Abstract
Background
Alcohol can lead to fatal and nonfatal overdose (OD) through its neurobiological inhibitory effects when used alone or with other drugs. Little research has examined alcohol OD characteristics in the context of concomitant drug use.
Methods
This study utilized alcohol OD data (defined as alcohol poisoning, passing out, or blacking out) collected in a large residential addiction treatment facility (N = 660). Latent class analysis identified classes of alcohol OD events based on concomitant drug use at the time of OD. We evaluated correlates of alcohol OD classes, including depression, emergency medical services, and hospitalization, using latent class regression.
Results
Only 20% of alcohol ODs involved alcohol alone. Marijuana was the most commonly used drug during the most recent alcohol OD (43.2%), followed by sedatives (27.9%), cocaine or crack (25.9%), prescription opioids (26.1%), and heroin (20%). The final latent class model included 3 classes: no/low drug involvement (61%), moderate drug involvement (33%), and high drug involvement (6%). Relative to the no/low drug involvement class, participants admitted to the hospital were 6.4‐fold more likely to be in the high drug involvement class (95% CI: 2.4 to 16.6) and 2.9‐fold more likely to be in the moderate drug involvement class (95% CI: 1.2 to 7.2). Participants receiving emergency medical services were more likely to be in the high drug involvement class (aOR: 2.2, 95% CI: 2.2, 1.1 to 4.5) and less likely to be in the moderate drug involvement class (aOR 0.39, 95% CI: 0.2 to 0.96).
Conclusions
Combining drug classes with alcohol prior to OD was common and associated with a higher likelihood of hospitalization. Overdose prevention efforts should address acute risks of alcohol ingestion with other drugs.
Keywords: Alcohol, Drug, Overdose, Depression, Hospitalization
This study analyzed alcohol overdose data (i.e. alcohol poisoning, passing out, or blacking out) collected from a residential treatment setting. Only 20% of alcohol overdoses involved alcohol alone. Latent class analysis revealed three alcohol overdose classes: no/low drug involvement (61%), moderate drug involvement (33%), and high drug involvement (6%). Membership in moderate and high drug involvement classes was associated with an increased likelihood of hospitalization. Overdose prevention efforts should address acute risks of alcohol ingestion with other drugs.

Alcohol is the most commonly used addictive substance in the United States (Center for Behavioral Health Statistics and Quality, 2016) and contributes to more than 88,000 deaths annually (Gonzales et al., 2014). Consuming large amounts of alcohol in a short period of time can lead to fatal or nonfatal alcohol overdose (OD), defined here as alcohol poisoning, blacking out, and/or passing out. Every day, 6 people in the United States die of an alcohol OD (Kanny et al., 2015). Alcohol OD occurs across a spectrum of severity. As blood alcohol content reaches a high enough concentration in the brain, areas that control memory formation shut down, resulting in blackout events (White, 2003). At higher blood alcohol concentrations, basic life‐support functions fail, resulting in loss of consciousness and impaired breathing, heart rate, temperature control, and gag reflex (National Institute on Alcohol Abuse and Alcoholism, 2015). Research indicates alcohol ODs are increasing in the United States (White et al., 2011). For example, emergency department (ED) visits related to acute alcohol consumption increased 51.5% between 2006 and 2014 (White et al., 2018). Likewise, drug ODs related to opioids, sedatives, cocaine, and other substances have also increased sharply over the past decade resulting in a national public health crisis (Scholl et al., 2018; Seth et al., 2018).
Concomitant alcohol and drug use increases OD likelihood and severity (Castle et al., 2016; Day, 2014; Jones et al., 2011; White and Irvine, 1999). Alcohol and other drugs interact through a variety of pathways, including neurobiological effects, pharmacological interactions, and cross‐tolerance (Hickman et al., 2008). Alcohol use also impairs decision making, which could lead to unintended drug use and/or higher quantities of use. Central nervous system depressants, such as alcohol, opioids, and benzodiazepines, are a particularly dangerous combination, due to their additive (and potentially multiplicative) effect on neurobiological inhibition and respiratory depression (White and Irvine, 1999). The interaction of these drugs increases respiratory depression and risk of mortality beyond what would be observed if a single drug class was used alone (Jones et al., 2011; White and Irvine, 1999). In fact, recent increases in ED admission for alcohol‐related adverse drug reactions are largely due to alcohol being consumed with other central nervous system depressants such as opioids, sedatives, and anxiolytics (Castle et al., 2016). Alcohol and stimulant drugs, such as cocaine, have synergistic effects that can potentiate acute adverse effects of both drugs, thereby increasing OD risk (Lange and Hillis, 2001; Santos et al., 2012). Even adverse effects of cannabis are enhanced by alcohol use, though research is limited and the pharmacokinetic interactions are poorly understood (Hartman et al., 2015).
