Abstract
Information about waitlist time has been reported as one of the single most frequently asked questions by individuals awaiting a transplant but data regarding waitlist time have not been processed in a useful way for pediatric candidates. To predict chance of receiving a deceased donor liver transplant (DDLT), we identified 6,471 pediatric (<18 years), non-status-1a, liver-only transplant candidates between 2006–2017 from the Scientific Registry for Transplant Recipients. Cox regression with shared frailty for DSA (Donor Service Area) level effect was used to model the association of blood type, weight, allocation PELD (Pediatric End-stage Liver Disease) and MELD (Model for End-stage Liver Disease), and DSA with chance of DDLT. Jackknife technique was used for validation. Median (IQR) waitlist time was 100 (34–309) days. Non-O Blood type, higher PELD/MELD score at listing, and DSA were associated with increased chance of DDLT, while age 1–5 years and 10–18 years was associated with lower chance of DDLT (p < 0.001 for all variables). Our model accurately predicted chance of transplant (C-statistic = 0.68) and was able to predict DDLT at specific follow-up times (e.g., 3 months). This model can serve as the basis for an online tool that would provide useful information for pediatric waitlist candidates.
Keywords: prediction, deceased donor liver transplant, calculator, pediatric
1. INTRODUCTION
Since 2002, the Organ Procurement and Transplantation Network has allocated livers based on a limited set of standardized criteria that are primarily centered around pre-transplant mortality risk as determined by the Pediatric End-stage Liver Disease (PELD) score for individuals under 12 years of age, or the Model for End-stage Liver Disease (MELD) score for individuals over 12 years.1 Blood type and organ size are additional objective criteria that are incorporated into the decision to allocate and accept an offer. Finally, the availability and the distribution of organs varies significantly across the country, with the chance of being transplanted varying as much as 4-fold depending on location.2–4
One critical consideration for patients awaiting transplantation is waitlist time. A recent analysis of patient and support networks maintained by the United Network for Organ Sharing (UNOS) and the Scientific Registry of Transplant Recipients (SRTR) determined that information about waitlist time was the most common type of data requested by transplant candidates, family and friends.5 However, despite the largely systematic approach to the allocation of livers, little information is available to candidates regarding individualized waitlist time estimates. A waitlist calculator for adult candidates was recently developed and shared on the UNOS website in order to provide individuals with historical information about waitlist time based on their allocation score, age, transplant center and blood type.6,7 With this tool, patients can input their characteristics (e.g., location, MELD score, blood type) and compare outcomes (i.e., probability of being transplanted) from similar historical patients; because this approach incorporated historical data rather than estimated probabilities from a model, information was only displayed when sufficient data from a set of characteristics was observed. Perhaps for this reason, no data are available for pediatric candidates.
Given the potential benefit of a calculator to provide information about waitlist time for pediatric candidates with chronic liver disease, we developed a model based on allocation score at listing, weight, blood type, and region. The purpose of this model was to develop an easy-to-use calculator that, like the adult calculator, is based on patient characteristics. We anticipated that these characteristics could yield a model that would accurately predict the chance of transplantation for pediatric candidates and would therefore provide these patients with the information they seek.
2. METHODS
2.1. Data Source
This study used data from the SRTR. The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the U.S. submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.8 The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
2.2. Study population
We identified 6,471 active pediatric (<18 years), non-status-1a, liver-only transplant candidates on the waitlist between January 1, 2006 and August 31, 2017 in the U.S. Candidates were excluded if they were registered for multi-organ transplants, or listed at multiple centers. We followed waitlisted candidates from initial listing to a deceased-donor liver transplant (DDLT), censoring at living donation, death, or removal due to other reasons.
2.3. Predictors associated with DDLT
Cox regression was used to model time-to-DDLT among waitlist candidates. The model adjusted for ABO blood type, as well as age (categorized as <1 year, 1–5 years, 6–10 years, 11–18 years) and initial allocation PELD/MELD (categorized as <15, 15–29, 30–34, ≥35, or status 1B with any PELD/MELD, irrespective of whether it was status 1B for standard criteria, or exception for malignancy or specific metabolic disorders). Age and PELD/MELD categories were chosen to be consistent with categories defined by the Scientific Registry of Transplant Recipients.9 PELD was used for candidates <12 years, and MELD was used for candidates ≥12 years. Allocation PELD/MELD is defined as whichever of the following is greater: the laboratory/calculated score or the score from exception points. We further investigated association of DDLT and primary diagnosis, categorized as: biliary atresia, non-status 1A acute hepatic necrosis (i.e., individuals listed with “acute hepatic necrosis” as the recipient diagnosis, but were not transplanted as status 1A because they failed to meet criteria such as intensive care unit admission; rather these recipients were transplanted using PELD/MELD scores), autoimmune hepatitis, primary sclerosing cholangitis (PSC), metabolic disease, malignancy, and other. However, primary diagnosis did not change overall DDLT prediction and thus was not used in the final model of DDLT prediction.
