcutaneous fat is also associated with insulin resistance.[22,23] In addition, it was shown that the increased inflammation[24] and cytokines[25] produced by fat tissue may lead to the development of diabetes. Some studies have proved that percentage body fat was associated with diseases including metabolic syndrome[26] and preeclampsia.[27] RamirezVelez et al[26] conducted a study in university students and found that percentage body fat was positively correlated to metabolic components that included glucose, high-density lipoprotein cholesterol, triglycerides, total cholesterol, waist circumference, and FMI. A study reported the changes in percentage body fat without in body weight was positively related to the changes in the glycated hemoglobin levels in diabetic patients.[28] These components are closely related to the development of GDM. It was clear that GDM or diabetes develops when a person has high glucose, high level of glycated hemoglobin, high-density lipoprotein cholesterol, high triglycerides and cholesterol, and large waist circumference. We have known that obesity was closely related to GDM. Obesity means that the body stores too much energy in the form of fat, rather than simply being overweight. At present, the most commonly used measure index of weight status is BMI, but it cannot distinguish between body fat and FFM, so it is difficult to detect muscular obesity and invisible obesity. Therefore, the application of BMI is limited, and percentage body fat can make up for this deficiency. In our study, we adopted the percentage body fat with the Japanese standard, which defined the percentage body fat below 25% as normal, percentage body fat higher than 25% as obesity.[29] This standard may not apply to the Chinese people which was a limitation in our study. Therefore, it is necessary to establish a percentage body fat standard for Chinese people. And percentage body fat was a good predicator of GDM, suggesting that pregnant women having a high percentage body fat should be implemented fat management.
In the human body, TBW consists of ECW and ICW. ECW mainly includes tissue interstitial fluid, plasma, lymph, cerebrospinal fluid, and so on. It accounts for 1/3 of TBW. The increase of ICW during pregnancy is likely to bring changes to the maternal body, including not only increases in mammary and uterine tissues, but also the growth of the fetus and placenta, because of maternal blood volume expansion and accumulation of amniotic fluid.[30] Our study revealed that ECW/ICW ration was a protective factor for GDM, which was inconsistent with the result reported by Xu et al,[31] who conducted a prospective cohort study enrolled 1135 women in 11 hospital in China. BIA and dietary surveys were used to determine body composition and the intake of nutrients in subjects at 21 to 24 weeks of gestation. They reported that ECW/ICW ratio increased GDM risk significantly compared with the lowest quartile. There may be some reasons for this difference. First, only low GDM risk pregnant women were included in this study, and their body water status may be different from that of pregnant women at high risk of GDM. In addition, our subjects were mainly pregnant women living in Sichuan province. The subjects of 2 studies had significant dietary differences, which may affect their body composition including fluid status. Second, the data we collected about the body composition of the pregnant women's body was at 13 to 20 weeks of gestation, while the body composition data collected in Xu's study was 21 to 24 weeks of gestation. The nutritional status of pregnant women varied greatly with the increase of gestational weeks. Our study showed that the value of ECW/ICW ratio fluctuated around 0.6 and peaked at 0.68. This value was lower than the 0.8 reported by Xu. Obviously, our subjects had lower ECW/ICW ratio. This finding revealed that in a certain range, ECW/ICW ratio may decrease GDM risk. And the relationship between body water and the risk of GDM needs further study.
In recent years, BIA has been applied in many hospitals in China to measure the body composition and nutrition status of pregnant women. It is a rapid, noninvasive, valid, inexpensive, and simple method to measure body composition using BIA in pregnancy. Some studies found that BIA had been highly correlated with doubly labeled water method,[32] and dual-energy x-ray absorptiometry[33] in measuring body composition. Studies have revealed the clinical significance of BIA in the measurement of body composition.[31,34,35] Body composition refers to fat, water and other components and percentage in the overall mass of the human body. As we all known, the water shows a relative and absolute increase within advancing gestational weeks and finally is the largest component of weight gain in pregnancy.[36] Many studies have confirmed the validity of BIA in measuring TBW, ECW, and ICW.[37–39] Multifrequency BIA can identify the human body consists of 5 different cylinders (legs, trunk, and arms) with different resistance, through which impedance can be measured separately to achieve segmented water analysis. This differs from other methods which take the human body as a single entity.[40] In general, BIA is reliable for measuring body composition.
