Abstract
Even though 8–25% of most populations studied globally are labeled as penicillin allergic, most diagnoses of penicillin allergy are made in childhood and relate to events that are either not allergic in nature, are low-risk for immediate hypersensitivity, or are a potential true allergy that has waned over time. Penicillin allergy labels directly impact antimicrobial stewardship by leading to use of less effective and broader spectrum antimicrobials and are associated with antimicrobial resistance. They may also delay appropriate antimicrobial therapy, and lead to increased risk of specific adverse healthcare outcomes. Operationalizing penicillin allergy de-labeling into a new arm of antimicrobial stewardship programs (ASPs) has become an increasing global focus. We performed an evidence-based narrative review of the literature of penicillin allergy label carriage, the adverse effects of penicillin allergy labels and current approaches and barriers to penicillin allergy de-labeling. Over the period 1928–2018 in Pubmed and Medline, search terms used included “penicillin allergy” or “penicillin hypersensitivity” alone or in combination with “adverse events”, “testing”, “evaluation”, “effects”, “label”, “de-labeling”, “prick or epicutaneous” and “intradermal” skin testing, “oral challenge or provocation” “cross-reactivity” and “antimicrobial stewardship.”
Keywords: allergy, de-labeling, label, penicillin, testing
Background:
Carriage of a label of penicillin allergy in the medical record is a common clinical entity; studies in the United Kingdom (UK), United States (US), and Australia estimate the prevalence to be between 8–25%.45–47 In contemporary clinical practice where the patient population has a lower overall historical risk of penicillin allergy, skin prick and intradermal skin testing to validated reagents followed by ingestion challenge to a penicillin such as amoxicillin demonstrates that only between 1–10% of those carrying a label of penicillin allergy are allergic, and in many recent studies this is 4% or less.17,48–50 It is clear that penicillin allergy labels should no longer be considered passive entities within the medical record and that systematic approaches need to be developed to manage the large global burden of over-labeled patients.46,51 Recent research has highlighted that 75% of children are labeled as penicillin allergic by age 3 and furthermore that there are specific risks that appear to be associated with an unverified penicillin allergy label.42,52–54 The potential negative sequelae associated with a penicillin allergy label include risk of antimicrobial treatment failure, antimicrobial resistance, adverse drug reactions from use of a broader spectrum or alternative antibiotic, and increased healthcare costs. 33,55–65 Thus, there is a great need to understand the circumstances surrounding the acquisition of penicillin allergy labels; to differentiate those with a true immunological basis from those which have an alternative etiology; and to determine the best strategies to safely de-label unnecessary penicillin allergy labels at an individual and population level. This paper will critically review the current state of science and evidence surrounding “penicillin allergy labels” including their origin, consequences, and it will provide a roadmap to strategically address penicillin allergy labels at both an individual and population level.
Acquisition of penicillin allergy labels:
The allergy section of a medical record is meant as a safeguard against patients being harmed by administration of a drug or exposure that they have failed to tolerate. Traditionally, the “allergy box” in the patient’s health record has been the place that all adverse reactions to drugs, foods and other substances are documented to help mitigate future harm from inadvertent re-exposure.66 A major challenge to realizing the utility of the allergy box is that the use of the term “allergy” largely ends up being both vague and unreconciled to both patients and healthcare providers. This undifferentiated approach also promotes misunderstanding about whether any individual drug adverse event was a true immune-mediated allergic reaction with a hard-stop for future avoidance, versus a potentially manageable side effect which does not preclude future use of the drug. Experts note that when record-takers note the name of a drug in the allergy section of the chart they frequently omit details66 or provide incomplete, incorrect or misleading details.67 Such data leaves future prescribers either at risk to make further erroneous conclusions or incapable of making reasonable conclusions, especially if patients can no longer recall the details of the associated event. It is not surprising that by adulthood almost one-third of penicillin allergy labels in the patient’s record lack specific details. Unfortunately, the label’s presence is enough to create uncertainty and to have negative consequences on the prescribing habits of healthcare providers.46,51
Most penicillin allergy labels are acquired in childhood, and many are acquired within the context of children being administered unnecessary antibiotics for viral infections. Studies suggest that 75% of penicillin allergy labels are obtained by the age of three years, and the prevalence of penicillin allergy is only slightly less in children compared to adults.42,52–54 In a retrospective US cohort, adverse drug events were reported in children at a rate of 1.6% per year over a 10 year study period; of these adverse drug events, 16% were found to correlate with administration of a penicillin or cephalosporin.53
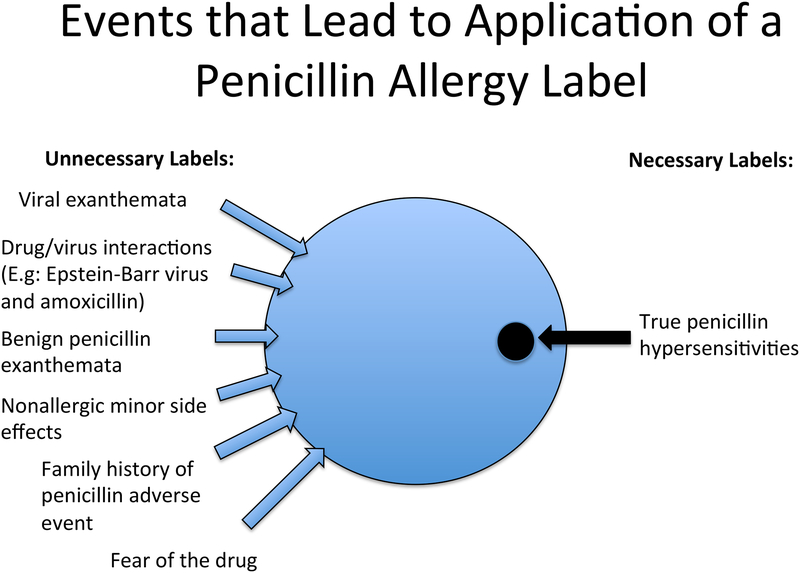
When a patient suffers an adverse outcome while taking penicillin in childhood, there is frequently uncertainty in both the underlying diagnosis and in causal attribution, leading to false labeling of a patient as “allergic.”68 Many times, the index reaction is clearly inconsistent with true allergy, such as nausea, vomiting, or diarrhea in isolation, or the documented reason for avoidance is a “family history of penicillin allergy” which has no relation to risk of true penicillin allergy.42 Cutaneous rashes are the most common pathway to an allergy label in childhood. Viral infections are the most common cause of cutaneous rashes in childhood, and virally-induced rashes or drug-virus interactions can be mistakenly diagnosed as penicillin allergy.28,69 Vyles et al. queried parents on the symptoms which led to a penicillin allergy diagnosis and noted 75% of the symptoms were low risk for an immunoglobulin E (IgE)-mediated allergy with the most frequent description of the event being a non-specific rash and/or pruritis.42 When evaluating the frequency and quality of penicillin-associated rash, Ibia et al. found that penicillin administration temporally correlated with a rash 2.72% of the time in children; of these, only one third of the rashes were clinically consistent with urticaria accompanied by itching and the remainder were not consistent with IgE-mediated hypersensitivity.70 Thus, while a concomitant rash with penicillin administration is common in childhood, clinical features consistent with IgE-mediated hypersensitivity occur in only a minority of these cases. Commonly, a penicillin allergy label is acquired due to an associated rash regardless of its true etiology or severity.71 (Figure 1)
Figure 1: Events that Lead to Application of a Penicillin Allergy Label.
The application of penicillin allergy labels results from events that are low risk for allergy in the vast majority of cases.42,52–54,68,70,71
A small subset of patients with penicillin allergy labels have a history consistent with a high risk reaction, such as anaphylaxis, severe cutaneous adverse reactions, or immune-mediated organ injuries (e.g. acute interstitial nephritis [AIN], drug-induced liver injury [DILI]). Depending on the specific type of reaction, older age, penicillin dose received, duration of treatment, route of drug administration, underlying genetic or metabolic factors, and the chemical properties of the drug (largely its protein reactivity), have all been reported as risk factors for true drug hypersensitivity.72 Yet, in the absence of clear comprehensive documentation of the primary penicillin adverse event in the medical record, it is frequently difficult to tell which labels must be taken seriously.66,67
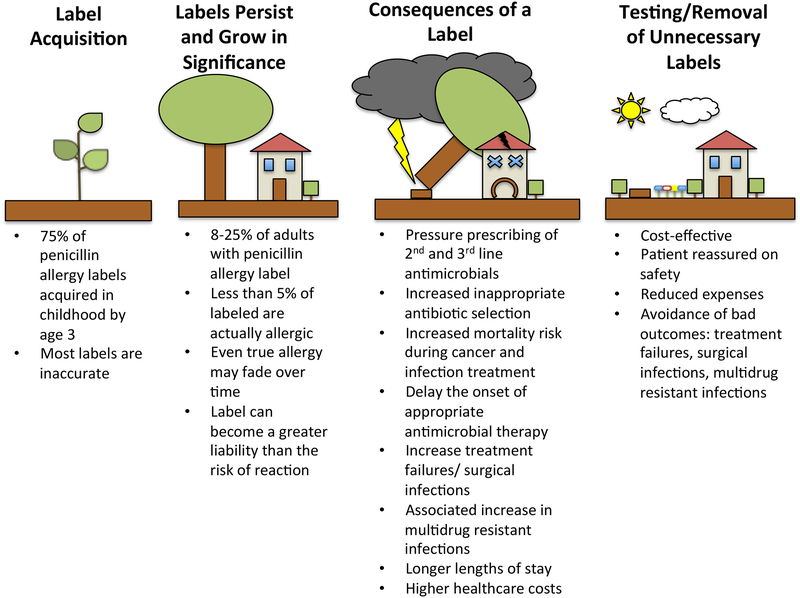
Important negative consequences of penicillin allergy labels (Figure 2):
Figure 2: A Penicillin Allergy Label is like a Tree Planted Too Close to Your House in Childhood.