To evaluate the role of geographic variability, models were developed that incorporated the region or the Donor Service Area (DSA). Shared-frailty was incorporated into the Cox regression to account for within-group correlation of candidates from the same geographic region where frailty represents the random effect of DSA on DDLT.10 In other words, Cox regression with shared frailty accounts for the fact that candidates from the same DSA share a similar chance of being transplanted compared to candidates not in that DSA. Given that a model with DSA and with region had identical C-statistics (i.e., C-statistic = 0.68 for both DSA and region), we chose to report geographical variation in DDLT as the DSA-specific hazard ratio (HR) of DDLT (vs national average DDLT). A likelihood ratio (LR) test was used to examine whether the geographical variation was statistically significant.
2.4. DDLT prediction
We estimated baseline cumulative hazard function for a reference candidate: blood type O, weight <10 kg, and PELD/MELD <10. We then applied the hazard ratios of predictors and DSAs from the shared-frailty Cox regression above. Consequently, we were able to calculate the cumulative hazard function of DDLT for any candidate according to his/her blood type, weight, allocation PELD/MELD at listing, and DSA. The predicted chance of DDLT is transformation of cumulative hazard function:
We examined the ability of the shared-frailty Cox regression model to correctly distinguish candidates with high chance of DDLT vs low chance of DDLT (discrimination) using Harrell’s C-statistic. We also categorized waitlist candidates into quintiles of the predicted chance of DDLT and compared the predicted to the observed DDLT chance within each quintile to examine the accuracy of DDLT prediction (calibration). This model then allowed us to estimate the chance of DDLT for an individual with any specific set of characteristics at 3 months, 6 months, and 12 months.
2.5. Validation of model
To examine the validity of DDLT prediction, we performed a jackknife validation technique where the chance of DDLT for each waitlist candidate was calculated based on a shared-frailty Cox regression built on the other candidates. Using the chance of DDLT regenerated, we estimated Harrell’s C-statistic, and compared the predicted-to-observed chance of DDLT.
2.6. Statistical analysis
Confidence intervals were reported using the method of Louis and Zeger.11 Statistical significance was assessed at the α = 0.05 confidence level. All analyses were performed using Stata 15.0/MP for Linux (College Station, TX).
3. RESULTS
3.1. Study population
Among 6,471 pediatric, non-status-1a liver-only transplant waitlist candidates, 52.0% were female (Table 1). The candidates included 74.4% Caucasian, 15.9% African-American, and 6.5% Asian candidates. Biliary atresia was the most common indication for a liver transplant (39.8%), whereas acute hepatic necrosis (3.6%), autoimmune hepatitis (3.0%), primary sclerosing cholangitis (2.5%), metabolic disease (14.0), and malignancy (9.6%) were much less common. Median (IQR) PELD/MELD score at listing was 13 (5–22) and weight was 12.2 (7.1–31.5) kg. Median (IQR) time on the waitlist was 100 (34–309) days. At 3 months after listing, 43% of candidates received a DDLT, 59% received a DDLT at 6 months, and 72% received a DDLT at 12 months.
Table 1.