In this study, we did not find the relationship between FMI and GDM in multiple analysis. FMI is determined by dividing tissue mass (kg) by height (m) squared. A recent study suggested that FMI is better than BMI in evaluating obesity.[41] A cross-sectional study involving 1687 volunteers revealed that FMI was positively correlated with metabolic syndrome components. Fat mass and obesity-associated gene that has a positive correlation with obesity was also correlated with FMI.[42] We also did not find that waist-hip ratio was associated with the development of GDM. However, some previous studies found that waist-hip increased the risk of the development of GDM.[43] Therefore, more studies are needed to clarify the relationship between body composition and GDM.
Our study had some limitations. First, we used a retrospective chart review design to collect data; therefore, it was not possible to obtain detailed lifestyle factors such as dietary habits in women during the period from pregnancy to being diagnosed with GDM. Second, the sample of this study was not representative enough because it was drawn from only 1 medical center in west China. The economic and cultural development of the country's eastern and western regions differ greatly. Third, BIA was more accurate in measuring hydration in human body, but less accurate in measuring FM because fat or fat-containing tissues produce a poor electrical pathway. Fourth, pre-pregnancy BMI was reported by patients themselves and there may be a slight deviation. Therefore, our observation of abnormal body composition to predict GDM should be used with caution in the population screening of low-risk subjects and needs to be validated in a multicenter, large sample.
These risk factors discussed above can provide guidance to identify GDM in pregnant women with a low GDM risk. Based on test results, patients with increased risk of GDM can be provided with clinical interventions about changes in diet, exercise, and the achievement of desirable body composition. The measurement of body composition can assist clinicians in early identification and diagnosis of GDM. Yet still, prospective, multicenter studies are needed to confirm the association between body composition and the development of GDM in Chinese pregnant women with low risk of GDM.
Acknowledgments
The authors are grateful to Dongtao Lin from College of Foreign Languages and Cultures of Sichuan University for editing the manuscript and Yanqiao Wu from West China Second University Hospital, Sichuan University for making suggestion and evaluation on the statistical part of the article.
Author contributions
Conceptualization: Bi-Ru Luo.
Formal analysis: Yan Wang.
Methodology: Bi-Ru Luo.
Software: Yan Wang.
Supervision: Bi-Ru Luo.
Writing – original draft: Yan Wang.
Writing – review and editing: Bi-Ru Luo.
Supplementary Material
Footnotes
Abbreviations: BIA = bioelectrical impedance analysis, BMI = body mass index, ECW = extracellular water, FFM = fat free mass, FM = fat mass, FMI = fat mass index, GDM = gestational diabetes mellitus, ICW = intracellular water, NGT = normal glucose tolerance, OGTT = oral glucose tolerance test, TBW = total body water.
