Abstract
Background:
Alcohol consumption despite aversive consequences is often a key component of an alcoholism diagnosis. Free-choice alcohol consumption despite bitter quinine adulteration in rodents has been seen following several months of free-choice drinking, but there has been little study of whether prolonged access to other palatable substances like saccharin yields quinine resistance. Selectively bred crossed High Alcohol Preferring (cHAP) mice average blood alcohol levels of over 250 mg/dl during free-choice access, considerably higher than other models. We hypothesized that higher intakes would yield more rapid development of quinine-resistant alcohol (QRA) drinking and quinine-resistant saccharin (QRS) drinking.
Methods:
All experiments used male and female cHAP mice. Experiment 1 compared mice with either 0 or 5 weeks alcohol drinking history, testing varying (0.032, 0.10, 0.32 g/L) quinine concentrations in ethanol. Experiment 2 examined whether innate QR may exist, comparing animals with a one or zero day drinking history. Experiment 3 examined the effect of varying histories (0, 2, or 5 weeks) of free-choice 10% alcohol drinking on QR alcohol consumption at high quinine concentrations. Finally, Experiment 4 investigated the development of QRS drinking.
Results:
We found that we could not detect a history effect in commonly used quinine concentrations, indicating that cHAP mice are innately quinine resistant to 0.10 g/L quinine. However, we were able to determine that a two-week drinking history was sufficient to induce QRA drinking in cHAP mice at extremely high quinine concentrations (0.74 and 0.32 g/L). However, the history effect was specific to QRA a saccharin drinking history did not yield QRS drinking.
Discussion:
These data suggest that an alcohol drinking history induces maladaptive behaviors, such as drinking in spite of negative consequences, a pattern not seen with saccharin. Furthermore, a strong genetic predisposition to drink may promote an innate aversion resistance compared to commonly used inbred strains.
Keywords: alcohol, quinine resistance, selectively bred
Introduction
Alcohol consumption in spite of negative consequences constitutes a major portion of the core symptoms of alcohol use disorder (American Psychiatric Association, 2013), as shown by a compulsion to drink even in the face of adverse legal, health, or social consequences. In some individuals alcohol consumption may become inflexible following a history of drinking; whereas other individuals may be innately susceptible to problematic drinking. To begin modeling this behavior in animals, researchers have used a variety of acquired (O’Tousa and Grahame 2016) or innate (Hopf et al. 2010) aversive flavors added directly to alcohol solutions to assess whether these might moderate drinking. Adding quinine, an innately aversive stimulus, to the alcohol solution, assesses the degree to which alcohol intake remains under aversive control. As such, quinine-resistant (QR) drinking has been shown to be a promising animal model of consumption in spite of negative consequences with strong face and construct validity (Hopf and Lesscher 2014).
Quinine resistant drinking has thus far required an alcohol history in order to develop. Previous studies have shown that long-term (2.5 – 4 months), intermittent ethanol (EtOH) drinking in rats (Fachin-Scheit et al. 2006; Hopf et al. 2010; Randall et al. 2017; Seif et al. 2013) was required for QR drinking. Notably, with BACs of < 50 mg/dl, these animals did not achieve the NIAAA definition of an alcohol binge (80 mg/dl) (Seif et al. 2013). In contrast, Lei et al. (2016) found that just 24-hour access to EtOH was sufficient to develop QR drinking in C57BL/6J mice. During two bottle choice access to alcohol, C57BL/6J mice also typically keep their blood alcohol levels (BALs) relatively low, about 60 mg/dl (Matson and Grahame 2013). Lesscher et al. (2010) used a scheduled access procedure in C57BL/6J mice, resulting in somewhat higher BALs (around 100 mg/dl), and obtained QR drinking in two weeks of daily 2-h drinking sessions. Although obtaining high BALs is not necessary for an alcoholism diagnosis, some of the core symptomology, including dependence and tolerance, implies exposure to high BALs over long periods of time. Mello and Mendelson (1970) reported BALs between 200 and 300 mg/dl in hospitalized human alcoholics permitted to drink freely. This raises the question of whether animal models achieving these BALs might be useful in modeling compulsive-like QR drinking, considering the high BALs frequently observed in alcoholics. To the extent that QR drinking is a symptom of disordered drinking, we hypothesize that higher BALs should either increase the intensity of QR drinking or facilitate the speed of its development.
Our lab has developed various lines of high alcohol preferring animals through selective breeding. The highest drinking line, the crossed High Alcohol Preferring (cHAP) mouse, was developed from a cross between the first and second High Alcohol Preferring lines (HAP1 and HAP2), which were selectively bred from an outbred progenitor (Oberlin et al. 2011). These animals reach average BALs of over 250 mg/dl during free-choice access (Matson and Grahame 2013), coming into contact with the BALs reported in humans by Mello and Mendelson. A previous study found that these mice become resistant to conditioned aversive flavors mixed with EtOH following five weeks of EtOH consumption (O’Tousa and Grahame 2016). Therefore, we hypothesized that they would develop a similar behavior, QR drinking, perhaps in less than 5 weeks.