Demographic and clinical characteristics of 6,471 pediatric waitlist candidates
| Age (months) at baseline, median (IQR) | 27 (8–127) |
| Weight (kg) at baseline, median (IQR) | 12.2 (7.1–31.5) |
| Female (%) | 3375 (52.2) |
| Race (%) | |
| Caucasian | 4812 (74.4) |
| African-American | 1031 (15.9) |
| Asian | 422 (6.5) |
| Other | 206 (3.2) |
| Blood type (%) | |
| O | 3204 (49.5) |
| A | 2132 (32.9) |
| B | 894 (13.8) |
| AB | 241 (3.7) |
| Primary diagnosis (%) | |
| Biliary atresia | 2575 (39.8) |
| Acute hepatic necrosis | 234 (3.6) |
| Autoimmune hepatitis | 192 (3.0) |
| Primary sclerosing cholangitis | 163 (2.5) |
| Metabolic disease | 905 (14.0) |
| Malignancy | 619 (9.6) |
| Other | 1783 (27.6) |
| PELD/MELD at baseline, median (IQR)* | 13 (5–22) |
| Status 1B at baseline | 353 (5.5) |
3.2. Predictors associated with DDLT
Blood type, weight, and PELD/MELD were associated with DDLT (Table 2). Compared to candidates with blood type O, those with blood type A (aHR: 1.171.251.34), B (aHR: 1.091.191.30), and AB (aHR: 1.711.982.29) were more likely to receive DDLT. Compared to individuals <1 year, candidates were less likely to receive a liver transplant if they were between 1–5 years (aHR: 0.780.850.92) or between 10–18 years (aHR: 0.560.620.68). Candidates with higher allocation PELD/MELD had greater chance of receiving DDLT, with the highest chance seen in candidates with PELD/MELD ≥35 (aHR: 3.003.664.48) and those with status 1b designation (aHR: 2.693.143.67). Compared to biliary atresia, acute hepatic necrosis (aHR: 0.390.490.62) and autoimmune hepatitis (aHR: 0.560.690.85) were associated with lower chance of DDLT, while malignancy (HR: 1.651.882.14) was associated with higher chance of DDLT.
Table 2.
Individual-level predictors associated with deceased donor liver transplant (DDLT).
| Adjusted HR of DDLT | p | |
|---|---|---|
| Blood type | ||
| O | Reference | |
| A | 1.171.251.34 | <0.001 |
| B | 1.091.191.30 | <0.001 |
| AB | 1.711.982.29 | <0.001 |
| Age | ||
| <1 year | Reference | |
| 1–5 years | 0.780.850.92 | <0.001 |
| 6–10 years | 0.840.931.03 | 0.1 |
| 10–18 years | 0.560.620.68 | <0.001 |
| PELD/MELD | ||
| <15 | Reference | |
| 15–29 | 1.561.681.44 | <0.001 |
| 30–34 | 2.082.312.23 | <0.001 |
| ≥35 | 3.003.664.48 | <0.001 |
| Status 1B | 2.693.143.67 | <0.001 |
| Primary diagnosis | ||
| Biliary atresia | Reference | |
| Acute hepatic necrosis | 0.390.490.62 | <0.001 |
| Autoimmune hepatitis | 0.560.690.85 | 0.001 |
| PSC | 0.700.871.07 | 0.2 |
| Metabolic disease | 0.971.081.20 | 0.1 |
| Malignancy | 1.651.882.14 | <0.001 |
| Other | 0.830.910.98 | 0.02 |
In addition to adjustment for these individual characteristics, significant geographical variation of DDLT was observed across DSA (LR test: p < 0.001). Compared to the national average, DDLT varied from 0.35% to nearly 2-fold higher than the national average depending on the DSA (Figure 1). The range of DDLT was similar across age groups (Supplemental Figure 1). Additionally, DDLT was independent of transplant volume in each DSA such that DSAs with high hazard of DDLT had similar volume as those with low hazard of DDLT (Supplemental Figure 2).
Figure 1.
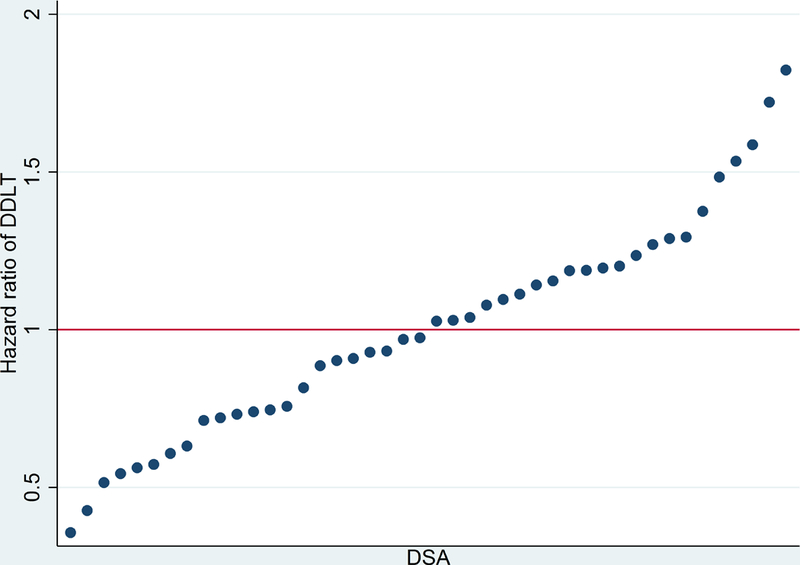
DSA- (Donor Service Area) specific hazard ratio of DDLT (deceased donor liver transplant) vs national average DDLT rate.