How to cite this article: Wang Y, Luo BR. The association of body composition with the risk of gestational diabetes mellitus in Chinese pregnant women. Medicine. 2019;98:42(e17576).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Boriboonhirunsarn D, Talungjit P, Sunsaneevithayakul P, et al. Adverse pregnancy outcomes in gestational diabetes mellitus. J Med Assoc Thai 2006;89Suppl 4:S23–8.. [PubMed] [Google Scholar]
- [2].Liao S, Mei J, Song W, et al. The impact of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) fasting glucose diagnostic criterion on the prevalence and outcomes of gestational diabetes mellitus in Han Chinese women. Diabet Med 2014;31:341–51.. [DOI] [PubMed] [Google Scholar]
- [3].Chen Y, Quick WW, Yang W, et al. Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manag 2009;12:165–74.. [DOI] [PubMed] [Google Scholar]
- [4].Lin P, Hung C, Chan T, et al. The risk factors for gestational diabetes mellitus: a retrospective study. Midwifery 2016;42:16–20.. [DOI] [PubMed] [Google Scholar]
- [5].Teede HJ, Harrison CL, Teh WT, et al. Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol 2011;51:499–504.. [DOI] [PubMed] [Google Scholar]
- [6].Hao M, Lin L. Analysis of the high risk factors of gestational diabetes mellitus in 820 case. Chin J Clin Obstet Gynecol 2015;6:421–3.. [Google Scholar]
- [7].Kalok A, Peraba P, Shah SA, et al. Screening for gestational diabetes in low-risk women: effect of maternal age. Horm Mol Biol Clin Investig 2018;34:20170071. [DOI] [PubMed] [Google Scholar]
- [8].Tatsukawa Y, Misumi M, Kim YM, et al. Body composition and development of diabetes: a 15-year follow-up study in a Japanese population. Eur J Clin Nutr 2018;72:374–80.. [DOI] [PubMed] [Google Scholar]
- [9].Shao J, Qi J. The relationship between body adiposity index and pregnancy-induced hypertension in third-trimester pregnant women. Blood Press Monit 2017;22:279–81.. [DOI] [PubMed] [Google Scholar]
- [10].Staelens AS, Vonck S, Molenberghs G, et al. Maternal body fluid composition in uncomplicated pregnancies and preeclampsia: a bioelectrical impedance analysis. Eur J Obstet Gynecol Reprod Biol 2016;204:69–73.. [DOI] [PubMed] [Google Scholar]
- [11].Bolognani CV, de Sousa Moreira Reis LB, de Souza SS, et al. Waist circumference in predicting gestational diabetes mellitus. J Matern Fetal Neonatal Med 2014;27:943–8.. [DOI] [PubMed] [Google Scholar]
- [12].Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cozzolino M, Serena C, Maggio L, et al. Analysis of the main risk factors for gestational diabetes diagnosed with International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria in multiple pregnancies. J Endocrinol Investig 2017;40:937–43.. [DOI] [PubMed] [Google Scholar]
- [14].Carolan M, Davey MA, Biro MA, et al. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery 2012;28:778–83.. [DOI] [PubMed] [Google Scholar]
- [15].Devlieger R, Benhalima K, Damm P, et al. Maternal obesity in europe: where do we standand how to move forward?: a scientific paper commissioned by the european board and college of obstetrics and gynaecology(ebcog). Eur J Obstet Gynecol Reprod Biol 2016;201:203–8.. [DOI] [PubMed] [Google Scholar]
- [16].Teng XH, Pan SL. The relationship among maternal age,risk factors an pregnancy outcomes: a retrospective cohort study. J Pract Obstet Gynecol 2017;33:692–6.. [Google Scholar]
- [17].Zhao J, Feng L. Management of pregnant women at advanced maternal age. Chin J Prac Gynecol Obstetr 2017;33:96–9.. [Google Scholar]
- [18].Suh Y, In-Kyu L, Kim D. Proinflammatory cytokines and insulin resistance in nonobsese women with high body fat and low fat free mass. Diabetes Metab J 2007;31:136–43.. [Google Scholar]
- [19].Samaras K, Botelho NK, Chisholm DJ, et al. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–9.. [DOI] [PubMed] [Google Scholar]
- [20].Wang DT, Peng DQ. The correlation of abdominal adipose tissue distribution and insulin resistance in type 2 diabetes mellitus. Chin J Diabetes 2015;23:587–91.. [Google Scholar]
- [21].Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev 2002;18Suppl 2:S5–9.. [DOI] [PubMed] [Google Scholar]