Despite the frequency and lethality of concomitant drug and alcohol use, alcohol is frequently overlooked in OD research and prevention efforts; thus, we sought to describe alcohol OD events in a large residential treatment sample and gain a better understanding of the role of concomitant drug use and its impact on OD outcomes to inform future OD prevention efforts. More specifically, the aims of this study were to characterize “typologies” or classes of alcohol OD based on concomitant drug use and toevaluate whether these classes were differentially associated with adverse OD outcomes such as hospital admission.
Materials and Methods
Study Population and Recruitment Procedures
This study used cross‐sectional data from patients completing screening measures for a randomized controlled trial of a prescription opioid OD prevention intervention (Clinical Trial registration #NCT02152397). Data were collected from 817 patients attending drug and alcohol residential rehabilitation in Michigan, which serves patients predominantly from the Flint, Detroit, and surrounding areas of Michigan. Patients at the residential treatment center were approached between October 2014 and January 2016, asked to provide informed consent, and self‐administered questionnaires. Patients were eligible for screening if they were 18 years of age or older and able to provide informed consent. We excluded 78 participants with incomplete data and 79 participants who had never experienced an alcohol OD, with 660 participants included in analysis. The Michigan Medicine institutional review board approved all study procedures.
Measures
Alcohol OD
The Overdose Experiences, Self and Witnessed—Alcohol (OESWA) was used to assess alcohol OD. This measure was adapted from a previous study (Tracy et al., 2005) and revised to assess alcohol OD. Participants were provided with a description/definition of alcohol OD: “The following questions are about times you drank too much alcohol. This is sometimes called ‘passing out,’ ‘blacking out,’ or ‘alcohol poisoning.’” They then reported their lifetime frequency of these types of events. Participants who reported one or more alcohol OD were asked about their most recent alcohol OD event, which is summarized in this analysis. Drug OD was assessed in a separate, parallel measure. Alcohol and drug OD event characteristics were reviewed to ensure that all alcohol ODs were unique and separate events independent from drug ODs.
For the most recent alcohol OD event, participants reported the type(s) of event they experienced (i.e., “passing out,” “blacking out,” “alcohol poisoning,” or “other”) in a “choose all that apply” format. Participants reported the drugs they took with alcohol prior to their most recent alcohol OD. Drugs were presented in a checklist format. We created binary indicators for 9 drugs reported as concomitantly used with alcohol during the most recently experienced alcohol OD. We formed a single binary indicator including methamphetamines and/or prescription stimulants and excluded inhalants from further analysis due to low frequency of use.
Outcomes of the most recent alcohol OD were assessed using a checklist of options that assessed whether the participant received help from anyone after their last alcohol OD, including whether they or someone else called 911, whether they went to the emergency room (ER), and whether they were admitted to the hospital. We created 2 binary indicators for alcohol OD outcomes—1 for hospitalization (yes/no) and the second for emergency medical involvement (yes/no). Emergency medical involvement was defined as calling 911 (they or another person called) or going to the ER (in or not in an ambulance).
Pain
Participants were asked “Have you been told by a doctor that you have chronic pain?” and “In what area of your body have you felt chronic pain in the last 6 months or longer?”. This question and response options were based on the Michigan Body Map (Brummett et al., 2016). Participants were classified as having chronic pain if they met either 1 of 2 criteria: They had been told they had chronic pain by a doctor, and/or they had chronic pain in one or more body sites in the past 6 months.
Depression
The patient health questionnaire‐9 (PHQ‐9; Kroenke et al., 2001) was administered to assess past 2‐week depressive symptom severity (α = 0.90). Responses to PHQ‐9 items were summed. For the analysis, we formed a binary variable that indicated presence of major depressive disorder (PHQ‐9 score > 9) versus no depression (PHQ‐9 score ≤ 9).