Because of their high drinking phenotype, we considered that cHAP mice may require a different concentration of quinine than has been used in other experiments in rats and other mouse strains. Quinine concentrations used in previous studies reporting quinine-resistant drinking ranged from as low as 0.005 g/L in Swiss mice (Fachin-Sheit et al., 2006) to 0.18 g/L in C57Bl/6J mice (Lesscher et al., 2010). In addition, because cHAP mice do not require a saccharin or sucrose fade in order to readily drink 10% EtOH, we considered that they may have a high baseline avidity for aversive flavors. We therefore hypothesized that the high-drinking cHAP line would require a stronger quinine concentration to demonstrate aversion-resistant drinking.
Beyond alcohol, only one study used a different conventional reinforcer, sucrose, to determine if a history of intake would lead to QR drinking. Dess (2000) found that three days of access to 2% sucrose was not sufficient. However, the ability of a more extended history of a reinforcer other than alcohol to engender QR drinking has not been previously investigated.
The goal of these studies was to better understand the development of QR drinking in mice that self-administer high doses of alcohol and expand the current literature to determine if QR drinking of appetitive tastants might be an endophenotype of alcohol use disorders. To accomplish this, we performed a quinine concentration-response study to determine an appropriate quinine concentration that would allow us to manipulate QRA with an alcohol drinking history. Based on these findings, we ran a single day study to determine if one day of alcohol drinking was sufficient to induce QRA or if cHAPs are innately resistant to quinine. Justified by the innate QR drinking seen at 0.10 g/L quinine and the lack of a drinking history effect at 0.32 g/L, we used an extremely high concentration of quinine, 0.74 g/L, in an attempt to explore whether a drinking history might allow consumption of alcohol even under extremely aversive conditions. Finally, we wanted to determine if these mice developed QR to saccharin as well, and tested this hypothesis at 0.32 g/L. Our findings demonstrate that our high-drinking cHAP line requires a higher concentration of quinine to test compulsive-like alcohol intake and that negative contrast can mediate an alcohol history. These findings did not generalize to QRS.
Materials and methods
Subjects
See Table 1 for a list of experiments and subjects. Mice within each experiment were assigned to groups counterbalanced for litter of origin and sex. Experiment 1 (concentration-response) assessed effects of 0 or 5 weeks of alcohol access (0W and 5W) on subsequent intake of quinine-adulterated solutions at varying concentrations. Experiment 2 (innate QR) used either 1 day or 0 days of alcohol access (1D and 0D) to measure quinine resistant alcohol intake. Mice in Experiment 3 (high quinine) had either a 0, 2, or 5 week alcohol history (0W, 2W, and 5W) before a QR test at high concentrations of quinine (0.74 and 0.32 g/L). Finally, Experiment 4 investigated whether quinine-resistant saccharin drinking (QRS) develops following 5 weeks of drinking 0.1% saccharin, with testing at the high 0.32 g/L quinine concentration.
Table 1:
Definition and subjects for each experiment
| Experiment | History | Quinine Concentration | n (# of males) |
|---|---|---|---|
| 1 | 0 or 5 weeks | 0.032, 0.10, 0.32 g/L | 35 (18) |
| 2 | 0 or 1 day | 0.10 g/L | 24 (12) |
| 3 | 0, 2, or 5 weeks | 0.74, 0.32 g/L | 36 (18) |
| 4 | 0, 2, or 5 weeks | 0.32 g/L | 36 (18) |
All animals were single-housed in standard Plexiglas cages with pine bedding and moved to the housing room at least 7 days prior to the first day of alcohol access, under a 12-hour reverse light cycle (lights off at 7 am) and had access to standard chow ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of IUPUI and conducted according to the NIH Guide for the Care and Use of Laboratory Animals.
Procedure – EtOH Drinking
Timelines for each individual experiment can be found above each graph. The two-bottle choice (2BC) procedure consisted of 24-hour access to a 25-mL bottle of tap water and a 50-mL bottle of either 10% EtOH (v/v) (Experiments 1 – 3) or 0.1% saccharin (w/v) (Experiment 4). Intake was measured three days a week for alcohol experiments and contents of the tubes were discarded and refilled once a week to maintain clean solutions. The side of the water bottle was alternated from left to right at each intake measurement.
Baseline Intake Testing
A baseline drinking day (BL1) was performed to establish the animals’ normal diurnal drinking pattern upon the cessation of the assigned drinking history (i.e., 0, 2, or 5 weeks). The baseline drinking day was the first alcohol exposure for 0W animals. The 2BC procedure continued, but the solutions were placed in 10-mL tubes for greater accuracy. Tubes were read every two hours during the dark cycle and once at the end of the light cycle, resulting in seven total readings (9 am, 11 am, 1 pm, 3 pm, 5 pm, 7 pm, and 7 am the following morning). Experiment 2 did not utilize a baseline day for the 0D animals, ensuring they were completely alcohol naïve before the QR test.