3.3. DDLT prediction
The shared-frailty Cox model predicted chance of DDLT for waitlist candidates based on their blood type, age, PELD/MELD, and listing DSA (C-statistic = 0.68). The predicted chance of DDLT was consistent with the observed chance of DDLT within quintiles of the predicted chance of DDLT (Figure 2). Based on this model, we were able to accurately predict the chance for DDLT. For example, a 2-year old pediatric candidate with blood type O, PELD/MELD score of 35 and listed in Maryland would have a chance of DDLT of 24.4% at 3 months, 37% at 6 months, and 51% at 12 months. Using a jackknife validation, the C statistic was 0.68 indicating that our model provided a valid prediction of the chance of DDLT. The model performed equally well for patients with biliary atresia (C-statistic = 0.68) and tumor (C-statistic = 0.66).
Figure 2.
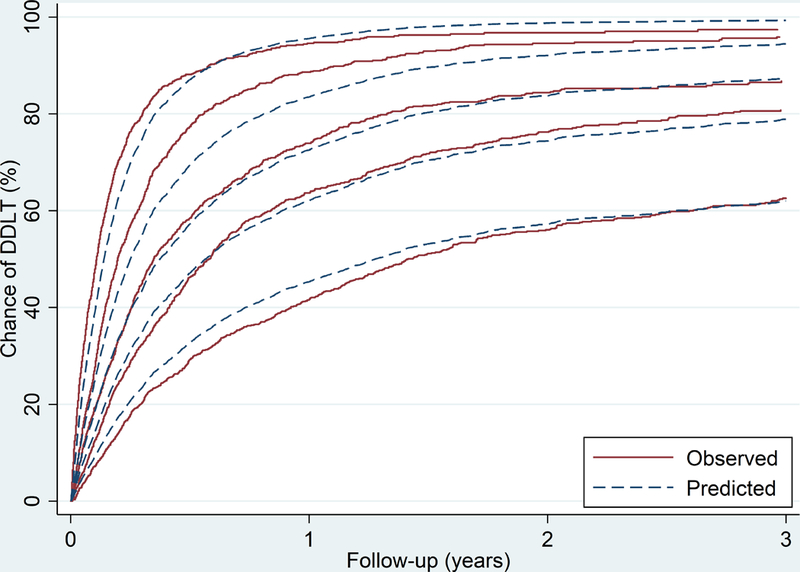
The predicted vs observed probability of deceased donor liver transplant, stratified by quintiles of the predicted probability.
4. DISCUSSION
We demonstrate that a model can be created for pediatric waitlist candidates that accurately predicts the chance of DDLT at specific timepoints based on an individual’s blood type, weight, and allocation score. DSA was also strongly predictive of DDLT and incorporated into the model. Our analysis censored for competing risks including waitlist mortality and living-donor liver transplantation. Consequently, if the calculator predicts a median time, for example, of 12 months, then the interpretation is that a patient has a 50% chance of being transplanted by that time, provided that they remain on the waitlist. This model, which provides an estimate could then serve to provide estimates about the chance of transplantation in an online tool for patients, families, and/or healthcare providers. Individuals could repeatedly impute their values (i.e., as PELD/MELD or age changes) to get different estimates of the chance of moving through the waitlist to get a DDLT.