Statistical Analysis
Prior to analysis, all continuous and categorical measures were evaluated for their distributional characteristics. Latent class analysis (LCA) was conducted to identify nonoverlapping alcohol OD subgroups (Lanza et al., 2007) based on the observed covariance patterns of substances used concomitantly with alcohol during the most recent alcohol OD. LCA models were analyzed for 2 to 6 classes. We selected the number of classes by examining fit statistics, class size, and model interpretability (Celeux and Soromenho, 1996; Lanza et al., 2007). The bootstrap likelihood ratio test (BLRT) (Dziak and Lanza, 2016; LCA Bootstrap SAS Macro (version 4.0), 2016; Nylund et al., 2007) was utilized to test whether successively adding 1 more class resulted in a better fitting model. After model selection, item response probabilities were examined in each class to assign labels representing alcohol OD classes and the prevalence of each class was summarized. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and the LCA procedure (Lanza et al., 2015; PROC LCA (version 1.3.2), 2015).
After selecting the number of alcohol OD classes, we described the distribution of the aforementioned covariates with class using participants' most likely class assignment. We then performed latent class regression to identify correlates of experiencing each alcohol OD type (Lanza et al., 2007), which models the alcohol OD classes as latent categorical outcomes using multinomial logistic regression (1 class was set as a referent group). We examined bivariate correlates of class with age (modeled as a continuous variable), self‐identified gender (male or female), race (black or other), comorbidities (self‐reported diagnosis of chronic pain and major depressive disorder at the time of the survey measured by the PHQ‐9), and OD outcomes (calling 911 or going to the ER and being hospitalized). We also assessed whether the associations of class with OD outcomes remained after adjustment for age and sex. In sensitivity analyses, we examined multivariable models additionally adjusted for race.
Results
Descriptive Results
In total, 660 of 739 participants reported one or more lifetime alcohol ODs (89.3%). Participants described their most recent alcohol‐related OD event as blacking out (50%), passing out (54%), and/or alcohol poisoning (11%; Table 1). Nearly three‐quarters (n = 448; 74%) of participants who experienced an alcohol OD were male. Most participants were white (n = 465; n = 74%). The median age was 36 years (interquartile range (IQR): 28 to 46 years). Approximately 20.5% of participants reported only using alcohol during their last alcohol OD (Fig. 1). Marijuana was the most commonly used drug during the most recent alcohol OD, with 43.2% of participants reporting marijuana involvement. Approximately one‐quarter used sedatives (27.9%), cocaine or crack (25.9%), or prescription opioids (26.1%), whereas heroin (20%), stimulants (17.7%), and energy drinks (17.6%) were less common. Other drugs were reported by 10% or fewer of participants.
Table 1.
Descriptive Results
| N, % (total = 660) or mean, SD | |
|---|---|
| Age (mean, SD) | 37.4 (11.0) |
| Male sex | 488 (74%) |
| African American racea | 131 (20%) |
| Evident depression (PHQ‐9 Score > 9)b | 295 (45%) |
| Chronic painc | 532 (81%) |
| Type of alcohol‐related eventd | |
| Passing out | 332 (50%) |
| Blacking out | 353 (54%) |
| Alcohol poisoning | 74 (11%) |
| Other type of event | 38 (6%) |
| Nonresponse | 65 (10%) |
| Overdose outcome | |
| No medical attention | 561 (85%) |
| Emergency medical attention (called 911; went to ER) | 85 (13%) |
| Hospitalization | 46 (7%) |
PHQ, Patient Health Questionnaire.
Referent group is white (n = 465/70%) and all others (n = 64/10%).
PHQ‐9 score mean (standard deviation) = 9.4 (6.8).
Ascertained via the Michigan Body Map.
Participants were asked to “choose all that apply.”
Figure 1.