Most penicillin allergy labels are applied in childhood, like a seed that grows up into a tree too close to the house (Label Acquisition). In adulthood, the justification for leaving such a tree next to the house is shaky (Labels Persist and Grow in Significance), as they can contribute to worsened outcomes during the storm of a healthcare encounter requiring antimicrobial treatment (Consequences of a Label). Removal of unnecessary penicillin allergy labels is likely to provide protection against adverse outcomes associated with its carriage (Testing/Removal of Unnecessary Labels).
In patients labeled as penicillin allergic, clinicians must weigh the benefits of prescribing penicillin or related beta-lactam antibiotics against the risk of an adverse reaction. In the setting of busy and demanding clinical practices, many times the penicillin allergy label is inadequately reconciled and prescribers choose an alternative antimicrobial with potentially lesser efficacy, greater unintended adverse effects, and increased costs during treatment.46,51 A conscious choice to avoid penicillin also leads to prescriber avoidance of other beta-lactam antimicrobials, especially cephalosporins.73
Alternative antimicrobials often have decreased efficacy compared to penicillin class antibiotics, hence carriage of a penicillin allergy label is not a passive or benign state. Patients with penicillin allergy labels have been shown to have an increased mortality risk from coexisting hematologic malignancies58 and infections from penicillin susceptible organisms such as methicillin-susceptible Staphylococcus aureus (MSSA).59 Blumenthal et al. have identified that penicillin allergy labels put patients at greater risk of post-operative surgical site infections, mediated by use of alternative antibiotics.74 Preoperative evaluation of penicillin allergy leads to increased utilisation of appropriate antimicrobials, theoretically lowering surgical site infection risk.60,61 Multiple investigators have shown that patients with penicillin allergy labels demonstrate increased length of hospital stay compared to the general population.33,55–58 Prolongation of hospitalizations appears to be mediated by increased treatment failures from less effective alternative antimicrobials.75,76 Additionally, MacFadden et al. demonstrated that patients who did not receive preferred beta-lactam therapy as a consequence of a beta-lactam allergy had a greater risk for a composite outcome of future adverse events in an adjusted model (aOR 3.1 95% CI 1.28–7.89).77 Increases in readmissions and reactions to alternative antimicrobials were the main drivers of this composite outcome.77 These treatment failures, adverse events, and prolongations of care have been shown to be modifiable by removal of the penicillin allergy label.60,61
Patients with penicillin allergy labels are also at greater risk for multidrug resistant infections and use of inappropriate antimicrobials during treatment. It has been shown across several countries and healthcare systems that patients with penicillin allergy labels experience increased rates of infection with multidrug resistant organisms such as Clostridioides difficile,33,55,58,62,63 methicillin-resistant Staphylococcus aureus (MRSA),33,62 and vancomycin-resistant Enterococcus species.33,63 This increase is likely mediated by use of alternative broad-spectrum antimicrobials that favor selection of these organisms.78 In the US, pregnant patients with penicillin allergy labels are more likely to receive antibiotics during labor which are ineffective for treatment of group B Streptococcus carriage.79
Decreased efficacy, treatment failures, and unintended adverse effects of alternative antimicrobials lead inexorably to increased costs during the delivery of healthcare. Li et al. have shown cost estimates from the UK indicating that use of alternative antimicrobials is more expensive than if patients were able to tolerate a penicillin.64 A retrospective study from a Canadian tertiary center by Picard et al. demonstrated greater expenditures per penicillin allergy patient during a one year period due to the use of non-beta lactam antibiotics.65 Prolongations of hospitalizations,33,55–58 surgical site infections,74 and treatment failures75,76 are costly outcomes that are increased in the context of a penicillin allergy label. Huang et al. report that patients with penicillin allergy labels and hematologic malignancies incur around $50,000 USD in additional costs during their hospitalizations.58
On the other hand, formal evaluation of penicillin allergy with skin testing has been estimated by Blumenthal et al. and Rimawi et al. to cost less per patient80 than it may save annually30 especially if a 2nd or 3rd line antibiotic is avoided.81 Vyles et al. estimated the hospital savings of removing inaccurate penicillin allergy diagnosis for a pediatric emergency room with 67,000 annual visits at $192,223 USD.41,42 Penicillin allergy label removal has been found to be cost effective, even in more expensive inpatient care settings.82 Thus, de-labeling of unnecessary penicillin allergies could be an important cost-effective strategy which simultaneously protects patients from adverse outcomes and reduces healthcare costs.
Penicillin allergy de-labeling as a crucial element of antimicrobial stewardship.
Antimicrobial stewardship programs (ASPs) aim to improve treatment outcomes while simultaneously reducing the creation and spread of multidrug resistant infections. Operationalizing penicillin allergy de-labeling into a new arm of ASP has therefore become an increasing global focus.31,83,84 Inappropriate prescribing,51 broad-spectrum antimicrobial utilisation,45,51,65,77,85,86 delayed time to appropriate antibiotic therapy87 and surgical site infections44 are ASP foci, and modified by penicillin allergy labels. A recent meta-analysis by Wu et al. demonstrated that an “antimicrobial allergy label” was associated with significant reductions in antibiotic guideline concordance, antibiotic appropriateness and beta-lactam utilisation, in conjunction with inferior hospital outcomes (increased readmissions, length-of-stay and antibiotic costs).88 Further, Blumenthal et al. recently demonstrated that the presence of a penicillin allergy in a large UK population was associated with a 1.69 fold increase risk of MRSA and 1.26 fold risk of Clostridioides difficile infection.32 The paradox is that a penicillin allergy label attached to a patient’s record with the intent of improving patient safety, harm minimization and reducing adverse events is now recognized to adversely impact key ASP targets – antimicrobial appropriateness, antimicrobial resistance and medication safety.
Any intervention that aligns antibiotic choice with guidelines should be able to significantly reduce antimicrobial resistance.40,89 Hence, the impacts of penicillin allergy label removal on antibiotic utilisation and antimicrobial resistance are avoidable, as over 90% of patients can have their penicillin allergy label removed by formal testing.90,91 Compounding the patient-level impacts of penicillin labels is the healthcare burden, where over 20% of all hospitalized patients in some studies report an antibiotic allergy, highest amongst cancer and haematology patients.51,92 Therefore the focus of ASPs should rightfully include penicillin de-labeling, in particular in the most vulnerable populations.93 Identification of patients that are low risk (e.g. childhood benign rashes) that are potentially amenable to direct oral challenge without preceding skin testing is vital to future ASPs. Benign childhood rashes are typically defined to include urticaria without pruritus and flushing with onset typically greater than 6 hours after the first dose of a penicillin and non-severe delayed onset exanthema without mucosal, systemic symptoms or organ involvement.69 The assessment of penicillin allergy and incorporation of testing into clinical practice is therefore supported by international ASP guidelines, 34 even if the optimal approach remains to be determined.
Current approaches to penicillin allergy labels and penicillin treatment:
Multiple strategies exist to approach a patient labeled as penicillin allergic. (Table 1) In a practical real world setting utilisation of each approach may be driven by availability of specialty services. Patients with a historically high pre-test probability of previous penicillin anaphylaxis (see Severe Immediate Symptoms, Table II) who have an immediate need for a penicillin benefit most from either desensitisation, validated prick and intradermal skin testing followed by oral challenge if negative, or from use of a comparably efficacious but structurally distinct antibiotic.
Table 1:
Approaches to Immediate Hypersensitivity Penicillin Allergy Labels in an Individual Patient
| Approach | De-labeling Approach | Strengths | Limitations | Level of Recommendation and Evidence |
|---|---|---|---|---|
| Select an alternative antibiotic50,61,67,82–88 | No |
|
|
2c, benefits of using alternative agents are unclear, and there are clearly known adverse effects reported across high quality clinical studies. Would suggest use of other approaches |
| Desensitisation at point of care89–95 | No |
|
|
2c for selected patient populations (see text) but not recommended for general population |
| De-label using history alone96–98 | Yes |
|
|
2c, randomized clinical trials of this approach are lacking but observational clinical studies have been performed showing benefit. Currently limited by unclear knowledge of when to use this approach |
| De-label using direct ingestion challenge36,99–105 | Yes |
|
|
2c, observational studies have been performed particularly in children showing benefit. Currently limited by unclear knowledge of when to use this approach and lack of large studies in adults |
| De-label using skin testing alone14,106–112 | Yes |
|
|
2c, randomized clinical trials of this approach are lacking but clinical studies have been performed showing benefit. |
| De-label using skin testing followed by ingestion challenge14,106–112 | Yes |
|
|
1b, absence of randomized double blind clinical trials of this approach, but a large body of historical evidence including large prospective cohort studies for its use as the current gold standard approach |
| Risk stratifying approach36,63,96,113–116 | Yes |
|
|
2c, randomized clinical trials of this combination approach are lacking but clinical and quasi-experimental design studies have been performed showing benefit. Possibility for this approach to become a new gold standard |
Level of evidence evaluated using the GRADE scoring system81: A “1” represents a strong recommendation, while a “2” represents weak recommendations/suggestions. “a, b, c,” represent the levels of available evidence, with “a” representing consistent evidence from well performed randomized, controlled trials or overwhelming evidence of some other form. Further research is unlikely to change our confidence in the estimate of benefit and risk. “b” represents evidence from randomized, controlled trials with important limitations (inconsistent results, methodologic flaws, indirect or imprecise), or very strong evidence of some other form. Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate. “c” represents evidence from observational studies, unsystematic clinical experience, or from randomized, controlled trials with serious flaws. Any estimate of effect is uncertain.
