Abstract
Light-sheet microscopy is an ideal technique for imaging large cleared samples; however, the community is still lacking instruments capable of producing volumetric images of centimeter-sized cleared samples with near-isotropic resolution within minutes. Here, we introduce the mesoscale selective plane-illumination microscopy (mesoSPIM) initiative, an open-hardware project for building and operating a light sheet microscope that addresses these challenges and is compatible with any type of cleared or expanded sample (www.mesospim.org).
Over the course of the past decade, tissue clearing methods have reached a high level of sophistication with a wide variety of approaches now available1. Common clearing techniques fall into two categories: Approaches using organic solvents, for example the DISCO family of protocols2–5, and methods using aqueous solutions such as CLARITY6 and CUBIC7, 8. To image samples processed with these methods, a wide range of commercial light-sheet microscopes can be utilized (Supplementary Note 1). Nonetheless, many users still experience significant shortcomings when using existing instruments to image cleared samples: For example, the imaging chamber, sample holders, and sample stages of microscopes designed for time-lapse imaging in developing embryos are usually undersized for cm-sized cleared samples. Even if the setup was specifically designed with clearing in mind, accommodating large samples can be a significant challenge: Modern clearing techniques can render a whole mouse central nervous system (CNS) or even entire mice4, 5 transparent; yet, there are no instruments capable of imaging such samples without cutting. In addition, many instruments achieve optimal image quality only for a limited selection of immersion media, often restricted by the specifications of existing microscope objectives. As typical refractive indices in light-sheet microscopy range from 1.33 (for water) to 1.56 (for a mixture of benzyl alcohol and benzyl benzoate, BABB9), this limits several commercial microscopes to a narrow subset of clearing techniques (Supplementary Table 1).
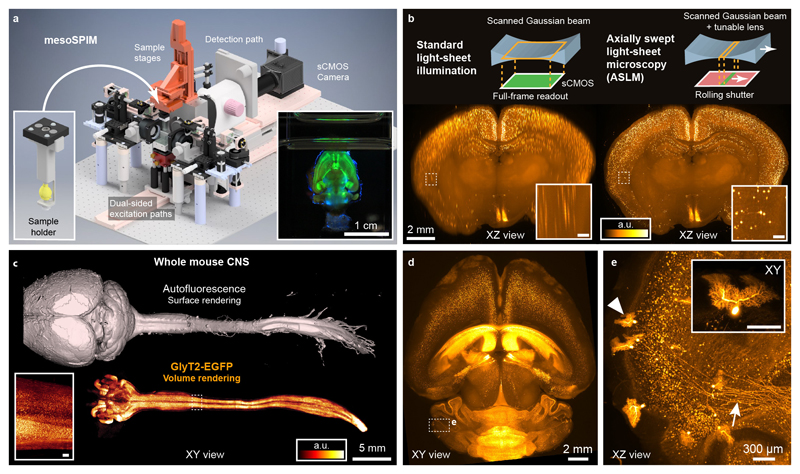
To overcome these limitations, we set out to design a modular light-sheet microscope that combines a large imaging volume, excellent image quality over large fields-of-view (FOV) with simple and versatile sample handling (Fig. 1a, Supplementary Note 2, Supplementary Video 1). To allow multi-view acquisitions, we adopted an instrument layout similar to the original selective plane illumination microscope (SPIM)10 with a horizontal detection path and a vertical sample rotation axis (Supplementary Fig. 1 and 2). The instrument is equipped with a zoom macroscope in the detection path which allows FOVs of 2-21 mm in combination with a sCMOS camera. This enables users to view large samples and then zoom in to reveal minute details such as individual axons (Fig. 1c-e). Therefore, we have termed the instrument the mesoscale selective plane-illumination microscope (mesoSPIM).