Quinine Adulteration Tests
Quinine adulteration began the following morning at 7 am and bihourly readings were conducted in an identical fashion to the baseline day. Quinine concentrations varied for each experiment and can be found in Table 1. In Experiment 1, mice had a series of three quinine test days of varying concentrations (0.032, 0.10, 0.32 g/L) in a Latin square design, separated by additional baseline days prior to each quinine test. Based on these results, Experiment 2 mice had access to water and 0.10 g/L quinine in 10% EtOH for a single 24-hour quinine test. In Experiment 3, the 10% EtOH bottle was replaced with 10% EtOH + 0.74 g/L quinine (QA1). Following the 24-hour quinine adulteration test, a second baseline day (BL2) was conducted. The quinine-adulterated EtOH tube was replaced with a 10% unadulterated EtOH tube and bihourly readings were performed. After the second baseline day, the mice had a second quinine test (QA2) with 0.32-g/L quinine added to the 10% EtOH tube. Experiment 4 (QRS) had one quinine drinking test (QA), using only the 0.32 g/L concentration, following the baseline drinking day (BL). Intake volumes were measured bihourly, identical to the baseline day.
Immediately following the quinine drinking test in Experiment 2, we conducted an indifference test to determine if the mice could still detect and reject quinine, or whether the innate QR found was instead due to quinine taste insensitivity. Mice had access to two sipper tubes, one containing 10% EtOH and the other 10% EtOH + 0.10 g/L quinine. Total intake was measured over a 12-hour period.
Solutions
In Experiments 1 & 3, mice underwent a 2BC procedure for 2 or 5 weeks with access to tap water and 10% ethanol (EtOH). In Experiment 4, mice were tested using 2BC access to 0.1% saccharin for 0, 2, or 5 weeks.
The quinine-adulterated solutions used on the test days were made by adding quinine hydrochloride (MP Biomedicals, LLC, Solon, OH) to 10% EtOH at concentrations ranging from 0.032 – 0.74 g/L, or by adding quinine to 0.1% saccharin at the 0.32 g/L concentration.
Statistical Analysis
Data were analyzed using SPSS software (SPSS, Version 22, Chicago, IL) and graphed using Prism software (Graphpad Prism, v. 6.0, La Jolla, CA). Significance was set at p < 0.05. The final day of intake history was analyzed using independent sample t-test, with history length as the factor (Experiments 3 and 4 only). Within each experiment, baseline and quinine test day data were first separately analyzed with a Sex X History (0, 2 or 5 weeks; 0 or 1 days) ANOVA. Barring interactions, we collapsed across sex to conduct a Time (9 am – 7 pm bi-hourly readings) X History (0, 2, or 5 weeks; 0 or 1 days) factorial ANOVA to assess different drinking trajectories across each test day. In the absence of a Time X History interaction, the data were collapsed across Time, and total intake on each test day (baseline vs quinine) was compared using a factorial ANOVA in order to look for different patterns of history effects on drinking during baseline vs quinine drinking tests. If there was a Time X History interaction, follow-up analyses were conducted at each time point to when History caused differences in drinking. For the inflexibility/indifference test, total EtOH intake and preference were compared by History (0 or 1 day) using independent samples t-test.
Results
Average daily EtOH or saccharin intake during the drinking histories for Experiments 1, 3 and 4 are documented in Table 2.
Table 2:
Average Consumption During History
| Experiment | Group | Average Daily Consumption (SEM) | Average Daily Preference (SEM) |
|---|---|---|---|
| 1 – Concentration-Response | 5W | 24.30 (0.674) g/kg/day | 0.88 (0.017) |
| 3 – High Quinine | 5W | 23.51 (0.940) g/kg/day | 0.94 (0.017) |
| 2W | 20.35 (0.780) g/kg/day | 0.89 (0.016) | |
| 4 – Saccharin | 5W | 641.31 (32.049) mL/kg/day | 0.95 (0.009) |
| 2W | 764.03 (28.959) mL/kg/day | 0.96 (0.007) |
5W = 5 week drinking history, 2W = 2 week drinking history
Experiment 1 – Quinine Concentration-Response
During the 5W drinking history, EtOH preference was greater than 50% (t’s > 11, p’s < 0.001; Table 2).
Baseline Drinking Tests
Although three “baseline” drinking tests were conducted (one before each of the quinine test days), we used BL1 as a comparison for all three QA tests because it was the first day of alcohol access for the 0W animals. This decision was supported by an absence of an interaction of BL day with either Time or History across the three days (Fs < 1.3, ps > 0.22).