Table 2:
Risk Stratification of Penicillin Allergy Labels by History
| History Elements that Favor Higher Risk of Penicillin Hypersensitivity | History Elements that Favor Low Risk of Penicillin Hypersensitivity | |
|---|---|---|
| Severe Delayed Symptoms at any point in the past: | Severe Immediate Symptoms (particularly within the last 5 years) | |
|
After administration of the first dose of a new treatment course with a penicillin, patient developed any of the following severe symptoms within one hour. Pre-test probability is highest when two or more occur together:
|
Low risk for allergy:
|
Current approaches to the diagnosis of delayed reactions associated with penicillins and other beta-lactams require standardization with regards to specific procedures and concentrations used and include delayed intradermal skin testing and patch testing.94 These procedures have had highest diagnostic utility for maculopapular exanthema, acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). When testing is performed on DRESS patients it is recommended that it be done off steroids and a minimum of 6 months from the acute episode.94 Currently in vitro and ex vivo testing such as ELISpot and lymphocyte transformation test have shown some diagnostic utility for delayed beta-lactam allergy but are subject to false positives and negatives, require widespread validation and are only available in specialized research centres.95 Patients whose histories are consistent with severe delayed cutaneous adverse reactions with systemic or mucosal involvement such as DRESS or Stevens-Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) should avoid direct challenges and desensitisation with the implicated and structurally related drugs and use the most efficacious structurally unrelated alternative antibiotic. (see Severe Delayed Symptoms, Table II.)
Patients with a low risk history or a history inconsistent with allergy can be challenged directly (see Favors Low Risk, Table II.) There are strengths, limitations and differing levels of evidence to support the use of each approach.96
De-labeling approaches to penicillin allergy:
Penicillin allergy de-labeling can take one of many interventional forms – including (i) assessment by history only, (ii) formal skin testing in combination with single or graded two dose ingestion challenge; (iii) single or graded two dose ingestion challenge without preceding skin testing. The most definitive test for de-labeling a penicillin allergy is tolerance of the drug on ingestion challenge. Tolerance of a graded two dose or single dose ingestion challenge is the gold standard to evaluate immediate hypersensitivity drug reactions.
Penicillin allergy assessment by history alone utilising validated point-of-care assessment tools,97,98 can risk stratify patients and potentially directly de-label those with clearly non-immune mediated reactions (20% of all reported penicillin allergies).99 These approaches currently target inaccurate penicillin allergy labels placed for reasons such as nausea, vomiting, diarrhea alone or a family history of allergy. They also target labeled patients where previous tolerance of penicillin can be readily demonstrated by chart review. It needs to be shown whether such approaches are convincing enough to have patients and physicians let go of penicillin allergy labels without a subsequent intentional drug ingestion. Devchand et al. recently validated an antibiotic allergy assessment tool that enables risk-stratification based upon reported phenotype97, which may aid in the identification of patients with low-risk phenotypes amenable to direct rechallenge.
Penicillin or aminopenicillin direct ingestion challenge has been employed successfully to definitively remove immediate hypersensitivity penicillin allergy labels.100–103 At present, direct ingestion has been done in carefully selected cohorts, predominantly healthy children or healthy adults, whose historical symptoms were at lower risk of true penicillin allergy.39,43,104,105 (Table 2) Direct provocation of a penicillin labeled patient without preceding skin testing appears to be an important tool in the de-labeling toolkit, as tolerance demonstrates the absence of immediate hypersensitivity. This approach has been particularly relevant in children where the pre-test risk of true anaphylaxis was very low.39 A rational approach for future studies would be to combine direct ingestion challenges with screening criteria validated on previous skin testing or challenge studies, to identify patients at low risk of an immediate hypersensitivity reaction a priori.97
Skin testing for penicillin allergy has long been known to add additional predictive utility for immediate hypersensitivity over clinical history alone.17,106 In those with a history of high risk reactions (e.g. widespread immediate urticaria upon first dose, anaphylaxis), skin testing has been successfully deployed to improve point-of-care prescribing, including in the intensive care unit (ICU),107 emergency department (ED),108 and inpatient wards.109,110 Sacco et al. performed a recent systematic review and meta-analysis of inpatient penicillin allergy testing (n = 24 studies), that demonstrated an increase in penicillin utilisation (9.9–49%) and decrease in vancomycin and fluoroquinolone usage post skin-testing.111 Heil et al. demonstrated that an infectious disease led testing service facilitated narrow spectrum antibiotic selection in an additional 63% of patients.112
The limitation of skin testing is that the pretest probability for positive penicillin allergy skin testing remains low, and allergy resources are typically scarce. In a large, prospective, inner-city cohort of consecutive adult patients being treated for sexually transmitted diseases, those reporting penicillin allergy had negative skin testing 93% of the time, and skin testing had a 97–99% negative predictive value for tolerating an oral challenge with penicillin.17 The likelihood of a positive challenge to a beta-lactam after negative skin testing has been observed by Goldberg et al. to be less than 4%, with around 5% still at risk for mild delayed onset rashes.113
Romano et al. have shown that among patients who have demonstrable beta-lactam allergy, 10% demonstrate loss of skin test reactivity every year,21,22 and once skin test reactivity to beta-lactams disappears, less than 1% of previously allergic patients will ever reacquire a true immune mediated beta-lactam allergy even when exposed to multiple courses of oral antibiotics.114,115 Similarly, amongst children with benign beta-lactam exanthema which are reproducible by direct provocation challenge, 89% will not react to the same challenge after 3 years.116
A future model of successful penicillin allergy evaluation will therefore need a collaborative, multimodal approach to de-labeling which incorporates decision support and risk stratification. Once risk is assessed, pathways will lead toward direct provocation challenges in low risk patients or skin testing in higher risk patients. One inpatient multi-centre ASP-led beta-lactam allergy testing service has already been shown to improve beta-lactam usage.117 A multidisciplinary ASP-led approach in Australia was able to remove a penicillin allergy label in 83% of patients and increase narrow-spectrum beta-lactam (aOR, 3.54; 95% CI, 1.98–6.33) and appropriate antibiotic usage (aOR, 12.27; 95% CI, 5.00–30.09) up to 90-days post testing.118 Such a multimodal “all of the above” approach has been used successfully in the US by Blumenthal et al. to reduce the effect of penicillin allergy labels in quasi-experimental study designs.76,119–121
Role of desensitisation and alternative antibiotics
In some instances, such as treatment of a patient with an acute life-threatening infection, direct de-labeling of the penicillin allergy may not be feasible or practical and favored approaches might be: (i) utilisation of a non-penicillin (structurally dissimilar beta-lactam or non-beta-lactam) or (ii) desensitisation. At the point-of-care if an allergy label is present and an alternative antimicrobial is available with no drop in treatment efficacy, use of that alternative agent may be an acceptable practice,122 but this is not true for all infections.121,123 An avoidance of penicillin leading to use of bacteriostatic, overly broad spectrum antimicrobials or less effective antibiotic, rather than a similar spectrum cephalosporin, is of higher concern. There is now sufficient evidence to conclude that use of alternative antimicrobials is likely the primary pathway by which the associated harm and expense of a penicillin allergy label is mediated to patients.62,74,80,121,124 Therefore, the current practice of using alternative antimicrobials to work around penicillin allergy labels needs to be critically reassessed and modified to include risk stratification. When appropriate and available, a referral to an allergy specialist for testing and de-labeling, if appropriate, should be utilized, especially in cases where future use of antimicrobials is anticipated.29,125,126
Desensitisation is another established procedure in which a patient who is allergic to a penicillin receives tolerable subtherapeutic doses (usually 1/10,000th of effective dose)127 of the drug delivered initially, with increasing doses at set intervals of time, until effective treatment doses are achieved and temporary tolerance is induced.128,129 Desensitisation is currently used in an expanding variety of drug hypersensitivity reactions as a method to induce a temporary drug tolerance which diminishes once the treatment is stopped.128 A desensitised patient can take the drug safely for a prescribed course, provided that the interval between any two doses does not exceed 4 drug half-lives.130 Very rarely patients will react during desensitisation and the protocol will need modification. This can also be an important clue that the reaction was real. Desensitisation is a temporary strategy to induce tolerance rather than a test; therefore, it cannot be expected to provide any information about the whether the patient has a true immunologically mediated adverse drug reaction or if the patient could tolerate the medication in the future. Routine post-desensitisation care of the “penicillin allergic” patient should therefore include subsequent formal drug allergy evaluation and appropriate testing >6 weeks following last receipt of the drug to clarify the need for the label.129,131
Desensitisation of a penicillin labeled patient is most useful under three scenarios:129
1. When the patient’s skin test status to a penicillin is known to be positive; 2. A clear, recent reaction concerning for an IgE-mediated reaction has been identified, the drug is indicated for a susceptible infection, and testing cannot be immediately performed.132; and/or 3. When a patient’s underlying medical conditions predispose them to instability or fragility, but penicillin is required for immediate treatment. Many physicians cite syphilis infection either during pregnancy or recalcitrant to alternative therapies 131 and treatment of susceptible and deep-seated staphylococcal or enterococcal infections133,134 as examples of indications for desensitisation.
The main arguments against using routine desensitisation for every penicillin allergy label are the time and resources used without having gained any information on the validity of the label or whether the patient is truly penicillin allergic. In penicillin allergy, the vast majority of labeled patients are unlikely to have true hypersensitivity reactions.17,113 Hence, frequent desensitisations in a patient who has never been skin tested or challenged represents a suboptimal approach that is unlikely to be cost-effective.