Figure 1. Example demonstrations of the mesoSPIM light-sheet mesoscope.
a) Overview of the mesoSPIM instrument (Version 4). Left inset: 3D-printed sample holder with magnetic quick-exchange mount. Alternatively, samples can be mounted in a cuvette. Right inset: Photograph of a Thy1-YFP mouse brain during image acquisition. b) Comparison of axial image quality achieved in a CLARITY-cleared VIP-tdTomato mouse brain for standard light-sheet illumination (left) and for the axially swept light-sheet mode, ASLM (right). Images are maximum intensity projections over 250-μm range. c) Whole-CNS imaging with the mesoSPIM. A whole central nervous system was dissected from a Glycine Transporter-2 EGFP (GlyT2-EGFP) mouse and cleared using the X-CLARITY protocol. The inset shows glycinergic neurons in a subregion of the spinal cord. d) Overview image of a CLARITY-cleared TPH2Cre-tdTomato mouse brain. e) Volume rendering of sparsely labeled Purkinje cells and their axonal projections (arrow) from the dashed box in (d). Inset: individual Purkinje cell (arrow head). Panels (d) and (e) use the same colorbar as (b). Scale bars of all insets: 200 μm. The imaging experiments were conducted once using animals aged 6 weeks (c) and 2 months (d).
To streamline sample handling, we use magnetic quick-exchange mounts for the immersion cuvettes and sample holders. These mounts allow rapid switching between different immersion media and samples within less than a minute (Supplementary Video 2). Samples are usually mounted in a cuvette11 or clamped in a 3D-printed holder (Fig. 1a, Supplementary Fig. 3).
The mesoSPIM light-sheet is generated by rapidly scanning a Gaussian beam in the vertical plane using a galvo scanner similar to a digital scanned light-sheet microscope (DSLM)12. This approach has several advantages when imaging large cleared samples: Firstly, it results in uniform image brightness as each part of the FOV is illuminated with the same intensity. Secondly, when changing the detection FOV using the detection zoom, the height of the light-sheet can be easily adapted by regulating the amplitude of the galvo waveform. Finally, DSLM illumination reduces shadow artefacts13: In light-sheet microscopy, any sample feature that absorbs or refracts the excitation light casts a shadow across the FOV. The resulting images are full of stripes along the illumination direction which complicates image analysis and visualization. Because in DSLM each part of the sample is illuminated with a scanned cone of excitation light, the shadow zone behind absorbing objects can be shortened by increasing the opening angle of the cone (equivalent to increasing the excitation NA). In the mesoSPIM, we use an NA of 0.15 to achieve homogenous illumination with minimal shadow artifacts.
A Gaussian beam with increased excitation NA does, however, have a reduced Rayleigh range which leads to axially blurred images outside of the narrow waist region of the light-sheet (Fig. 1b). As we deemed uniform axial resolution to be absolutely necessary to achieve the highest possible data quality, we integrated axially scanned light-sheet microscopy (ASLM)14 in the mesoSPIM (Fig. 1b, see Supplementary Note 3, and Supplementary Videos 3-5). In our ASLM implementation, we shift the excitation beam waist through the sample using an electrically tuneable lens (ETL) and synchronize this motion with the rolling shutter of the sCMOS camera. Therefore, only the axially most confined region of the light-sheet contributes to image formation comparable to earlier approaches using mechanical translation of the sample15. In ASLM mode the mesoSPIM achieves an axial resolution of 5.57 ± 0.03 μm (FWHM, n = 2170 beads, nD=1.45) across a 3.29-mm FOV and 6.52 ± 0.07 μm (FWHM, n = 322 beads, nD=1.45) across a FOV of 13.29 mm (Supplementary Note 4, Supplementary Figure 4). These features enable us to image a whole mouse brain (≈ 1 cm3) with isotropic sampling (6.5 μm) within 7-8 minutes resulting in a relatively small dataset (12-16 GB).