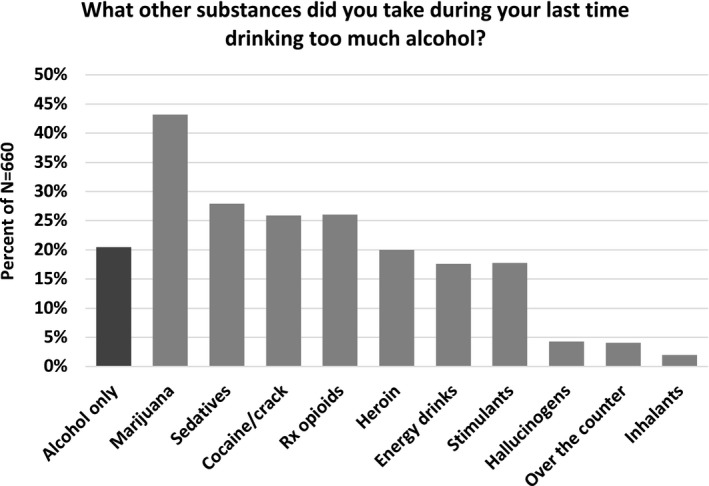
Prevalence of drug use during the most recent alcohol overdose for all drug types assessed.
Most (81%) of the participants reported chronic pain and 45% were depressed (PHQ‐9 score > 9) at the time of the survey. Hospitalization during the most recent alcohol OD was rare (7% of participants), and calling 911 or going to the ER occurred after 13% of alcohol OD events.
Latent Class Analysis
Fit indices identified different optimal numbers of latent classes (Table 2). The Bayesian information criteria (BIC) were optimized with a 3‐class model, whereas BLRT was optimized using a 4‐class model. Entropy and Akaike Information criteria (AIC) were maximized using a 6‐class model. We therefore compared the interpretability of the 3‐ and 4‐class models. The 3‐class model had 1 class with approximately 33% of participants. The 4‐class model split these participants into 2 classes, 1 of which contained only 3% of participants. As this was too small a class to examine in further analysis, we chose the 3‐class model.
Table 2.
Fit Statistics of the Latent Class Analysis to Characterize Drugs During the Most Recent Alcohol Overdose Among 660 Patients in a Residential Rehabilitation Facility
| Number of classes | Log‐likelihood | AIC | BIC | Entropy | Bootstrap likelihood ratio testa |
|---|---|---|---|---|---|
| 2 classes | −2,455.70 | 413.06 | 498.42 | 0.81 | – |
| 3 classes | −2,411.36 | 344.37 | 474.65 | 0.73 | 0.0099 |
| 4 classes | −2,392.09 | 325.83 | 501.03 | 0.79 | 0.0099 |
| 5 classes | −2,387.49 | 336.63 | 556.75 | 0.71 | 1.0 |
| 6 classes | −2,365.75 | 313.15 | 578.19 | 0.82 | – |
AIC, Akaike information criteria; BIC, Bayesian information criteria.
p‐Value corresponds to the bootstrap likelihood ratio test result for the model in the table row relative to the model with one fewer classes.
Bolded values indicate the optimal fitting model based on each statistic.
Most participants (61%) were assigned to a class with no/low drug involvement for their most recent alcohol OD. In this class, the median number of drugs used in addition to alcohol was 1 (IQR = 0 to 1). The most commonly reported substance involved was marijuana (31% of participants; Fig. 2). The next largest class (33% of participants) was a moderate drug involvement class. In this class, the median number of drugs used in addition to alcohol was 3 (IQR = 2 to 4). The most common drugs used with alcohol were marijuana (57%), prescription sedatives (53%), and prescription opioids (47%). Approximately 6% of participants were assigned to a high drug involvement alcohol OD class. In this class, the median number of drugs used in addition to alcohol was 7 (IQR = 6 to 8); the most common drugs used with alcohol were prescription opioids (99%), cocaine (96%), marijuana (92%), and prescription sedatives (91%).
Figure 2.
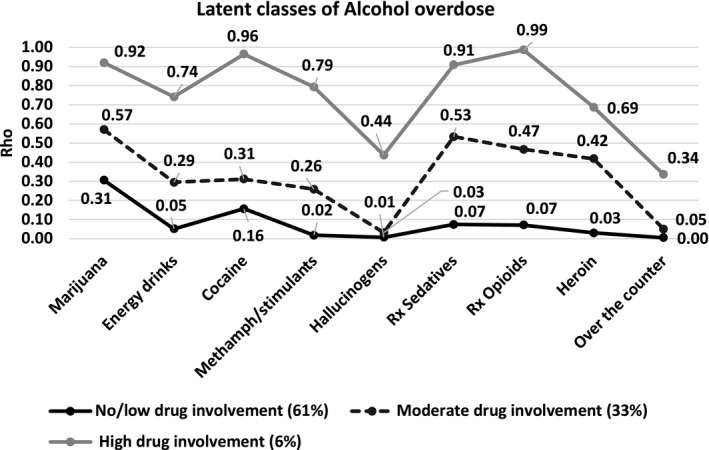
Latent class model of alcohol overdose subtypes among a residential rehabilitation sample: item response probabilities of the 3‐class model.