Specific challenges to allergy de-labeling:
Currently there are important barriers and limitations that must be considered to address the large burden of patients carrying a penicillin allergy label. Access to penicillin skin testing is not universally available.135 Internationally, not all allergists offer penicillin skin testing as part of their routine practice.136 Allergy and Immunology training programs do not universally offer drug and antibiotic allergy training that can be implemented into routine clinical practice.137
The burden of proof to convince a patient that a penicillin allergy label is no longer needed may vary between childhood and adulthood. Tonson de la Tour et al. demonstrated that a 2 day challenge and delabeling in children led to a 69% utilization of subsequent penicillin treatment within the next three years.116 Vyles et al. demonstrated that 73% of parents felt comfortable giving penicillin derivatives to their children after skin testing and single dose oral challenge delabelling.41,43 Labrosse et. al demonstrated that parents were convinced of penicillin safety at high rates by negative direct provocation testing, and to a greater degree by a multiple day challenge compared to a single dose challenge.138 Labels that are removed in adulthood may require greater effort to assuage a patient’s desire to avoid that drug. Adult patients have previously received negative conditioning and reinforcement about avoidance of penicillin. They can be fearful of label removal and reluctant to take penicillin despite being told it is safe after a negative evaluation, which would suggest that earlier intervention is needed. In a study by Gerace et al., 41% of patients who underwent outpatient penicillin allergy testing with or without challenge continued to avoid all penicillins in the absence of post-testing physician counseling.139 Using a more intensive program, Bourke et al found that an inpatient drug allergy delabeling service resulted in 75% of patients following allergy label modifications.90 Ratzon et al. found that adult patients were more convinced by a multiple day challenge than by a single dose challenge for delabeling.140
Unfortunately, while most penicillin allergies are currently acquired in childhood, decisions about whether to test a child’s antibiotic allergy label are often deferred, as antibiotic alternatives may appear to be readily available and parents see testing as uncomfortable or painful for their child.57,69 However, Lucas et al. have recently shown that penicillin labels are associated with adverse outcomes such as longer hospital stay even in childhood.57 Deferral of penicillin allergy testing may also lead to the acquisition of additional drug labels as alternatives are utilized over time, leading to the scenario of a multi-drug allergy labeled patient who has run out of treatment options.29,125,126 Overcoming any reluctance or inertia to testing penicillin allergy earlier in childhood or adolescence will likely be crucial in reducing the “stickiness” of labels that have been reinforced for decades.
There is also still some debate as to the length of provocation challenge needed for allergy label removal. Both single step and graded same day drug challenges are utilized for de-labeling in current practice, but graded challenges may not provide much additional utility over single dose challenges in exchange for the increased resources required.141 Multiple day challenges may provide additional diagnostic utility for delayed rashes compared to a single dose with prolonged patient follow up, but published series suggest this benefit is also small.142 Immediate clinical use of a penicillin following de-labeling is beneficial to future patient confidence for use. Multi-day challenges also have the potential to decrease patient anxiety and penicillin avoidance, but this will need to be weighed against the downside of increased antimicrobial exposure.138,140
There are no validated blood testing strategies or biomarkers to aid in penicillin allergy de-labeling that are sufficiently sensitive or specific enough to use alone in clinical practice. It is known that IgE specific for drugs are mechanistically involved in Type I immediate hypersensitivity reactions. Unfortunately, testing for penicillin-specific IgE currently lacks both sensitivity and specificity to detect true drug allergy, does not cover the penicillin minor determinants, and has a negative predictive value significantly less than 100%.129,143,144 Partially, this may reflect a limited window in which penicillin-specific IgE is detectable after a reaction, followed by a rapid decrease in circulating antibody concentrations.145,146 Similarly the basophil activation test, a flow cytometry based test that measures drug induced activation of basophils by examining alterations in the expression of basophil markers such as CD63 and CD203c is not available at many centers and does not have the negative predictive value needed for use in clinical practice.147 Because of these limitations, current commercial versions of specific IgE tests and investigational tests such as the basophil activation test do not provide sufficient clinical utility to support their use in de-labeling.148
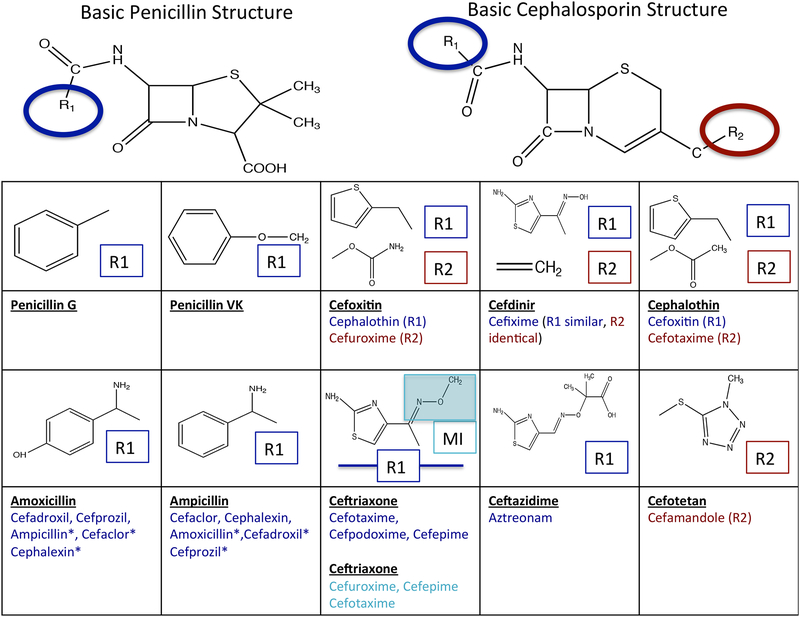
Current skin testing and oral challenge protocols to de-label penicillin are not standardized across practices within countries, much less internationally, and do not always account for the new knowledge of beta-lactam cross-reactivity. Oral tolerance of amoxicillin is sufficient to remove all penicillin allergy labels. However, in an amoxicillin labeled patient, skin testing using major and minor penicillin determinants may miss some patients with selective side-chain specific aminopenicillin allergies who will react to amoxicillin and ampicillin based compounds upon provocation, and this is particularly true in Europe and other countries such as Australia where 1/3 or more of patients may demonstrate selective aminopenicillin skin test reactivity.18,149 Selective reactions to clavulanic acid have been described for which an intradermal skin testing strategy exists in Europe and Australia but not in North America, which may reflect differing sensitization patterns.24,150 While parenteral amoxicillin/clavulanic acid is used in many countries around the world, including Europe and Australia, only oral administration is approved by the FDA for use in the US.151 When skin testing to amoxicillin and/or ampicillin is negative in the setting of an immediate reaction to amoxicillin-clavulanate within the last 5 years in particular, a selective reaction to clavulanate should be considered. 24,150 Simultaneous sensitization to both amoxicillin and clavulanic acid have been reported in Spain.152 Cephalosporin labels must be considered separately from penicillin labels, using knowledge of cross reactive families, as side chain cross-reactivity patterns are likely to be a primary driver in cephalosporin reactions and are particularly relevant to cross-reactivity between aminopenicillin and aminocephalosporins.35,153 (Figure 3) Selective aminopenicillin reactions and cross-reactivity between aminopenicillins and cephalosporins appear to be more prevalent in Europe and Australia than in the US although because of lack of widespread use of multiple penicillin and cephalosporin reagents and cephalosporin ingestion challenge in the US this has not been fully examined.31,35,135,150,153 A recent prospective multicenter open label investigation of penicillin skin testing in 455 patients with histories of immediate reactions to penicillin was conducted across 13 allergy centres in the US using a penicillin skin testing kit containing penicilloyl-polylysine, tripartite minor determinant mixture (penicillin G, penicilloate and penilloate) and amoxicillin. In this study 4/63 (6.3%) of skin test positive patients reacted to amoxicillin alone with negative skin tests to the other reagents.154
Figure 3: Understanding Cross Reactivity Amongst Beta Lactams Based Upon R1, R2 Side Chains or Shared Methoxyimino (MI) Grouping is Important to De-labeling Efforts.
For example, consider a patient with two allergy labels, one to penicillin and the other to ceftriaxone. Tolerance of an oral challenge with amoxicillin would prove the safety of all penicillins in this patient by challenging the patient with both the basic penicillin structure and the aminopenicillin side chain. This challenge would not effectively determine the safety of ceftriaxone in a patient labeled allergic to ceftriaxone, however, due to differing side chains and basic structure. Hence, the penicillin allergy label could be removed, but additional basic cephalosporin and ceftriaxone specific side chain specific testing would be needed to determine the safety of ceftriaxone prior to ceftriaxone label removal. 18,35,149,153
Future Directions/ Opportunities for Improvement:
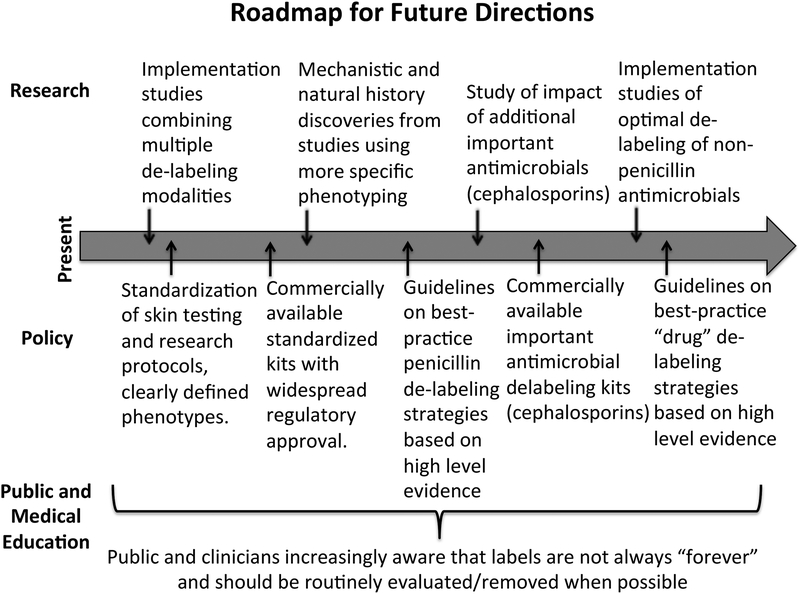
Despite these limitations, energy and enthusiasm to tackle the problem of inappropriate penicillin allergy labeling is currently high, given the demonstrable effects of penicillin allergy labels on antimicrobial stewardship and healthcare outcomes. Within this window of opportunity, there are multiple ways in which we can improve upon our testing, research, and implementation. (Figure 4, Table 3)
Figure 4: Roadmap for Future Directions in Penicillin De-labeling Research.