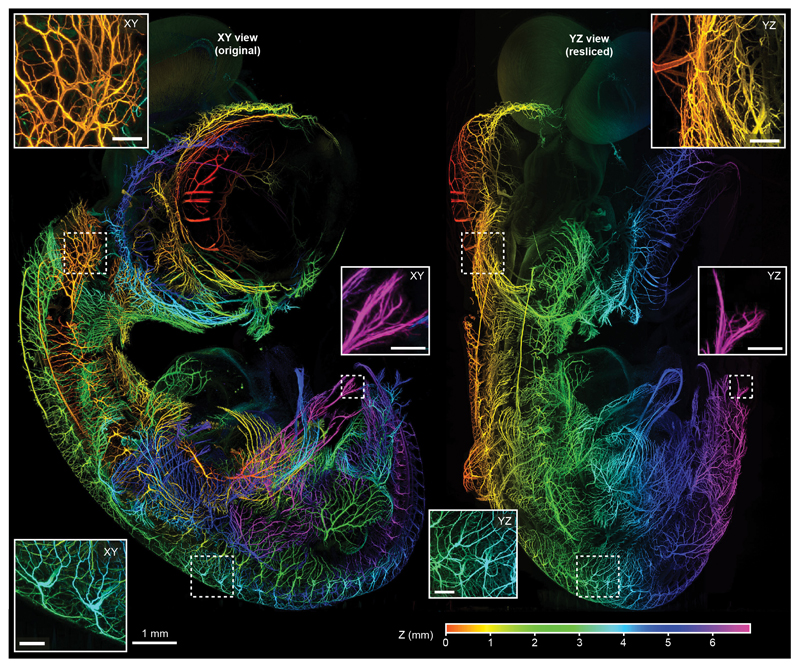
The microscope software (mesoSPIM-control, Supplementary Software) is written in Python and allows users to specify sequences of z-stacks using a table-based acquisition manager (Supplementary Note 5, Supplementary Fig. 5, Supplementary Video 6). The software can also be used to acquire large-scale tiling acquisitions, for example to visualize fine neurites in the developing nervous system of a 7-day old chicken embryo resulting in a 880 GB dataset (Figure 2, Supplementary Video 7). To achieve optimum optical sectioning in ASLM, the amplitude and offset of the ETL waveform need to be adapted when changing the excitation wavelength, zoom, or the immersion medium and can even depend on the local refractive properties of the sample. Therefore, mesoSPIM-control allows users to select configuration files with default ASLM settings for different immersion media and to manually optimize ASLM parameters (Supplementary Video 8).
Figure 2. Large scale-dataset acquired with the mesoSPIM:
Depth-coded original XY (left) and resliced YZ (right) projections of a dataset taken from a 7-day old chicken embryo (neurofilament labeling) cleared using BABB. Throughout the dataset (acquired at 1.6×1.6×2 μm3 sampling), neurites are visible in great detail. Because of the ASLM mode the same holds true for the original (transverse) and the resliced (axial) direction. The assignment of color to Z-position is similar for both the XY and YZ view. Scale bars of all insets: 200 μm. The imaging experiment was conducted once.
With a travel range of 44 × 44 × 100 mm, large samples such as a whole mouse CNS can be imaged in their entirety (Fig. 1c, Supplementary Video 9). After acquiring overview datasets, users can zoom in and record multidimensional data at higher resolution by mosaic acquisitions, for example revealing cellular distribution and long-range axonal projections of Purkinje cells in the mouse cerebellum (Fig. 1d-e, Supplementary Videos 10-12).
We tested the instrument in combination with all major clearing techniques (Supplementary Note 6) including active7 and passive11 CLARITY (Fig 1b-e, Supplementary Fig. 6 and 7, Supplementary Video 13) and CUBIC-X9 (Supplementary Fig. 8). Among organic solvent methods, we tested iDISCO4 (Supplementary Fig. 9, Supplementary Video 14) and BABB2 (Fig. 2, Supplementary Fig. 10). To demonstrate multi-view acquisitions with the mesoSPIM, we imaged a BABB-cleared chicken embryo from multiple directions (Supplementary Fig. 11) and fused the resulting datasets using BigStitcher16 (Supplementary Fig. 12). Given its flexible sample holders, the mesoSPIM is compatible with a wide range of sample types ranging from Drosophila melanogaster (Supplementary Fig. 13, Supplementary Video 15) to cleared human cortex processed using MASH17 (Supplementary Fig. 14, Supplementary Videos 16 and 17).
Inspired by the openSPIM18 and openSPIN19 projects, the mesoSPIM hardware documentation and software are freely available. Depending on the configuration, a mesoSPIM requires a budget of $170000-$240000 (Supplementary Table 2) and can be installed in a day if all parts are available (Supplementary Video 18). Currently, five mesoSPIM setups are in operation across Europe and several more instruments are under construction. The mesoSPIM is the ideal instrument to quickly bridge scales from the μm- to the cm-level which enables it to serve as an excellent tool for detailed three-dimensional anatomical investigations in neuroscience and developmental biology. We have designed the mesoSPIM as a versatile and modular imaging platform and expect that it will be extended towards even larger samples, combined with novel clearing methods, and integrated with other imaging modalities such as optical projection tomography20.
Supplementary Material
Editor’s summary.
The mesoSPIM is an open hardware axially scanned light-sheet microscope for the rapid imaging of large cleared samples with isotropic resolution.