Bivariate Associations
Participants who were female, younger, nonblack race, and experiencing significant depression during the time of the survey were more likely to be assigned to the moderate or high drug involvement classes than the no/low drug involvement alcohol OD class (Table 3). Participants who were hospitalized after their most recent alcohol OD were more likely to have a high drug involvement OD than a no/low drug involvement during their last alcohol OD. The association between emergency medical involvement and alcohol OD reached our threshold for inclusion in multivariable analysis (p = 0.06).
Table 3.
Bivariate associations of alcohol overdose classes with covariates
| Covariate | No/Low drug involvement class | Moderate drug involvement class | High drug involvement class | |||
|---|---|---|---|---|---|---|
| n (%)a | Odds ratio (95% CI) | n (%) | Odds ratio (95% CI) | n (%) | Odds ratio (95% CI) | |
| Male (vs. female) | 338 (81%) | – | 121 (60%) | 0.41 (0.25, 0.67) | 29 (67%) | 0.58 (0.27, 1.26) |
| Ageb | 42 (34, 49) | – | 27 (24, 33) | 0.87 (0.84, 0.91) | 28 (24, 35) | 0.91 (0.87, 0.95) |
| Black race (vs. white or other race) | 118 (31%) | – | 5 (2%) | 0.07 (0.01, 0.47) | 8 (6%) | 0.35 (0.14, 0.89) |
| Chronic pain (vs. no chronic pain) | 341 (80%) | – | 151 (78%) | 1.02 (0.61, 1.72) | 40 (95%) | 3.85 (0.83, 17.9) |
| Depression (vs. no depression) | 129 (31%) | – | 133 (64%) | 3.37 (2.15, 5.29) | 33 (77%) | 8.41 (3.43, 20.6) |
| ER/911 (vs. no ER visit or 911 call) | 59 (13%) | – | 16 (9%) | 0.65 (0.28, 1.49) | 10 (23%) | 2.28 (0.99, 5.23) |
| Hospitalization (vs. not hospitalized) | 20 (5%) | – | 19 (10%) | 1.95 (0.85, 4.50) | 7 (16%) | 4.02 (1.47, 10.9) |
CI, Confidence Interval; IQR, Interquartile Range.
The number and percent in each cell reflect those within each class of drug involvement that have the given characteristic.
Summarized as median (IQR).
No/Low drug involvement class is reference class.
Multivariable Analysis
Multivariable multinomial logistic regression models were used to assess whether the associations of OD outcomes (emergency medical attention and hospitalization) remained after adjustment for 2 demographic factors that were also strongly associated with class status: age and sex (Table 4). Participants who received emergency medical attention were 2.2‐fold more likely to be in the high drug involvement class (95% CI: 1.1 to 4.5) and were less likely to be in the moderate drug involvement class (aOR: 0.39, 95% CI: 0.16 to 0.96) than those who did not receive emergency services after adjustment. Hospitalized participants were 6.4‐fold more likely (95% CI: 2.4 to 16.6) to be in the high drug involvement class and 2.9‐fold more likely (95% CI: 1.19 to 7.20) to be in the moderate drug involvement class than nonhospitalized participants after adjustment for age and sex.
Table 4.
Results of Multivariable Regression of 3 Factors on Class Membership for Alcohol Overdose Classes Adjusted for Age and Sex
| Variables | Moderate drug involvement class | High drug involvement class |
|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Model 1 | ||
| ER/911 | 0.39 (0.16, 0.96) | 2.24 (1.11, 4.54) |
| Age | 0.87 (0.83, 0.91) | 0.93 (0.89, 0.96) |
| Male | 0.71 (0.40, 1.25) | 0.78 (0.39, 1.56) |
| Model 2 | ||
| Hospitalization | 2.93 (1.19, 7.20) | 6.37 (2.44, 16.6) |
| Age | 0.89 (0.87, 0.92) | 0.90 (0.86, 0.94) |
| Male | 0.63 (0.37, 1.05) | 0.82 (0.38, 1.73) |
CI, confidence interval.