An additional important milestone includes research to identify factors that lead to reintroduction of penicillin allergy labels into a patient’s chart.
Table 3:
Characteristics that Favor or Impede Penicillin Allergy De-labeling: A SWOT Analysis
| Helpful to Achieving the Objective | Harmful to Achieving the Objective | |
|---|---|---|
|
Internal Origin (Attributes of the Institution/Organization) |
Strengths
|
Weaknesses
|
|
External Origin (Attributes of the Environment) |
Opportunities
|
Threats
|
In light of the emerging knowledge of beta-lactam allergy cross-reactivity patterns,18,35 there is a need for easier access to skin testing reagents, standardized international skin testing protocols and research protocols. Results from this standardization will lead to more precise phenotyping in routine clinical practice. Since true beta-lactam allergy with a positive skin test is somewhat rare, collaborative research networks aimed at a deeper understanding of penicillin allergy epidemiology and mechanisms are needed once standardized testing is implemented.
On the implementation side, the awareness that most patients carry unnecessary penicillin allergy labels highlights the need to further validate clinical tools to correctly risk stratify patients with low risk penicillin allergy history who may be appropriate for direct ingestion challenge.
Finally, there is a growing need for insight into factors from both a patient and healthcare provider perspective that impede the effectiveness of penicillin allergy de-labeling strategies and lead to reintroduction of penicillin allergy labels into a patient’s chart. The work, resources and effort of de-labeling is in vain if the results are unconvincing to patients and treating clinicians during future healthcare encounters.
Conclusions:
Penicillin allergy labels are highly prevalent, largely inaccurate and their carriage may lead to unnecessary treatment and inferior outcomes with alternative agents as well as adverse public health outcomes such as antibiotic resistance. Operationalizing penicillin allergy de-labeling as an aspect of ASP has become an increasing global focus. There is a need for validated approaches that optimally combine the use of history and risk stratification with validated allergy testing approaches such as ingestion challenge with or without preceding formal skin testing to tackle penicillin allergy efficiently across disparate healthcare systems. At the same time, there is great promise for penicillin allergy evaluation and de-labeling as an individual and public health strategy to reduce adverse healthcare outcomes, improve antimicrobial stewardship, and decrease healthcare costs.
Major Milestone Discoveries Text Box:
1928 - Discovery of penicillin1
1940 - Usage of penicillin in military2
1941 - Widespread usage of penicillin2
1941–1947 - Unusual cutaneous and systemic reactions to penicillin described3–7
1944–1946 - First cases of skin test positive against penicillin described4,5
1948–1949 - First cases of penicillin anaphylaxis8
1950s - Early use of penicillin reagents in skin testing9
1960s - Implication of serologic factors in penicillin allergy10
1960s – First cases suggesting cross-reactivity between penicillins and cephalosporins.11–13
1972 – Amoxicillin introduced in widespread usage
1960s-1990s - Early and established use of penicillin reagents in skin testing;16 several papers established the high negative predictive value of skin testing when combined with oral challenge17
1990 - Side chain reactions associated with amino penicillins described and more prevalent in Southern Europe 18–20
1990- Reports of waning skin test reactivity to penicillin and other reagents over time21,22
1995- First description of selective allergic reaction to clavulanic acid in Southern Europe.24
2005 - Penicillin reagents (Allergopharma) removed from market leaving void of commercially available and validated penicillin major and minor determinants: This necessitated use of alternative skin testing strategies25
2008 - Penicillin major and minor determinant reagent (Diater®) available for testing in Europe and special access in some other countries (e.g. Australia)26
2010 - Benzyl penicilloyl polylysine (Pre-Pen®) (Allerquest) available as
commercial reagent on US market27
2010 - Early reports of the burden of over-labelling of penicillin allergy28.
2010 - First direct challenges of children with benign nonimmediate reactions to penicillin without preceding skin testing28
2012- Early reports of the impact of penicillin labels on antimicrobial stewardship29,30
2013 - First reports of term “penicillin allergy de-labelling”31
2014–2018 - Impact of penicillin labels on Clostridioides difficile32,33
2016 - Recognition of the need to address penicillin allergy by Centers for Disease Control, Infectious Disease Society of America antimicrobial stewardship guidelines34
2016–2018 – More recent articles on penicillin and cephalosporin cross reactivity highlight specific R1 cross reactivity patterns and very low rate of cross-reactivity based on beta-lactam ring (<2%) 35,36
2016–2018 - Impact of penicillin labels on time to antibiotic administration37–39
2017 - Impact of penicillin over-labelling on antibiotic resistance40
2018 - Impact of penicillin labels on surgical site infections44
Future Research Perspectives Text Box:
Areas of Greatest Need:
Development of large collaborative drug allergy research networks
Standardized clinical phenotyping of penicillin reactions.
Standardization of skin testing protocols for determination of immediate and delayed hypersensitivity phenotypes and beta-lactam cross-reactivity pattern.
Develop and standardization of sensitive and specific in vitro testing tools for penicillin allergy
Validation and implementation of point-of-care tools to identify “low risk” penicillin allergy
Evidence base for de-labeling penicillin allergy patients, using risk stratification to direct patients for allergy label removal using direct oral challenge versus skin testing followed by challenge.
Long-term outcomes from controlled intervention studies of penicillin allergy label removal.
Development of “toolkit” for routine integration of penicillin and antibiotic allergy management into antimicrobial steward programs.
Understanding the immunopathogenesis of and mechanisms of sensitization, cross-reactivity and waning of immunity to penicillins and other beta-lactam antimicrobials and differences in regional epidemiology
Qualitative studies to examine behavioral factors that drive differences in the effectiveness of penicillin allergy de-labeling.
Funding Sources:
Dr. Stone receives funding from 5T32 GM007569–41 from NIH/NIGMS.
Dr. Phillips receives funding related to this project from: National Institutes of Health (1P50GM115305–01, 1P30AI110527–01A1, R21AI139021 and R34AI136815), National Health and Medical Research (NHMRC) Foundation of Australia.
Dr. Trubiano is supported by an NHMRC Early Career Fellowship and funding from The National Centre for Infections in Cancer and Austin Medical Research Foundation.
Abbreviations:
- UK
United Kingdom
- US
United States
- MRSA
methicillin-resistant Staphylococcus aureus
- ASPs
Antimicrobial stewardship programs
- IgE
Immunoglobulin E
- ICU
intensive care unit
- ED
emergency department
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest to disclose.