Our model contains variables similar to the SRTR calculator for adult candidates including allocation score, blood type and location.6,7 Our finding that non-O blood types were associated with greater DDLT is consistent with several other reports in adult and pediatric candidates.12–14 However, whereas the adult calculator did not incorporate patient size despite some evidence that it may influence transplantation rate, we chose to include patient age into our model given its increased importance in surgical decision-making for pediatric candidates as well as statistical evidence that this variable predicts likelihood of transplantation.13–15 Finally, while geographic variability in DDLT is well-understood to occur for adult candidates, information on regional variability for DDLT for pediatric candidates is scarce and if present, may be due to regional variability in obtaining exception points.16–18 In our model, we incorporate the use of allocation scores (i.e., the score with exception points if greater than the calculated laboratory score) which therefore bypasses any center-level variability with exception points. Even with the incorporation of exception points into our model, we continue to see substantial regional differences in chance of DDLT, that are more likely related to other geographic or center-level characteristics such as the (in)balance of donor availability and need in different areas, or the aggressiveness with which some centers may pursue segmental grafts or marginal grafts.
Similar to the adult calculator, we chose not to incorporate center-level characteristics that would potentially improve prediction such as the use of splitting, extended criteria donors, and other practices that might increase the chance of DDLT. Our model also incorporates DSAs rather than centers, as use of centers would lead to even smaller subgroups with more unstable estimates. Instead, we chose to emphasize patient ease of use by incorporating only variables that a patient could easily know or obtain. This approach, common in prediction modeling compared to etiologic modeling, emphasizes parsimony.19,20
Our calculator could serve to mitigate stress and anxiety that has been documented in the pre-transplant period in several ways. First, a lack of information about waitlist time has been shown to be directly related to perceptions of hopelessness, loss of control and potentially depression.21,22 Second, parents have acknowledged anger towards their transplant center as the waitlist time grows longer and their child has not yet received an offer.21,22 Third, uncertainty surrounding waitlist time can make it difficult to plan other aspects of one’s life such as travel.21,22 And finally, greater understanding about waitlist time may impact medical decision-making such as the role and benefit for living donation.23 Given these uncertainties, some limited evidence exists that stress in the pre-transplant period can be reduced through increased education and preparation about what to expect in this phase of care, although the ability to reduce stress has not been consistently demonstrated.24,25 Following future development of an online calculator that would be publicly available, it will be necessary to better understand whether use of this calculator actually improves stress and quality of life while waiting for a liver transplant even as it provides information that patients indicate they want.
Another important concern that must be explored is whether individuals would interpret correctly the information that is provided by a calculator. While shared decision-making – the process of integrating a patient’s goals/concerns with medical information of knowledge about options, benefits and harms – is a stated aim of the Institute of Medicine, there is substantial evidence that individuals frequently misunderstand medical information surrounding decision-making, risk and probability.26,27 At the same time, there is emerging literature regarding what strategies work best for communicating health information such as text, graphs, or infographics.28,29 Therefore, once the calculator becomes publicly available, we will evaluate which method of communication is most readily understood by users.
One final concern is that any changes in the allocation system would potentially influence time to transplantation. In December, 2018, UNOS approved a new allocation system based on circles around donor hospitals. This system is expected to improve allocation of deceased organs for pediatric candidates and, as such, it is possible that our calculator will overestimate how long it will take for a candidate to receive an organ. It will be necessary to continue to evaluate and optimize the prediction model as new data becomes available.
In conclusion, our model accurately predicts chance of transplant for pediatric candidates on the waitlist. This model can serve as the basis for an online tool that would provide frequently requested information to candidates and caregivers during the pre-transplant period.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of, or interpretation by, the SRTR or the U.S. Government. The authors would like to acknowledge the Gilead Foundation and Larry Ackman for funding the creation of the waitlist calculator.
Dr. Mogul is supported by grant 5K08HS023876-02 from the Agency for Healthcare Research and Quality. Dr. Massie is supported by grant K01DK101677 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Segev is supported by grants K24DK101828 and R01DK111233 from the National Institute of Diabetes and Digestive and Kidney Diseases.
abbreviations:
- DDLT
deceased donor liver transplantation
- DSA
Donor Service Area
- PELD
Pediatric End-stage Liver Disease
- MELD
Model for End-stage Liver Disease
- UNOS
United Network for Organ Sharing
- SRTR
Scientific Registry for Transplant Recipients
Footnotes
DISCLOSURE
The authors report no conflicts of interest.
REFERENCES
- 1.Learn How Organ Allocation Works - OPTN. https://optn.transplant.hrsa.gov/learn/about-transplantation/how-organ-allocation-works/ Accessed May 8, 2018.