No/Low drug involvement class is the reference class.
We also re‐assessed the association of depression with class after adjustment for age and sex. Participants experiencing depression at the time of the survey were 2.8‐fold more likely (95% CI: 1.8 to 4.3) to be in the moderate drug involvement class and 7.0‐fold more likely (95% CI: 3.2 to 14.9) to be in the high drug involvement class than participants without depression after adjustment for age and sex.
While race was significantly associated with class in bivariate models, models adjusted for race, age, and gender simultaneously did not converge, likely because of the strong and imprecise inverse associations of race with the moderate and high drug involvement classes and because the sample included few African American women (n = 24). These models did converge with the introduction of a strong Bayesian prior (Lanza et al., 2015) for the regression coefficient estimates, and we observed little change in the results for the associations of emergency medical attention, hospitalization, and depression with class (data not shown). Similarly, the estimates remained similar after adjustment for age and race alone (without adjustment for gender), which did not require a stabilizing prior.
Discussion
This is the first study to describe classes of alcohol OD based on concomitant drug use in a residential treatment sample. Nine of 10 patients reported experiencing one or more alcohol ODs in their lifetime. Strikingly, only 20% of self‐reported alcohol ODs resulted from alcohol use alone. We found identifiable patterns in alcohol ODs based on the number/types of drugs combined with alcohol. Our analysis revealed 3 classes: (i) an alcohol OD class with low or no drug involvement, (ii) a moderate drug involvement class, and (iii) a high drug involvement class. The no/low drug involvement class was the largest (61% of the sample) and was characterized by ODs attributed to alcohol use alone or alcohol and marijuana co‐use. The moderate drug involvement class (33% of the sample) included ODs that frequently involved marijuana, prescription opioids, street opioids, and/or sedatives. The high drug involvement class was the smallest class (6% of the sample), and included the highest probabilities of combining alcohol across multiple drug types. The majority of ODs in this class (>90%) involved some combination of marijuana, cocaine, prescription sedatives, and/or prescription opioids.
Membership in the high drug involvement class was strongly associated with increased odds of receiving emergency medical attention and hospital admission. Membership in the moderate drug involvement class was associated with increased likelihood of hospitalization but decreased likelihood of receiving emergency medical attention. Clearly, “alcohol ODs” that involved other drugs, particularly central nervous system depressants, were associated with more severe OD outcomes that required medical attention. These findings are consistent with research indicating that alcohol is involved in 1 in 5 OD deaths from opioid‐based pain relievers and benzodiazepines in the United States (Jones et al., 2014) and research showing that drug involvement increases the likelihood of inpatient admission for acute alcohol‐related ED visits by more than 2‐fold (White et al., 2018). The finding that hospitalized individuals were more likely to be in the moderate drug involvement class, yet individuals who received emergency medical attention were less likely to be in the moderate drug involvement class, is counter‐intuitive. This may reflect measurement error resultant from assessments requiring participants to select all OD outcomes from a list. Though patients could select more than 1 outcome (e.g., went to ED and admitted to hospital), they may have only selected the final or more serious outcome (i.e., hospital admission). In addition, adding covariates to latent class models can influence the formation of classes (Vermunt, 2010). In our analysis, adding emergency medical attention shifted some individuals from the moderate to low drug involvement alcohol OD class, whereas models with hospitalization and depression were less impacted by the addition of covariates. Thus, the change in formation of latent classes may account for some of the discrepant findings we report and should be verified in future work.
Current depression was associated with both moderate drug involvement and high drug involvement in alcohol ODs. The elevated odds of depression in these classes raises the question of whether some ODs in these classes were suicide attempts. In these cases, increased likelihood of hospital admission could also reflect psychiatric hospital admission. We did not assess this variable, but cannot rule out the possibility. Future research on alcohol OD events could extend these findings by assessing the role of suicidal ideation and intent and by using longitudinal data to characterize the bidirectional relationships between depression and alcohol use.