Over the course of the 12-h BL1 test (data not shown), there was a main effect of Time (F(5,150) = 6.437, p < 0.001), as demonstrated by higher drinking early in the dark cycle, as well as a main effect of History (F(1,30) = 23.852, p < 0.001). Since Time did not interact with History (Fs < 2, ps > 0.9), we collapsed across time to confirm an effect of Drinking History on total daily alcohol intake (t(33) = 5.218, p < 0.001). Mice with a 5-week drinking history drank more unadulterated EtOH than the alcohol naïve mice.
Quinine Adulteration Tests
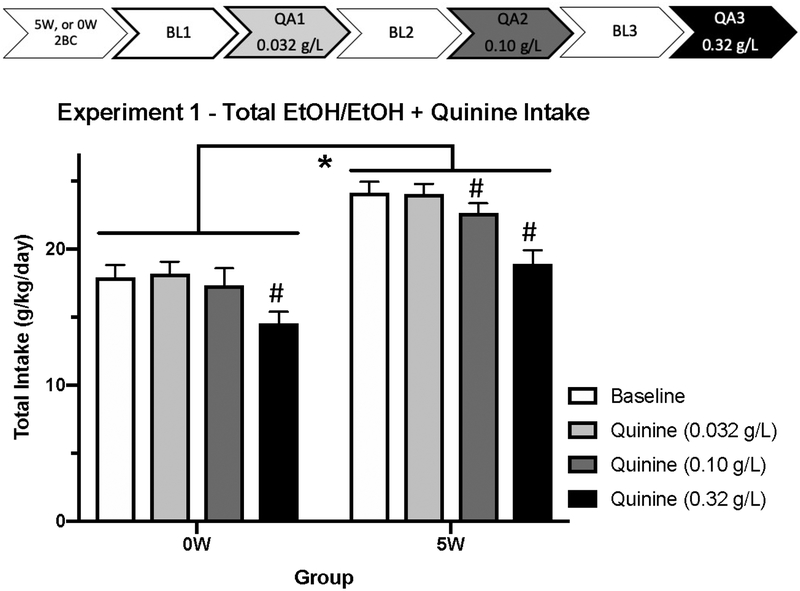
We utilized a Latin square design to test the three concentrations of quinine: low (0.032 g/L), medium (0.10 g/L), and high (0.32 g/L). Order of concentrations did not interact with Time or Group (Fs < 1.04, ps > 0.40) so data from all tests were collapsed across Order. At 0.032 g/L quinine in EtOH, there was no interaction between Time and History or Sex throughout the day (Fs < 0.72, ps > 0.61), therefore we examined total intake across the entire day (see Figure 1). There was an effect of History, with 5W mice drinking more than 0W (t(33) = 6.164, p < 0.001), echoing the effect of drinking history observed during BL1. Comparing total intake between the BL and the lowest (0.032 g/L) quinine concentration, we found quinine did not attenuate drinking in either History group (ts < .29, ps > 0.77, indicating that both groups were resistant to quinine’s aversive effects at this low concentration. For the 0.10 g/L (medium quinine) test day, we again found that Time did not interact with History or Sex (Fs < 0.72, ps > 0.61), so further analyses were performed on total daily intake. 5W mice had a higher total intake than the 0W group, t(33) = 3.829, p < 0.002). Interestingly, when compared to their intake on the BL1 day, 0W mice did not appear to reduce intake (t(16) = 0.695, p = 0.497), but the 5W animals drank less (t(17) = 2.745, p = 0.014, indicating that only the 0W, but not the 5W, mice were quinine resistant at this concentration. At the highest quinine concentration, 0.32 g/L, we again collapsed across Time as the drinking trajectory did not change as a function of Sex or History (Fs < 0.72, ps > 0.61). Throughout the entire High QA test, mice with a drinking history consumed more EtOH + quinine than naïve animals, t(33) = 3.340, p < 0.003). Both History groups reduced drinking significantly from the BL1 test day (ts > 4.88, ps < 0.001), suggesting that animals were not resistant to quinine at this concentration, even following 5 weeks of drinking.
Figure 1:
Experiment 1 alcohol intake (g/kg) during Baseline (10% 2BC) and Quinine-Adulteration tests (0.032 – 0.32 g/L quinine). Overall, animals with 5 weeks of drinking experience (5W) drank more than animals with one day of experience (0W; * indicates p < 0.05 for 5W compared to 0W, ns = 17 – 18 per history group), but groups did not differ in sensitivity to quinine at these concentrations. # indicates that quinine suppressed alcohol intake relative to the quinine-free baseline test, p < 0.05
Experiment 2 – Innate QR
Baseline Drinking Test
For the 1D animals’ first exposure to EtOH, baseline day alcohol drinking (BL) demonstrated a similar trajectory of drinking across the day as mice in Experiments 1 & 3, as demonstrated by a trend toward a main effect of Time (F(5,55) = 2.206, p = 0.067).