- 2.Massie AB, Caffo B, Gentry SE, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2011;11(11):2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2013;13(8):2052–2058. doi: 10.1111/ajt.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaffhausen CR, Bruin MJ, Chesley D, et al. What patients and members of their support networks ask about transplant program data. Clin Transplant. 2017;31(12). doi: 10.1111/ctr.13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waiting List Calculator. https://www.srtr.org/reports-tools/waiting-list-calculator/ Accessed May 8, 2018.
- 7.Hart A, Schladt DP, Zeglin J, et al. Predicting Outcomes on the Liver Transplant Waiting List in the United States: Accounting for Large Regional Variation in Organ Availability and Priority Allocation Points. Transplantation. 2016;100(10):2153–2159. doi: 10.1097/TP.0000000000001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2018;18 Suppl 1:172–253. doi: 10.1111/ajt.14559 [DOI] [PubMed] [Google Scholar]
- 10.Kalbfleisch J, Prentice R. The Statistical Analysis of Failure Time Data. 2nd ed Hoboken, NJ: J. Wiley; 2002. [Google Scholar]
- 11.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostat Oxf Engl. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink MA, Berry SR, Gow PJ, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22(1):119–124. doi: 10.1111/j.1440-1746.2006.04422.x [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Schaubel DE, Messersmith EE, Guidinger MK, Merion RM. Factors that affect deceased donor liver transplantation rates in the United States in addition to the Model for End-stage Liver Disease score. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2012;18(12):1456–1463. doi: 10.1002/lt.23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogul DB, Luo X, Chow EK, et al. Impact of Race and Ethnicity on Outcomes for Children Waitlisted for Pediatric Liver Transplantation. J Pediatr Gastroenterol Nutr. 2018;66(3):436–441. doi: 10.1097/MPG.0000000000001793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2010;10(12):2658–2664. doi: 10.1111/j.1600-6143.2010.03326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2013;13(8):2052–2058. doi: 10.1111/ajt.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvalaggio PR, Neighbors K, Kelly S, et al. Regional variation and use of exception letters for cadaveric liver allocation in children with chronic liver disease. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2005;5(8):1868–1874. doi: 10.1111/j.1600-6143.2005.00962.x [DOI] [PubMed] [Google Scholar]
- 19.Shmueli G To explain or predict? Stat Sci. 2010;25(3):289–310. [Google Scholar]
- 20.van Diepen M, Ramspek CL, Jager KJ, Zoccali C, Dekker FW. Prediction versus aetiology: common pitfalls and how to avoid them. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2017;32(suppl_2):ii1–ii5. doi: 10.1093/ndt/gfw459 [DOI] [PubMed] [Google Scholar]
- 21.Gold LM, Kirkpatrick BS, Fricker FJ, Zitelli BJ. Psychosocial issues in pediatric organ transplantation: the parents’ perspective. Pediatrics. 1986;77(5):738–744. [PubMed] [Google Scholar]
- 22.Brown J, Sorrell JH, McClaren J, Creswell JW. Waiting for a liver transplant. Qual Health Res. 2006;16(1):119–136. doi: 10.1177/1049732305284011 [DOI] [PubMed] [Google Scholar]
- 23.Molinari M, Matz J, DeCoutere S, El-Tawil K, Abu-Wasel B, Keough V. Live liver donors’ risk thresholds: risking a life to save a life. HPB. 2014;16(6):560–574. doi: 10.1111/hpb.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharif F, Mohebbi S, Tabatabaee H-R, Saberi-Firoozi M, Gholamzadeh S. Effects of psycho-educational intervention on health-related quality of life (QOL) of patients with chronic liver disease referring to Shiraz University of Medical Sciences. Health Qual Life Outcomes. 2005;3:81. doi: 10.1186/1477-7525-3-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey DE, Hendrix CC, Steinhauser KE, et al. Randomized trial of an uncertainty self-management telephone intervention for patients awaiting liver transplant. Patient Educ Couns. 2017;100(3):509–517. doi: 10.1016/j.pec.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alston C, Paget L, Halvorson G, et al. Communicating with Patients on Health Care Evidence. :17. [Google Scholar]
- 27.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schapira MM, Nattinger AB, McHorney CA. Frequency or probability? A qualitative study of risk communication formats used in health care. Med Decis Mak Int J Soc Med Decis Mak. 2001;21(6):459–467. doi: 10.1177/0272989X0102100604 [DOI] [PubMed] [Google Scholar]
- 29.Lipkus IM, Hollands JG. The visual communication of risk. J Natl Cancer Inst Monogr. 1999;(25):149–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