References:
- 1.Fleming A On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. Bulletin of the World Health Organization. 2001;79(8):780–790. [PMC free article] [PubMed] [Google Scholar]
- 2.du Vigneaud V, Carpenter F, Holley R, Livermore A, Rachele J. Synthetic Penicillin. Science. 1946;104(2706):431–450. [DOI] [PubMed] [Google Scholar]
- 3.Keefer C, Blake F, Marshall E, et al. Penicillin in the treatment of infections: A report of 500 cases. Journal of the American Medical Association. 1943;122(18):1217–1224. [Google Scholar]
- 4.Welch H, Rostenberg A Jr. Hypersensitivity of the tuberculin type to crystalline penicillin sodium. Journal of the American Medical Association. 1944;126(1):10–12. [Google Scholar]
- 5.Kolodny M, Denhoff E. Reactions in penicillin therapy. Journal of the American Medical Association. 1946;130(16):1058–1061. [DOI] [PubMed] [Google Scholar]
- 6.Jaslowitz H Reaction to Penicillin. British medical journal. 1945;2(4430):767–767. [PMC free article] [PubMed] [Google Scholar]
- 7.Farrington J, Riley K, Olansky S. Untoward reactions and cutaneous testing in penicillin therapy. Southern medical journal. 1948;41(7):614–620. [DOI] [PubMed] [Google Scholar]
- 8.Waldbott G Anaphylactic death from penicillin. Journal of the American Medical Association. 1949;139(8):526–527. [Google Scholar]
- 9.Berger A, Eisen B. Feasibility of skin testing for penicillin sensitivity: A study of one thousand cases. Journal of the American Medical Association. 1955;159(3):191–193. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz R, Vaughan J. Immunologic Responsiveness of Man to Penicillin. Jama. 1963;186:1151–1157. [DOI] [PubMed] [Google Scholar]
- 11.Scholand J, Tennenbaum J, Cerilli G. Anaphylaxis to cephalothin in a patient allergic to penicillin. Jama. 1968;206(1):130–132. [PubMed] [Google Scholar]
- 12.Crieco M Cross-allergenicity of the penicillins and the cephalosporins. Archives of internal medicine. 1967;119(2):141–145. [DOI] [PubMed] [Google Scholar]
- 13.Rothschild P, Doty D. Cephalothin Reaction After Penicillin Sensitization. Jama. 1966;196(4):372–373. [Google Scholar]
- 14.Johansson S, Bennich H, Wide L. A new class of immunoglobulin in human serum. Immunology. 1968;14(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizaka K, Ishizaka T. Physicochemical properties of reaginic antibody. 1. Association of reaginic activity with an immunoglobulin other than gammaA- or gammaG-globulin. The Journal of allergy. 1966;37(3):169–185. [DOI] [PubMed] [Google Scholar]
- 16.Levine B, Redmond A. Minor haptenic determinant-specific reagins of penicillin hypersensitivity in man. International archives of allergy and applied immunology. 1969;35(5):445–455. [DOI] [PubMed] [Google Scholar]
- 17.Gadde J, Spence M, Wheeler B, Adkinson N Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. Jama. 1993;270(20):2456–2463. [PubMed] [Google Scholar]
- 18.Romano A, Torres M, Fernandez J, et al. Allergic reactions to ampicillin. Studies on the specificity and selectivity in subjects with immediate reactions. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1997;27(12):1425–1431. [PubMed] [Google Scholar]
- 19.Blanca M, Fernandez J, Miranda A, et al. Cross-reactivity between penicillins and cephalosporins: clinical and immunologic studies. The Journal of allergy and clinical immunology. 1989;83(2 Pt 1):381–385. [DOI] [PubMed] [Google Scholar]
- 20.Torres M, Gonzalez F, Mayorga C, et al. IgG and IgE antibodies in subjects allergic to penicillins recognize different parts of the penicillin molecule. International archives of allergy and immunology. 1997;113(1–3):342–344. [DOI] [PubMed] [Google Scholar]
- 21.Blanca M, Torres M, Garcia J, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. The Journal of allergy and clinical immunology. 1999;103(5 Pt 1):918–924. [DOI] [PubMed] [Google Scholar]
- 22.Romano A, Gaeta F, Valluzzi R, Zaffiro A, Caruso C, Quaratino D. Natural evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity to cephalosporins. Allergy. 2014;69(6):806–809. [DOI] [PubMed] [Google Scholar]
- 23.Linares T, Marcos C, Gavilan M, Arenas L. Hypersensitivity to penicillin V with good tolerance to other beta-lactams. Journal of investigational allergology & clinical immunology. 2007;17(1):50–51. [PubMed] [Google Scholar]
- 24.Fernandez-Rivas M, Perez Carral C, Cuevas M, Marti C, Moral A, Senent C. Selective allergic reactions to clavulanic acid. The Journal of allergy and clinical immunology. 1995;95(3):748–750. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Bada JL, Montanez MI, Torres MJ, et al. Skin testing for immediate hypersensitivity to betalactams: comparison between two commercial kits. Allergy. 2006;61(8):947–951. [DOI] [PubMed] [Google Scholar]
- 26.Nolan R, Puy R, Deckert K, O’Hehir R, Douglass J. Experience with a new commercial skin testing kit to identify IgE-mediated penicillin allergy. Internal medicine journal. 2008;38(5):357–361. [DOI] [PubMed] [Google Scholar]
- 27.FDA. FDA Approved Drug Products. Drugs@FDA: FDA Approved Drug Products 2018; https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/050114s008ltr.pdf. Accessed 11-20-2018, 2018.
- 28.Caubet J, Kaiser L, Lemaitre B, Fellay B, Gervaix A, Eigenmann P. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. The Journal of allergy and clinical immunology. 2011;127(1):218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caimmi S, Sanfiorenzo C, Caimmi D, Bousquet P, Chiron R, Demoly P. Comprehensive allergy work-up is mandatory in cystic fibrosis patients who report a history suggestive of drug allergy to beta-lactam antibiotics. Clinical and translational allergy. 2012;2(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimawi R, Cook P, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. Journal of hospital medicine. 2013;8(6):341–345. [DOI] [PubMed] [Google Scholar]
- 31.Trubiano J, Phillips E. Antimicrobial stewardship’s new weapon? A review of antibiotic allergy and pathways to ‘de-labeling’. Current opinion in infectious diseases. 2013;26(6):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. Bmj. 2018;361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. The Journal of allergy and clinical immunology. 2014;133(3):790–796. [DOI] [PubMed] [Google Scholar]
- 34.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(10):e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romano A, Gaeta F, Arribas Poves M, Valluzzi R. Cross-Reactivity among Beta-Lactams. Current allergy and asthma reports. 2016;16(3):24. [DOI] [PubMed] [Google Scholar]
- 36.Romano A, Valluzzi RL, Caruso C, Maggioletti M, Quaratino D, Gaeta F. Cross-Reactivity and Tolerability of Cephalosporins in Patients with IgE-Mediated Hypersensitivity to Penicillins. The journal of allergy and clinical immunology. In practice. 2018;6(5):1662–1672. [DOI] [PubMed] [Google Scholar]
- 37.Moussa Y, Shuster J, Matte G, et al. De-labeling of beta-lactam allergy reduces intraoperative time and optimizes choice in antibiotic prophylaxis. Surgery. 2018:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Conway E, Lin K, Sellick J, et al. Impact of Penicillin Allergy on Time to First Dose of Antimicrobial Therapy and Clinical Outcomes. Clinical therapeutics. 2017;39(11):2276–2283. [DOI] [PubMed] [Google Scholar]
- 39.Mill C, Primeau M, Medoff E, et al. Assessing the Diagnostic Properties of a Graded Oral Provocation Challenge for the Diagnosis of Immediate and Nonimmediate Reactions to Amoxicillin in Children. JAMA pediatrics. 2016;170(6):e160033. [DOI] [PubMed] [Google Scholar]
- 40.Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990–1001. [DOI] [PubMed] [Google Scholar]
- 41.Vyles D, Chiu A, Routes J, et al. Antibiotic Use After Removal of Penicillin Allergy Label. Pediatrics. 2018;141(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vyles D, Chiu A, Simpson P, Nimmer M, Adams J, Brousseau D. Parent-Reported Penicillin Allergy Symptoms in the Pediatric Emergency Department. Academic pediatrics. 2017;17(3):251–255. [DOI] [PubMed] [Google Scholar]
- 43.Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau D. Allergy Testing in Children With Low-Risk Penicillin Allergy Symptoms. Pediatrics. 2017;140(2). [DOI] [PubMed] [Google Scholar]
- 44.Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clin Infect Dis. 2018;66(3):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penicillin Macy E. and beta-lactam allergy: epidemiology and diagnosis. Current allergy and asthma reports. 2014;14(11):476. [DOI] [PubMed] [Google Scholar]
- 46.Trubiano J, Cairns K, Evans J, et al. The prevalence and impact of antimicrobial allergies and adverse drug reactions at an Australian tertiary centre. BMC infectious diseases. 2015;15:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerr J Penicillin allergy: a study of incidence as reported by patients. The British journal of clinical practice. 1994;48(1):5–7. [PubMed] [Google Scholar]
- 48.Sogn D, Evans R 3rd, Shepherd G, et al. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Archives of internal medicine. 1992;152(5):1025–1032. [PubMed] [Google Scholar]
- 49.Borch J, Andersen K, Bindslev-Jensen C. The prevalence of suspected and challenge-verified penicillin allergy in a university hospital population. Basic Clin Pharmacol Toxicol. 2006;98(4):357–362. [DOI] [PubMed] [Google Scholar]
- 50.Blanca M, Romano A, Torres M, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64(2):183–193. [DOI] [PubMed] [Google Scholar]
- 51.Trubiano JA, Chen C, Cheng AC, et al. Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. The Journal of antimicrobial chemotherapy. 2016;71(6):1715–1722. [DOI] [PubMed] [Google Scholar]
- 52.Trubiano J, Adkinson N, Phillips E. Penicillin Allergy Is Not Necessarily Forever. Jama. 2017;318(1):82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le J, Nguyen T, Law A, Hodding J. Adverse drug reactions among children over a 10-year period. Pediatrics. 2006;118(2):555–562. [DOI] [PubMed] [Google Scholar]
- 54.Romano A, Caubet J. Antibiotic allergies in children and adults: from clinical symptoms to skin testing diagnosis. The journal of allergy and clinical immunology. In practice. 2014;2(1):3–12. [DOI] [PubMed] [Google Scholar]
- 55.MacFadden D, LaDelfa A, Leen J, et al. Impact of Reported Beta-Lactam Allergy on Inpatient Outcomes: A Multicenter Prospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(7):904–910. [DOI] [PubMed] [Google Scholar]
- 56.Macy E, Contreras R. Adverse reactions associated with oral and parenteral use of cephalosporins: A retrospective population-based analysis. The Journal of allergy and clinical immunology. 2015;135(3):745–752 e745. [DOI] [PubMed] [Google Scholar]