Quinine Adulteration Test
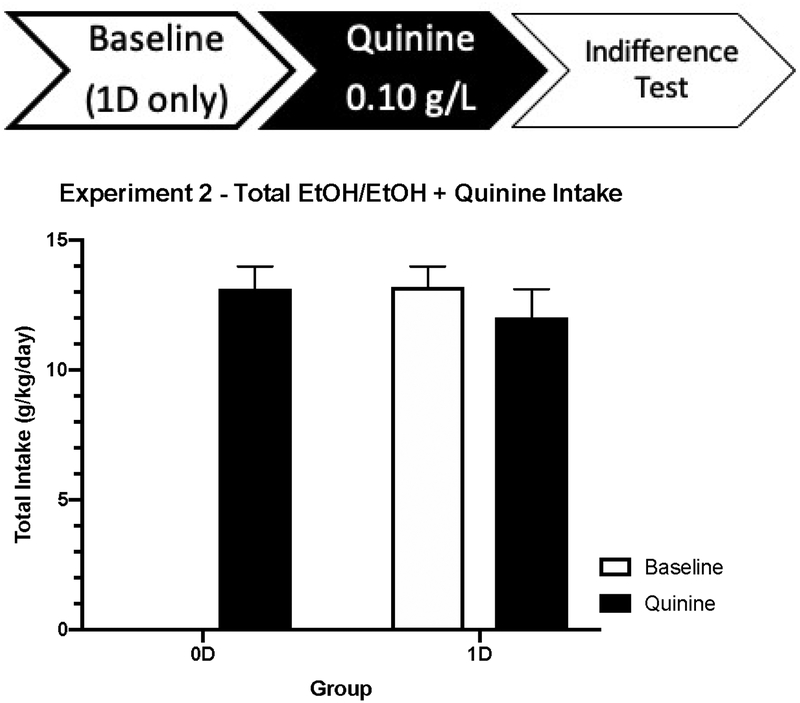
On the quinine adulteration test (QA), Drinking History did not interact with either Time or Sex (p’s > 0.18), thus we analyzed total intake for each day (Figure 2). The overall effect of Drinking History on EtOH + quinine intake was not significant, t(22) = 0.796, p = 0.434, suggesting that one day of alcohol history did not impact EtOH + quinine intake. When comparing the 1D BL test day to the 0D QR test, consumption of the EtOH + 0.10 g/L quinine was not reduced, (t(11) = 1.029, p = 0.326) indicating quinine resistance.
Figure 2:
Experiment 2 alcohol intake (g/kg) during Baseline (10% 2BC) and 0.10 g/L Quinine Adulteration tests. Baseline day and quinine day consumption are shown for Group 1D (n = 12), while only the quinine test is shown for group 0D (n = 12), as this was their first alcohol drinking day. Intake did not differ between groups, showing that cHAP mice do not require any alcohol drinking experience to demonstrate quinine-resistant drinking.
Indifference Test
The final test was the indifference test, pitting bitter ethanol against unadulterated ethanol. One possible explanation of high quinine-alcohol acceptance would be an inability to taste quinine, rather than high motivation to drink alcohol. However, mice still preferred plain 10% EtOH over the quinine-adulterated EtOH, as demonstrated by a preference of 0.69, greater than 0.50 (t[23] = 3.039, p < 0.01; Table 3).
Table 3:
Preference for Unadulterated EtOH During Inflexibility/Indifference Test (Experiment 2)
| Group | Preference for EtOH |
|---|---|
| Total | 0.69 (0.062) |
| 0D | 0.68 (0.097) |
| 1D | 0.70 (0.080) |
0D = 0 day drinking history, 1D = 1 day drinking history
Experiment 3 – High Quinine
In Experiment 1A, one female mouse from the 2W group was sacrificed during the study due to extreme weight loss and was therefore removed from all analyses.
Baseline Drinking Test 1
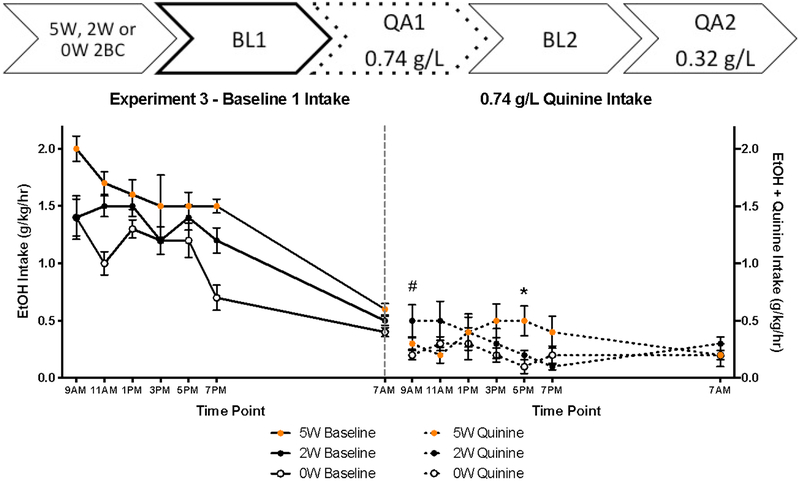
Alcohol consumption escalated over the two-bottle choice history. As a result, when averaged over their entire drinking histories, intake was higher in 5W than 2W groups (t[22] = 2.59, p = 0.017, Table 2). During Baseline Day 1 (BL1), intake was affected by Drinking History, F(2, 34) = 17.56, p < 0.001, with post hoc analyses indicating that the 5W drank more than 2W and 0W animals, as well as 2W animals drinking more than the 0W mice (p’s < 0.05) (Figure 3). Because alcohol intake did not interact with sex, we collapsed across sex for subsequent analyses. As expected, mice drank ethanol at the highest rate early in the day, as shown by a main effect of time, F(5, 140) = 4.608, p = 0.001, but no interaction with drinking history was seen.