Future research should clarify how individuals classify and understand their own alcohol ODs. All participants in this study reported alcohol ODs and drug ODs separately in the larger assessment battery. All alcohol and drug OD events were cross‐checked to ensure they did not represent the same event. Thus, participants classified many OD events as alcohol ODs even though they involved other drug use. At this time, it is unclear what would lead a person to classify a polysubstance OD as an “alcohol OD” relative to a “drug OD.” Future research could evaluate whether this is related to the quantity of the drugs or alcohol consumed, the relative timing of drug ingestion, or which drug the individual uses more frequently. Likewise, when drug ODs are studied, asking about alcohol use and alcohol OD is recommended, as our results suggest people may view alcohol and drug ODs in different categories. For the purposes of this analysis, we assessed the most recent alcohol OD. Other studies may broaden this timeframe to capture several OD events per individual to characterize the full spectrum of alcohol ODs experienced by an individual.
Limitations and Future Directions
Limitations of this study include the use of a cross‐sectional sample recruited from a single treatment facility. A large multisite or nationally representative sample is needed to establish the larger epidemiological trends in alcohol ODs and outcomes. The sample was predominantly white race, limiting our analysis of the impact of race/ethnicity on outcomes. We used a novel measure of alcohol OD as there is no validated assessment available in the literature. Depression and chronic pain were assessed at the time of study enrollment. Therefore, these comorbidities should be interpreted with caution and, at most, as correlates that may serve as proxies for depression and chronic pain at the time of the OD event. We did not have data on healthcare coverage and thus were unable to adjust for the impact this could have had on access to prescription medications and OD outcomes.
When studying alcohol OD, a broad definition such as the one used in this study may be useful for assessing ODs across the range of severity. In our study, we defined alcohol OD to include “blacking out” and “passing out.” This is a rather inclusive definition of an “OD,” yet still revealed a number of high risk events, some serious enough to result in hospital admission. Careful definition, whether asking about alcohol or drug OD, is important. We recommend clinicians and researchers consider asking about times individuals “passed out” or “blacked out” due to drug or alcohol use, not just times they “nodded out” or “lost consciousness.” Without thorough inquiry, it is possible OD events of varying levels of severity will be overlooked.
This study suggests that ongoing OD prevention efforts and surveillance activities should focus not just on opioid and drug use, but incorporate alcohol use as well. Clinicians could provide messages about the role of alcohol use in OD when distributing naloxone or dispensing opioid‐based pain relievers and sedatives. Likewise, outpatient addiction clinics and residential treatment facilities are in an ideal position to provide clinical interventions and messaging about alcohol's contribution to OD risk as well. Such efforts, and others, could hopefully curtail the national rise in alcohol‐related ODs observed nationally (Castle et al., 2016; White et al., 2018), thereby saving lives and preventing costly emergency department visits and hospital admissions.
Role of Funding Source
Data collection was funded by National Institute of Drug Abuse (R34DA035331). This work is, however, the sole responsibility of the authors and does not reflect the positions of the National Institutes of Health. The funding agency did not have direct involvement in the study design; collection, analysis, or interpretation of data; writing of the paper; or in the decision to submit this paper for publication. During her work on this project, Dr. Fernandez was supported by a K23 award from NIAAA (K23AA023869), and Dr. Gicquelais was supported by National Institute of Allergy and Infectious Diseases HIV Epidemiology and Prevention Sciences Training Program at Johns Hopkins Bloomberg School of Public Health (2T32AI102623‐06).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors wish to thank the staff and clients at the addiction treatment facility that hosted our research project. We also wish to thank Laura Thomas and Emily Yeagley for their oversight of the data collection.
References
- Brummett CM, Bakshi RR, Goesling J, Leung D, Moser SE, Zollars JW, Williams DA, Clauw DJ, Hassett AL (2016) Preliminary validation of the Michigan Body Map. Pain 157:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle I‐JP, Dong C, Haughwout SP, White AM (2016) Emergency department visits for adverse drug reactions involving alcohol: United States, 2005 to 2011. Alcohol Clin Exp Res 40:1913–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeux G, Soromenho G (1996) An entropy criterion for assessing the number of clusters in a mixture model. J Classif 13:195–212. [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2016) Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health, HHS Publication No. SMA 16‐4984.
- Day C (2014) Benzodiazepines in Combination with Opioid Pain Relievers or Alcohol: Greater Risk of More Serious ED Visit Outcomes Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed] [Google Scholar]
- Dziak JJ, Lanza ST (2016) LCABootstrap SAS macro users’ guide (version 4.0). Available from: http://methodology.psu.edu. Accessed November 27, 2018.