Acknowledgements
This work was supported by grants from the Swiss National Science Foundation (grant nos. 31003A-149858, 31003B-170269 to F.H.; no. 31003A_170037 to T.K.; nos. 31003A-153448, 31003A_173125, CRSII3_154453 and NCCR Synapsy no. 51NF40-158776 to A.H.), the European Research Council (ERC Advanced Grant no. BRAINCOMPATH, project no. 670757 to F.H.), an ERC Starting Grants (InterWiring, project no. 679175 to T.K. and MULTICONNECT, project no. 639938 to A.R.), the Dutch science foundation (NWO VIDI Grant, project no. 14637 to A.R.), a PhD fellowship by the Swiss Foundation for Excellence in Biomedical Research (to R.K), a gift from a private foundation with public interest through the International Foundation for Research in Paraplegia (to A.H. and S.P.), and a Distinguished Scientist Award of the Nomis Foundation (to A.A.). In addition, we would like to thank D. Göckeritz-Dujmovic and S. Bichet for help with sample preparation and M. Wieckhorst for help with custom electronics.
Footnotes
Code availability
The mesoSPIM software and documentation are available as Supplementary Software. Updated versions can be found on Github (https://github.com/mesoSPIM). mesoSPIM-control is licensed under the GNU General Public License v3.0 (GPL v3).
Data availability
Data was deposited to the Image Data Resource (http://idr.openmicroscopy.org) under accession number IDR0066.
Author information – Contributions
F.F.V. and F.H. designed the project. F.F.V designed the microscope, wrote control software and documentation, coordinated the mesoSPIM initiative, and analyzed data. F.F.V., E.P., and P.B. imaged samples. D.K., E.P., R.K.,M.S, L.E., A. v.d. B, K. H., N.F., T.T., N.R., H-U. Z., T.K., P.P., R.P., D.H., B.R, S.H., A.S., A.R. prepared samples for imaging. F.F.V, S.P., E. P., D.K., R.A.A.C, F.M., U.Z., L.B. A.H., C.L., A.A. set up mesoSPIM instruments. F.F.V and F.H. wrote the manuscript with input from all coauthors.
Competing interests
None to disclose.
Peer review information
Rita Strack was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
References
- 1.Richardson DS, Lichtman JW. Cell. 2015;162:246–257. doi: 10.1016/j.cell.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ertürk A, et al. Nature Protocols. 2012;7:1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- 3.Renier N, et al. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Pan C, et al. Nat Methods. 2016;13:859–867. doi: 10.1038/nmeth.3964. [DOI] [PubMed] [Google Scholar]
- 5.Cai R, et al. Nat Neurosci. 2019;22:317–327. doi: 10.1038/s41593-018-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung K, et al. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susaki EA, et al. Cell. 2014;157:726–739. doi: 10.1016/j.cell.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Murakami TC, et al. Nature Neuroscience. 2018;1 [Google Scholar]
- 9.Dodt H-U, et al. Nature Methods. 2007;4:331–336. doi: 10.1038/nmeth1036. [DOI] [PubMed] [Google Scholar]
- 10.Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Science. 2004;305:1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- 11.Tomer R, Ye L, Hsueh B, Deisseroth K. Nat Protocols. 2014;9:1682–1697. doi: 10.1038/nprot.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EHK. Science. 2008;322:1065–1069. doi: 10.1126/science.1162493. [DOI] [PubMed] [Google Scholar]
- 13.Fahrbach FO, Simon P, Rohrbach A. Nat Photon. 2010;4:780–785. [Google Scholar]
- 14.Dean KM, Roudot P, Welf ES, Danuser G, Fiolka R. Biophysical Journal. 2015;108:2807–2815. doi: 10.1016/j.bpj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buytaert JAN, Dirckx JJJ. Journal of Biomedical Optics. 2007;12 doi: 10.1117/1.2671712. 014039-014039-014013. [DOI] [PubMed] [Google Scholar]
- 16.Hörl D, et al. Nat Methods. 2019;16:870–874. doi: 10.1038/s41592-019-0501-0. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand S, Schueth A, Herrler A, Galuske R, Roebroeck A. Sci Rep. 2019;9 doi: 10.1038/s41598-019-47336-9. 10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitrone PG, et al. Nat Methods. 2013;10:598–599. doi: 10.1038/nmeth.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gualda EJ, et al. Nat Methods. 2013;10:599–600. doi: 10.1038/nmeth.2508. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe J, et al. Science. 2002;296:541–545. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.