- 57.Lucas M, Arnold A, Sommerfield A, et al. Antibiotic allergy labels in children are associated with adverse clinical outcomes. The journal of allergy and clinical immunology. In practice. 2018. [DOI] [PubMed] [Google Scholar]
- 58.Huang K, Cluzet V, Hamilton K, Fadugba O. The Impact of Reported Beta-Lactam Allergy in Hospitalized Patients With Hematologic Malignancies Requiring Antibiotics. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;67(1):27–33. [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Kim K, Kim H, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrobial agents and chemotherapy. 2008;52(1):192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDanel D, Azar A, Dowden A, et al. Screening for Beta-Lactam Allergy in Joint Arthroplasty Patients to Improve Surgical Prophylaxis Practice. The Journal of arthroplasty. 2017;32(9S):S101–S108. [DOI] [PubMed] [Google Scholar]
- 61.Park M, Markus P, Matesic D, Li J. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2006;97(5):681–687. [DOI] [PubMed] [Google Scholar]
- 62.Blumenthal K, Lu N, Zhang Y, Li Y, Walensky R, Choi H. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. Bmj. 2018;361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishna M, Huissoon A, Li M, et al. Enhancing antibiotic stewardship by tackling “spurious” penicillin allergy. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2017;47(11):1362–1373. [DOI] [PubMed] [Google Scholar]
- 64.Li M, Krishna M, Razaq S, Pillay D. A real-time prospective evaluation of clinical pharmaco-economic impact of diagnostic label of ‘penicillin allergy’ in a UK teaching hospital. Journal of clinical pathology. 2014;67(12):1088–1092. [DOI] [PubMed] [Google Scholar]
- 65.Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. The journal of allergy and clinical immunology. In practice. 2013;1(3):252–257. [DOI] [PubMed] [Google Scholar]
- 66.Nebeker J, Barach P, Samore M. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795–801. [DOI] [PubMed] [Google Scholar]
- 67.Inglis J, Caughey G, Smith W, Shakib S. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Internal medicine journal. 2017;47(11):1292–1297. [DOI] [PubMed] [Google Scholar]
- 68.Edwards I, Aronson J. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–1259. [DOI] [PubMed] [Google Scholar]
- 69.Norton A, Konvinse K, Phillips E, Broyles A. Antibiotic Allergy in Pediatrics. Pediatrics. 2018;141(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ibia E, Schwartz R, Wiedermann B. Antibiotic rashes in children: a survey in a private practice setting. Archives of dermatology. 2000;136(7):849–854. [DOI] [PubMed] [Google Scholar]
- 71.National Clinical Guideline Centre (UK). Drug Allergy: Diagnosis and Management of Drug Allergy in Adults, Children and Young People. London: 2014. [PubMed] [Google Scholar]
- 72.Adkinson N Jr. Risk factors for drug allergy. The Journal of allergy and clinical immunology. 1984;74(4 Pt 2):567–572. [DOI] [PubMed] [Google Scholar]
- 73.Macy E, Blumenthal K. Are Cephalosporins Safe for Use in Penicillin Allergy without Prior Allergy Evaluation? The journal of allergy and clinical immunology. In practice. 2018;6(1):82–89. [DOI] [PubMed] [Google Scholar]
- 74.Blumenthal K, Ryan E, Li Y, Lee H, Kuhlen J, Shenoy E. The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(3):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeffres M, Narayanan P, Shuster J, Schramm G. Consequences of avoiding beta-lactams in patients with beta-lactam allergies. The Journal of allergy and clinical immunology. 2016;137(4):1148–1153. [DOI] [PubMed] [Google Scholar]
- 76.Blumenthal K, Wickner P, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. The Journal of allergy and clinical immunology. 2017;140(1):154–161 e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacFadden DR, LaDelfa A, Leen J, et al. Impact of Reported Beta-Lactam Allergy on Inpatient Outcomes: A Multicenter Prospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(7):904–910. [DOI] [PubMed] [Google Scholar]
- 78.Su T, Broekhuizen B, Verheij T, Rockmann H. The impact of penicillin allergy labels on antibiotic and health care use in primary care: a retrospective cohort study. Clinical and translational allergy. 2017;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Briody V, Albright C, Has P, Hughes B. Use of Cefazolin for Group B Streptococci Prophylaxis in Women Reporting a Penicillin Allergy Without Anaphylaxis. Obstetrics and gynecology. 2016;127(3):577–583. [DOI] [PubMed] [Google Scholar]
- 80.Blumenthal K, Li Y, Banerji A, Yun B, Long A, Walensky R. The Cost of Penicillin Allergy Evaluation. The journal of allergy and clinical immunology. In practice. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones B, Bland C. Penicillin skin testing as an antimicrobial stewardship initiative. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2017;74(4):232–237. [DOI] [PubMed] [Google Scholar]
- 82.Sacco K, Bates A, Brigham T, Imam J, Burton M. Clinical outcomes following inpatient penicillin allergy testing: A systematic review and meta-analysis. Allergy. 2017;72(9):1288–1296. [DOI] [PubMed] [Google Scholar]
- 83.Ressner RA, Gada SM, Banks TA. Antimicrobial Stewardship and the Allergist: Reclaiming our Antibiotic Armamentarium. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(3):400–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling Inpatient Penicillin Allergies: Tools for Antimicrobial Stewardship. The Journal of allergy and clinical immunology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Archives of internal medicine. 2000;160(18):2819–2822. [DOI] [PubMed] [Google Scholar]
- 86.van Dijk SM, Gardarsdottir H, Wassenberg MW, Oosterheert JJ, de Groot MC, Rockmann H. The High Impact of Penicillin Allergy Registration in Hospitalized Patients. The journal of allergy and clinical immunology. In practice. 2016. [DOI] [PubMed] [Google Scholar]
- 87.Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The Cost of Self-Reported Penicillin Allergy: A Systematic Review. The journal of allergy and clinical immunology. In practice. 2018. [DOI] [PubMed] [Google Scholar]
- 88.Wu JH, Langford BJ, Schwartz KL, et al. Potential Negative Effects of Antimicrobial Allergy Labelling on Patient Care: A Systematic Review. Can J Hosp Pharm. 2018;71(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- 89.Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. The Cochrane database of systematic reviews. 2017;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bourke J, Pavlos R, James I, Phillips E. Improving the Effectiveness of Penicillin Allergy De-labeling. The journal of allergy and clinical immunology. In practice. 2015;3(3):365–334 e361. [DOI] [PubMed] [Google Scholar]
- 91.Trubiano JA, Adkinson NF, Phillips EJ. Penicillin Allergy Is Not Necessarily Forever. Jama. 2017;318(1):82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang KG, Cluzet V, Hamilton K, Fadugba O. The Impact of Reported Beta-Lactam Allergy in Hospitalized Patients with Hematologic Malignancies Requiring Antibiotics. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018. [DOI] [PubMed] [Google Scholar]
- 93.Trubiano JA, Grayson ML, Thursky KA, Phillips EJ, Slavin MA. How antibiotic allergy labels may be harming our most vulnerable patients. The Medical journal of Australia. 2018;208(11):469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phillips E, Bigliardi P, Bircher A, et al. Controversies in drug allergy: Testing for delayed reactions. The Journal of allergy and clinical immunology. 2019;143(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mayorga C, Ebo DG, Lang DM, et al. Controversies in drug allergy: In vitro testing. The Journal of allergy and clinical immunology. 2019;143(1):56–65. [DOI] [PubMed] [Google Scholar]
- 96.Kavanagh B The GRADE system for rating clinical guidelines. PLoS Med. 2009;6(9):e1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Devchand M, Urbancic K, Khumra S, et al. Pathways to Improved Antibiotic Allergy and Antimicrobial Stewardship Practice - The Validation of a Beta-Lactam Antibiotic Allergy Assessment Tool. The journal of allergy and clinical immunology. In practice. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Staicu ML, Brundige ML, Ramsey A, et al. Implementation of a penicillin allergy screening tool to optimize aztreonam use. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2016;73(5):298–306. [DOI] [PubMed] [Google Scholar]
- 99.Trubiano JA, Cairns KA, Evans JA, et al. The prevalence and impact of antimicrobial allergies and adverse drug reactions at an Australian tertiary centre. BMC infectious diseases. 2015;15:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. The journal of allergy and clinical immunology. In practice. 2017;5(3):813–815. [DOI] [PubMed] [Google Scholar]
- 101.Vezir E, Dibek Misirlioglu E, Civelek E, et al. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(1):50–54. [DOI] [PubMed] [Google Scholar]
- 102.Iammatteo M, Alvarez Arango S, Ferastraoaru D, et al. Safety and Outcomes of Oral Graded Challenges to Amoxicillin without Prior Skin Testing. The journal of allergy and clinical immunology. In practice. 2018. [DOI] [PubMed] [Google Scholar]
- 103.Confino-Cohen R, Rosman Y, Meir-Shafrir K, et al. Oral Challenge without Skin Testing Safely Excludes Clinically Significant Delayed-Onset Penicillin Hypersensitivity. The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(3):669–675. [DOI] [PubMed] [Google Scholar]
- 104.Confino-Cohen R, Rosman Y, Meir-Shafrir K, et al. Oral Challenge without Skin Testing Safely Excludes Clinically Significant Delayed-Onset Penicillin Hypersensitivity. The journal of allergy and clinical immunology. In practice. 2017;5(3):669–675. [DOI] [PubMed] [Google Scholar]
- 105.Tucker M, Lomas C, Ramchandar N, Waldram J. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. The journal of allergy and clinical immunology. In practice. 2017;5(3):813–815. [DOI] [PubMed] [Google Scholar]
- 106.Tannert L, Mortz C, Skov P, Bindslev-Jensen C. Positive Skin Test or Specific IgE to Penicillin Does Not Reliably Predict Penicillin Allergy. The journal of allergy and clinical immunology. In practice. 2017;5(3):676–683. [DOI] [PubMed] [Google Scholar]
- 107.Arroliga ME, Vazquez-Sandoval A, Dvoracek J, Arroliga AC. Penicillin skin testing is a safe method to guide beta-lactam administration in the intensive care unit. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;116(1):86–87. [DOI] [PubMed] [Google Scholar]