- [22].Tumurbaatar B, Poole AT, Olson G, et al. Adipose tissue insulin resistance in gestational diabetes. Metab Syndr Relat Disord 2017;15:86–92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kelley DE, Thaete FL, Troost F, et al. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000;278:E941–8.. [DOI] [PubMed] [Google Scholar]
- [24].Wolf M, Sauk J, Shah A, et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care 2004;27:21–7.. [DOI] [PubMed] [Google Scholar]
- [25].Valsamakis G, Kumar S, Creatsas G, et al. The effects of adipose tissue and adipocytokines in human pregnancy. Ann N Y Acad Sci 2010;1205:76–81.. [DOI] [PubMed] [Google Scholar]
- [26].RamirezVelez R, CorreaBautista JE, SandersTordecilla A, et al. Percentage of body fat and fat mass index as a screening tool for metabolic syndrome prediction in Colombian University Students. Nutrients 2017;9:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sween LK, Althouse AD, Roberts JM. Early-pregnancy percent body fat in relation to preeclampsia risk in obese women. Am J Obstet Gynecol 2015;212: 84.e1. [DOI] [PubMed] [Google Scholar]
- [28].Sohmiya M, Kanazawa I, Kato Y. Seasonal changes in body composition and blood HbA(1c) levels without weight change in male patients with type 2 diabetes treated with insulin. Diabetes Care 2004;27:1238–9.. [DOI] [PubMed] [Google Scholar]
- [29].Nagamine S, Suzuki S. Anthropometry and body composition of Japanese young men and women. Hum Biol 1964;36:8–15.. [PubMed] [Google Scholar]
- [30].Lof M, Forsum E. Evaluation of bioimpedance spectroscopy for measurements of body water distribution in healthy women before, during, and after pregnancy, Journal of Applied Physiology. J Appl Physiol 2004;96:967–73.. [DOI] [PubMed] [Google Scholar]
- [31].Xu Q, Gao ZY, Li LM, et al. The association of maternal body composition and dietary intake with the risk of gestational diabetes mellitus during the second trimester in a cohort of chinese pregnant women. Biomed Environ Sci 2016;29:1–1.. [DOI] [PubMed] [Google Scholar]
- [32].Liu J, Pu J, Sun R, et al. Comparison of bioelectrical impedance analysis and isotope dilution method in assessment of body cmpositon. Clin J Prev Med 2008;42:244–7.. [PubMed] [Google Scholar]
- [33].Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res 2012;32:479–85.. [DOI] [PubMed] [Google Scholar]
- [34].Piuri G, Ferrazzi E, Bulfoni C, et al. Longitudinal changes and correlations of bioimpedance and anthropometric measurements in pregnancy: simple possible bed-side tools to assess pregnancy evolution. J Matern Fetal Neonatal Med 2017;30:2824–30.. [DOI] [PubMed] [Google Scholar]
- [35].Larciprete G, Valensise H, Vasapollo B, et al. Body composition during normal pregnancy: reference ranges. Acta Diabetol 2003;40Suppl 1:S225–32.. [DOI] [PubMed] [Google Scholar]
- [36].McCartney CP, Pottinger RE, Harrod JP. Alterations in body composition during pregnancy. Am J Obstet Gynecol 1959;77:1038–53.. [DOI] [PubMed] [Google Scholar]
- [37].Zhou SG, Chen W. Human body water composition measurement: methods and clinical application. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018;40:603–9.. [DOI] [PubMed] [Google Scholar]
- [38].Diouf A, Diongue O, Nde M, et al. Validity of bioelectrical impedance analysis in predicting total body water and adiposity among Senegalese school-aged children. Plos One 2018;13:e0204486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lukaski HC, Hall CB, Siders WA. Validity of bioelectrical impedance vector anlysis (BIVA) to assess total body water (TBW) in women before, during pregnancy and post partum (PP). Faseb J 2007;21:A7. [Google Scholar]
- [40].Maria F, Niamh D, Keating AM, et al. Maternal body composition measurements as a predictor of GDM. Am J Obstet Gynecol 2017;216Suppl 1:358. [Google Scholar]
- [41].Bao JP, Yu KL, Li YL, et al. Lean mass index and fat mass index of Lingao,China. J Guangxi Normal Univ 2017;35:142–7.. [Google Scholar]
- [42].Pecioska S, Zillikens MC, Henneman P, et al. Association between type 2 diabetes loci and measures of fatness. Plos One 2010;5:e8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Madhavan A, Kumari RB, Sanal MG. A pilot study on the usefulness of body mass index and waist hip ratio as a predictive tool for gestational diabetes in Asian Indians. Gynecol Endocrinol 2008;24:701–7.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