Figure 3:
Experiment 3 bi-hourly alcohol intake during Baseline and 0.74 g/L Quinine Adulteration tests after 0, 2, or 5 weeks of alcohol drinking history. The addition of quinine strongly attenuated drinking in all three history groups, but mice with a drinking history showed somewhat higher intake at certain time points (*5W different from 0W; #2W trending different from 0W at p = 0.051; n = 11 – 12/dot).
Quinine Adulteration Test 1
QA1 was conducted the day after BL1 using 0.74 g/L of quinine (Figure 3, right panel). This was apparently extremely aversive, because regardless of drinking history, mice did not exceed the critical alcohol consumption rate of about 1 g/kg per hour, which roughly indicates the rate of metabolism and which would therefore lead to intoxication (Matson et al, 2013). Nonetheless, there was a Time X Drinking History interaction, F(5.912, 85.725) = 2.35, p = 0.039, which suggests that quinine suppressed alcohol intake differently over the course of the day depending on how long mice had been drinking. We therefore assessed Drinking History effects at each time point. 5W mice drank more than 0W at 5 pm only (t[15.345] = 2.385, p = 0.030) and 2W mice showed a strong trend toward increased intake compared to 0W mice at the 9 am reading (t(21) = 2.072, p = 0.051).
Baseline Drinking Test 2
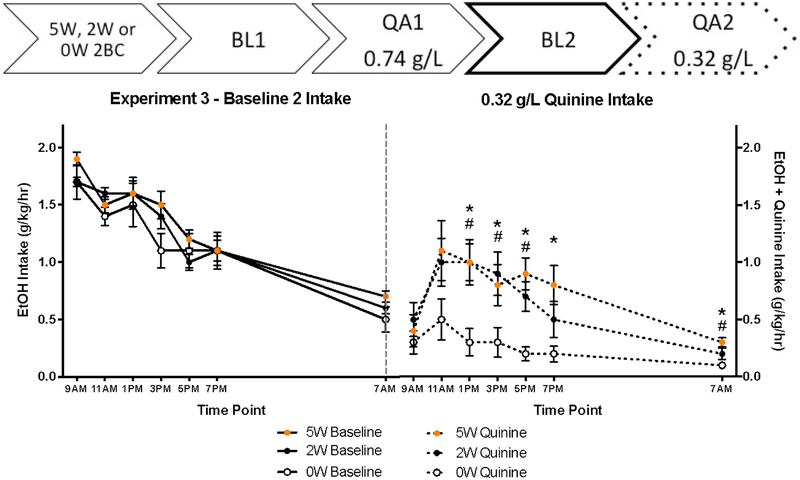
Because consumption was so low, a floor effect may have obscured the actual magnitude of quinine resistance. Subsequently we opted to use a lower concentration of quinine (0.32 g/L) during an additional test (QA2) after a second, quinine-free alcohol baseline day (BL2). On BL2, mice in the 3 drinking history groups drank similar amounts (Figure 4, left panel). Drinking History no longer affected total alcohol consumption, p = 0.061, and there was no Time X History interaction, p = 0.821.
Figure 4:
Experiment 3 bi-hourly alcohol intake during Baseline and 0.32 g/L Quinine Adulteration tests. While quinine adulteration reduced drinking in all animals compared to the baseline day, both 2W and 5W mice showed a reduced sensitivity to quinine, indicating that a 2 week EtOH drinking history was sufficient to induce quinine resistance. (*5W different from 0W; # 2W different from 0W; n = 11 – 12/dot).
Quinine Adulteration Test 2
On the QA2 test when 0.32 g/L quinine was added to the 10% EtOH, we found profound quinine-resistant drinking in both long-term drinking groups (2W and 5W) relative to 0W mice. QR drinking was supported by a Time X Drinking History interaction (F[7.113, 99.589] = 2.380, p = 0.026, Figure 4, right panel), and additional analyses showed that both the 5W and 2W history groups consumed more quinine-adulterated EtOH than the 0W group at 1 pm, 3 pm, 5 pm, and 7 am, however, only the 5W differed from the 0W (t’s > 2.31, p’s < 0.03) at the 7 pm reading.