- 108.Marwood J, Aguirrebarrena G, Kerr S, Welch SA, Rimmer J. De-labelling self-reported penicillin allergy within the emergency department through the use of skin tests and oral drug provocation testing. Emerg Med Australas. 2017;29(5):509–515. [DOI] [PubMed] [Google Scholar]
- 109.Macy E, Roppe LB, Schatz M. Routine Penicillin Skin Testing in Hospitalized Patients with a History of Penicillin Allergy. The Permanente journal. 2004;8(3):20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: Effect on antibiotic selection and cost. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;117(1):67–71. [DOI] [PubMed] [Google Scholar]
- 111.Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: A systematic review and meta-analysis. Allergy. 2017. [DOI] [PubMed] [Google Scholar]
- 112.Heil EL, Bork JT, Schmalzle SA, et al. Implementation of an Infectious Disease Fellow-Managed Penicillin Allergy Skin Testing Service. Open forum infectious diseases. 2016;3(3):ofw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goldberg A, Confino-Cohen R. Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2008;100(1):37–43. [DOI] [PubMed] [Google Scholar]
- 114.Ponvert C, Weilenmann C, Wassenberg J, et al. Allergy to betalactam antibiotics in children: a prospective follow-up study in retreated children after negative responses in skin and challenge tests. Allergy. 2007;62(1):42–46. [DOI] [PubMed] [Google Scholar]
- 115.Solensky R, Earl H, Gruchalla R. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Archives of internal medicine. 2002;162(7):822–826. [DOI] [PubMed] [Google Scholar]
- 116.Tonson la Tour A, Michelet M, Eigenmann P, Caubet J. Natural History of Benign Nonimmediate Allergy to Beta-Lactams in Children: A Prospective Study in Retreated Patients After a Positive and a Negative Provocation Test. The journal of allergy and clinical immunology. In practice. 2018;6(4):1321–1326. [DOI] [PubMed] [Google Scholar]
- 117.Leis JA, Palmay L, Ho G, et al. Point-of-care Beta-lactam Allergy Skin Testing by Antimicrobial Stewardship Programs: A Pragmatic Multicenter Prospective Evaluation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017. [DOI] [PubMed] [Google Scholar]
- 118.Trubiano JA, Thursky KA, Stewardson AJ, et al. Impact of an Integrated Antibiotic Allergy Testing Program on Antimicrobial Stewardship: A Multicenter Evaluation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;65(1):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blumenthal K, Shenoy E, Varughese C, Hurwitz S, Hooper D, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2015;115(4):294–300 e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blumenthal K, Shenoy E, Hurwitz S, Varughese C, Hooper D, Banerji A. Effect of a drug allergy educational program and antibiotic prescribing guideline on inpatient clinical providers’ antibiotic prescribing knowledge. The journal of allergy and clinical immunology. In practice. 2014;2(4):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blumenthal K, Parker R, Shenoy E, Walensky R. Improving Clinical Outcomes in Patients With Methicillin-Sensitive Staphylococcus aureus Bacteremia and Reported Penicillin Allergy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(5):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller M, Fish D, Barber G, et al. A comparison of safety and outcomes with cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bloodstream infections. J Microbiol Immunol Infect. 2018. [DOI] [PubMed] [Google Scholar]
- 123.Vogler S, Pavich E. Pyelonephritis treatment in the community emergency department: Cephalosporins vs. first-line agents. Am J Emerg Med. 2018;36(11):2054–2057. [DOI] [PubMed] [Google Scholar]
- 124.Blumenthal K, Shenoy E, Huang M, et al. The Impact of Reporting a Prior Penicillin Allergy on the Treatment of Methicillin-Sensitive Staphylococcus aureus Bacteremia. PloS one. 2016;11(7):e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phillips E, Mallal S. Drug hypersensitivity in HIV. Current opinion in allergy and clinical immunology. 2007;7(4):324–330. [DOI] [PubMed] [Google Scholar]
- 126.Petroni D, Aitken M, Ham E, et al. Approach to the evaluation of adverse antibiotic reactions in patients with cystic fibrosis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;117(4):378–381. [DOI] [PubMed] [Google Scholar]
- 127.Khan D, Solensky R. Drug allergy. The Journal of allergy and clinical immunology. 2010;125(2 Suppl 2):S126–137. [DOI] [PubMed] [Google Scholar]
- 128.Castells M A New Era for Drug Desensitizations. The journal of allergy and clinical immunology. In practice. 2015;3(4):639–640. [DOI] [PubMed] [Google Scholar]
- 129.Joint Task Force on Practice Parameters. Drug allergy: an updated practice parameter. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;105(4):259–273. [DOI] [PubMed] [Google Scholar]
- 130.Legendre D, Muzny C, Marshall G, Swiatlo E. Antibiotic hypersensitivity reactions and approaches to desensitization. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(8):1140–1148. [DOI] [PubMed] [Google Scholar]
- 131.Pham M, Ho H, Desai M. Penicillin desensitization: Treatment of syphilis in pregnancy in penicillin-allergic patients. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017;118(5):537–541. [DOI] [PubMed] [Google Scholar]
- 132.Wendel G Jr., Stark B, Jamison R, Molina R, Sullivan T. Penicillin allergy and desensitization in serious infections during pregnancy. The New England journal of medicine. 1985;312(19):1229–1232. [DOI] [PubMed] [Google Scholar]
- 133.Kwong J, Urbancic K, Trubiano J. Beyond penicillin - rapid desensitization for specific flucloxacillin hypersensitivity. Antimicrobial agents and chemotherapy. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sullivan T Antigen-specific desensitization of patients allergic to penicillin. The Journal of allergy and clinical immunology. 1982;69(6):500–508. [DOI] [PubMed] [Google Scholar]
- 135.Trubiano J, Beekmann S, Worth L, et al. Improving Antimicrobial Stewardship by Antibiotic Allergy Delabeling: Evaluation of Knowledge, Attitude, and Practices Throughout the Emerging Infections Network. Open forum infectious diseases. 2016;3(3):ofw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gerace K, Karlin E, McKinnon E, Phillips E. Varying penicillin allergy testing practices in the United States: A time for consensus. The journal of allergy and clinical immunology. In practice. 2015;3(5):791–793. [DOI] [PubMed] [Google Scholar]
- 137.Derrick M, Williams K, Shade L, Phillips E. A survey of drug allergy training opportunities in the United States. The journal of allergy and clinical immunology. In practice. 2018;6(1):302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Labrosse R, Paradis L, Lacombe-Barrios J, et al. Efficacy and Safety of 5-Day Challenge for the Evaluation of Nonsevere Amoxicillin Allergy in Children. The journal of allergy and clinical immunology. In practice. 2018;6(5):1673–1680. [DOI] [PubMed] [Google Scholar]
- 139.Gerace K, Phillips E. Penicillin allergy label persists despite negative testing. The journal of allergy and clinical immunology. In practice. 2015;3(5):815–816. [DOI] [PubMed] [Google Scholar]
- 140.Ratzon R, Reshef A, Efrati O, et al. Impact of an extended challenge on the effectiveness of beta-lactam hypersensitivity investigation. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;116(4):329–333. [DOI] [PubMed] [Google Scholar]
- 141.Mawhirt S, Fonacier L, Calixte R, Davis-Lorton M, Aquino M. Skin testing and drug challenge outcomes in antibiotic-allergic patients with immediate-type hypersensitivity. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017;118(1):73–79. [DOI] [PubMed] [Google Scholar]
- 142.Garcia Rodriguez R, Moreno Lozano L, Extremera Ortega A, Borja Segade J, Galindo Bonilla P, Gomez Torrijos E. Provocation Tests in Nonimmediate Hypersensitivity Reactions to beta-Lactam Antibiotics in Children: Are Extended Challenges Needed? The journal of allergy and clinical immunology. In practice. 2018. [DOI] [PubMed] [Google Scholar]
- 143.Blanca M, Mayorga C, Torres M, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy. 2001;56(9):862–870. [DOI] [PubMed] [Google Scholar]
- 144.Fontaine C, Mayorga C, Bousquet P, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy. 2007;62(1):47–52. [DOI] [PubMed] [Google Scholar]
- 145.Hjortlund J, Mortz C, Stage T, Skov P, Dahl R, Bindslev-Jensen C. Positive serum specific IgE has a short half-life in patients with penicillin allergy and reversal does not always indicate tolerance. Clinical and translational allergy. 2014;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Opstrup M, Poulsen L, Malling H, Jensen B, Garvey L. Dynamics of plasma levels of specific IgE in chlorhexidine allergic patients with and without accidental re-exposure. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2016;46(8):1090–1098. [DOI] [PubMed] [Google Scholar]
- 147.Rive C, Bourke J, Phillips E. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34(1):15–38. [PMC free article] [PubMed] [Google Scholar]
- 148.Macy E, Goldberg B, Poon K. Use of commercial anti-penicillin IgE fluorometric enzyme immunoassays to diagnose penicillin allergy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;105(2):136–141. [DOI] [PubMed] [Google Scholar]
- 149.Macy E, Ngor E. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. The journal of allergy and clinical immunology. In practice. 2013;1(3):258–263. [DOI] [PubMed] [Google Scholar]
- 150.Blanca-Lopez N, Perez-Alzate D, Ruano F, et al. Selective immediate responders to amoxicillin and clavulanic acid tolerate penicillin derivative administration after confirming the diagnosis. Allergy. 2015;70(8):1013–1019. [DOI] [PubMed] [Google Scholar]
- 151.Food and Drug Administration. AUGMENTIN. 2011. Accessed 2 28 2019, 2019.
- 152.Salas M, Laguna J, Dona I, et al. Patients Taking Amoxicillin-Clavulanic Can Become Simultaneously Sensitized to Both Drugs. The journal of allergy and clinical immunology. In practice. 2017;5(3):694–702 e693. [DOI] [PubMed] [Google Scholar]
- 153.Trubiano J, Stone C, Grayson M, et al. The 3 Cs of Antibiotic Allergy-Classification, Cross-Reactivity, and Collaboration. The journal of allergy and clinical immunology. In practice. 2017;5(6):1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: A prospective, multicenter, open label evaluation of a comprehensive penicillin skin test kit. The journal of allergy and clinical immunology. In practice. 2019. [DOI] [PubMed] [Google Scholar]