- Gonzales K, Roeber J, Kanny D, Tran A, Saiki C, Johnson H, Yeoman K, Safranek T, Creppage K, Lepp A, Miller T, Tarkhashvili N, Lynch KE, Watson JR, Henderson D, Christenson M, Geiger SD, Centers for Disease Control and Prevention (CDC) (2014) Alcohol‐attributable deaths and years of potential life lost—11 States, 2006–2010. MMWR Morb Mortal Wkly 63:213–216. [PMC free article] [PubMed] [Google Scholar]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, Huestis MA (2015) Controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clin Chem 61:850–869. [DOI] [PubMed] [Google Scholar]
- Hickman M, Lingford‐Hughes A, Bailey C, Macleod J, Nutt D, Henderson G (2008) Does alcohol increase the risk of overdose death: the need for a translational approach. Addiction 103:1060–1062. [DOI] [PubMed] [Google Scholar]
- Jones AW, Kugelberg FC, Holmgren A, Ahlner J (2011) Drug poisoning deaths in Sweden show a predominance of ethanol in mono‐intoxications, adverse drug–alcohol interactions and poly‐drug use. Forensic Sci Int 206:43–51. [DOI] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA (2014) Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse‐related emergency department visits and drug‐related deaths ‐ United States, 2010. MMWR Morb Mortal Wkly Rep 63:881–885. [PMC free article] [PubMed] [Google Scholar]
- Kanny D, Brewer RD, Mesnick JB, Paulozzi LJ, Naimi TS, Lu H (2015) Vital signs: alcohol poisoning deaths — United States, 2010–2012. Morb Mortal Wkly Rep 65:1238–1242. [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB (2001) The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange RA, Hillis LD (2001) Cardiovascular complications of cocaine use. N Engl J Med 345:351–358. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Collins LM, Lemmon DR, Schafer JL (2007) PROC LCA: a SAS procedure for latent class analysis. Struct Equ Model 14:671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Dziak JJ, Huang L, Wagner A, Collins LM (2015) PROC LCA & PROC LTA users’ guide (Version 1.3.2). Available from: http://methodology.psu.edu
- LCA Bootstrap SAS Macro (version 4.0), 2016. Software.
- National Institute on Alcohol Abuse and Alcoholism (2015) Alcohol overdose: the dangers of drinking too much. https://www.niaaa.nih.gov/sites/default/files/publications/overdoseFact.pdf
- Nylund KL, Asparouhov T, Muthen BO (2007) Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model 14:535–569. [Google Scholar]
- PROC LCA & PROC LTA (version 1.3.2), 2015. Software. https://www.methodology.psu.edu/downloads/proclcalta/
- Santos S, Brugal MT, Barrio G, Castellano Y, Domingo‐Salvany A, Espelt A, Bravo MJ, De La Fuente L (2012) Assessing the effect of patterns of cocaine and alcohol use on the risk of adverse acute cocaine intoxication. Drug Alcohol Rev 31:439–446. [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G (2018) Drug and opioid‐involved overdose deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep 67:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S (2018) Overdose deaths involving opioids, cocaine, and psychostimulants — United States, 2015–2016. Morb Mortal Wkly Rep 67:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy M, Piper TM, Ompad D, Bucciarelli A, Coffin PO, Vlahov D, Galea S (2005) Circumstances of witnessed drug overdose in New York City: implications for intervention. Drug Alcohol Depend 79:181–190. [DOI] [PubMed] [Google Scholar]
- Vermunt JK (2010) Latent class modeling with covariates: two improved three‐step approaches. Polit Anal 18:450–469. [Google Scholar]
- White AM (2003) What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health 27:186–196. [PMC free article] [PubMed] [Google Scholar]
- White AM, Hingson RW, Pan I‐J, Yi H‐Y (2011) Hospitalizations for alcohol and drug overdoses in young adults ages 18–24 in the United States, 1999–2008: results from the nationwide inpatient sample. J Stud Alcohol Drugs 72:774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972. [PubMed] [Google Scholar]
- White AM, Slater ME, Ng G, Hingson R, Breslow R (2018) Trends in alcohol‐related emergency department visits in the United States: results from the nationwide emergency department sample, 2006 to 2014. Alcohol Clin Exp Res 42:352–359. [DOI] [PubMed] [Google Scholar]