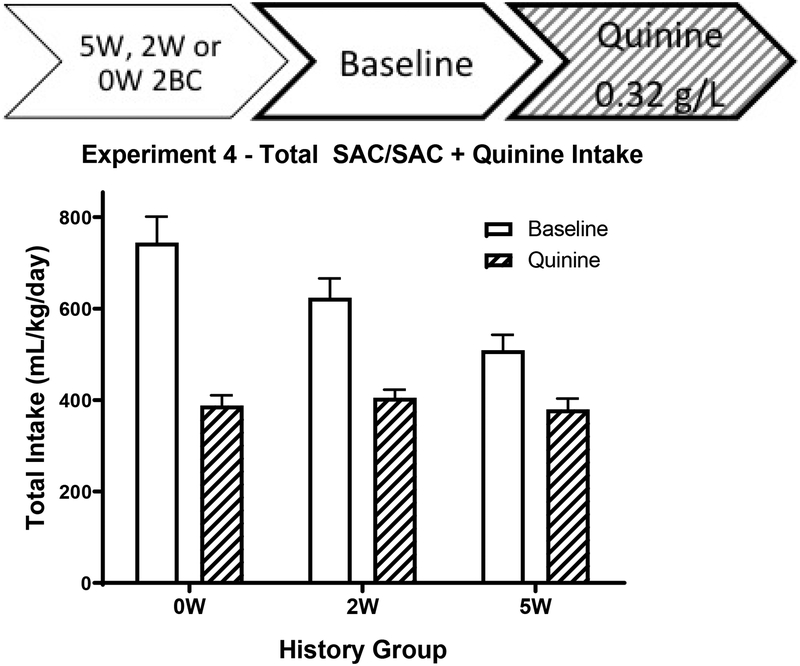
Experiment 4: Saccharin Drinking History
Following robust QR alcohol drinking in Experiment 3, we used a similar procedure to probe for the development of QR SAC drinking using 0.1% saccharin. Unlike with alcohol, longer-term access to saccharin actually caused decreased intake, as 2W mice drank more SAC than 5W mice, t(21) = 2.82, p = 0.010 (Table 2), suggesting novelty of the saccharin solution leads to greater intake.
Baseline Drinking Test
This pattern was repeated on the baseline day, when the 0W animals showed the highest SAC intake, especially early in the day. This was shown by a main effect of Drinking History, F(3.33, 108.65 = 15.02, p < 0.001, as well as a by a Drinking History x Time interaction, F(10, 160) = 2.45, p = 0.024 (Figure 5).
Figure 5:
Experiment 5 Quinine Test
Total daily consumption of 0.1% SAC (solid) and 0.1% SAC + 0.32 g/L quinine (stripes) in cHAP mice. All groups drank less SAC once quinine was added, but there was no difference in SAC + quinine intake between the different history lengths. 5 weeks of 0.1% SAC drinking does not induce quinine resistance in cHAP mice. (n = 11 – 12/bar)
Quinine Adulteration Test
Interestingly, once quinine was introduced the following day, the Drinking History X Time interaction disappeared, p = 0.533, along with any main effect of Drinking History, p = 0.703, consistent with the lack of any development of QR SAC drinking in cHAP mice. Indeed, quinine adulteration reduced total intake in all three drinking history groups (t’s > 6.9, p’s < 0.001). Drinking History had no effect on water intake on either baseline or quinine test (p’s > 0.718). Overall, longer-term saccharin access decreased intake in all lines tested, and no QR saccharin drinking was observed.
Discussion
This comprehensive study demonstrates innate quinine resistant alcohol drinking in selectively bred cHAP mice at quinine concentrations (0.032, 0.10 g/L) commonly used in QR research. Because mice were not indifferent to quinine in alcohol, this innate QR drinking cannot be attributed to deficiencies in taste perception. Alcohol drinking history effects as seen in previous studies could only be detected at very high (0.74 or 0.32 g/L) quinine challenge concentrations. Extended access to 0.1% saccharin did not lead to quinine-resistant saccharin intake even when testing at a high, 0.32 g/L quinine concentration. Although we replicated the quinine-resistant drinking that others have observed with alcohol (Lei et al. 2016; Lesscher et al. 2010; Seif et al. 2013), we extended the findings to demonstrate innate QR resistance as well as an effect of an alcohol drinking history at higher quinine concentrations. We also demonstrated that access to a non-pharmacological reinforcer like saccharin does not lead to aversion resistance in the same cHAP population. These data suggest that a preclinical model of family history positive subjects may be predisposed to compulsive-like alcohol, but not saccharin consumption, and have a higher tolerance for aversive stimuli.
Experiment 1 sought to find an appropriate quinine concentration that would reduce QR intake in alcohol naïve animals but would not affect intake in mice with an alcohol history. We chose to use a relatively moderate history of 5 weeks, in hopes that it would be sufficient to induce compulsive-like intake, similar to that observed in a number of previous studies (Fachin-Scheit et al. 2006; Hopf et al. 2010; Randall et al. 2017; Seif et al. 2013, Lesscher et al, 2010; Lei et al., 2016). Experiment 1 showed that quinine-induced suppression of alcohol intake was more modest than observed in other studies using naïve mice. Thus in Experiment 3, we elected to test at higher concentrations of 0.32 and 0.74 g/L. Overall, our findings in this study were unexpected, as the lower concentrations resulted in classic QR drinking, as indicated by no change in intake from baseline to the quinine test day, but also demonstrated no effect of drinking history. However, at the 0.74 and 0.32 g/L concentrations, we saw an overall reduction in intake once quinine was introduced regardless of alcohol history, which does not follow the traditional definition of quinine-resistant drinking. Importantly, at the 0.32 g/L concentration, 2W and 5W history groups were still reaching pharmacologically relevant levels of EtOH + quinine intake (Figure 4), exceeding 0W intake rates, and therefore demonstrating acquired QR drinking.
In Experiment 2, we therefore decided to further probe the 0.10 g/L concentration to determine whether a 1-day drinking history was sufficient to allow mice to acquire quinine resistance. Mice drank the same amount of quinine-alcohol solution regardless of history, demonstrating innate quinine resistance for the first time, to our knowledge. Lei et al. (2016) found no innate quinine resistance at 0.032 g/L in B6 mice, suggesting that the selectively bred cHAPs differ from the widely used inbred strain. These findings may indicate a predisposition to compulsive-like behavior in individuals with a family history of alcohol use disorder. For future studies, the results of Experiment 2 indicate that 0.10 g/L does not pose a strong enough “challenge” for cHAP animals, and use of a higher concentration of quinine is necessary to further investigate acquired quinine-resistance in this line.
Choosing the 0.74 and 0.32 g/L quinine concentration in Experiment 3 posed an issue for interpretation: if the 0.32 g/L quinine concentration reduced consumption from baseline in Experiment 1, can this behavior be classified as quinine-resistant alcohol consumption? The classic definition would say no, given its reliance on a lack of suppression of intake by quinine (Hopf & Lesscher, 2010). Here, we have defined QR as an effect of drinking history on intake of the bitter quinine-alcohol solution: over time, animals become more willing to ingest this aversive solution. We did not define it as a failure to suppress consumption of alcohol when quinine is added because at the high concentration we used, we were able to detect quinine’s suppressive effect on alcohol consumption even in long-term drinkers. Furthermore, despite the suppression of consumption at the 0.32 g/L concentration, animals were still reaching pharmacologically relevant levels of intoxication. Although we also observed drinking history effects at lower concentrations, they were not as pronounced, and may have been artificially diminished by a ceiling effect for alcohol drinking in mice with a drinking history. Testing at higher quinine concentrations thus allowed us to uncover an acquired quinine resistance less prominent than that seen with less bitter solutions. Overall, these findings demonstrate innate quinine resistance at low concentrations, operating in conjunction with acquired quinine resistance at higher concentrations.
Interestingly, the pattern of alcohol + 0.32 g/L quinine intake did not match between Experiments 1 and 3. While both sets of mice reduced drinking once quinine was introduced, the 0W mice in Experiment 3 showed an almost complete attenuation of EtOH + quinine, more similar to their intake on the 0.74 g/L quinine test day than to the 0.32 g/L quinine test day in Experiment 1. The 0W animals appeared to be uniquely sensitive to the very aversive 0.32 g/L quinine concentration in a way that only affected the 2W and 5W animals at the first 9 am time point. This suggests either that the drinking history in the 2W and 5W animals led to their drinking through the aversive 0.32 g/L quinine, despite the higher concentration they were exposed to earlier, or that the 0W animals were strongly affected by their early experience with the 0.74 g/L QA test (which came on their second day of alcohol drinking), and were thus sensitized to the aversive effects of the 0.32 g/L concentration tested subsequently. These findings in Experiment 3 showed the strongest evidence that a history of alcohol consumption affects quinine-resistant alcohol intake. The 0.74 g/L challenge may be a useful technique to explore phenotypes not observed in traditional QR experiments.
Another factor that could contribute to differences between our findings and previous literature is our drinking procedure. A majority of studies utilized a limited access paradigm throughout their alcohol history in order to induce high levels of alcohol drinking (Hopf et al. 2010; Lei et al. 2016; Lesscher et al. 2010; Seif et al. 2013). However, since our mice are selectively bred for high alcohol consumption on a 2-bottle choice paradigm, we chose to use this procedure to maximize their alcohol consumption. This also resulted in greater cumulative exposure to ethanol during the history phase, as our mice had longer daily access to alcohol. Therefore, the length of alcohol history is not directly comparable between experiments.
A history of saccharin intake did not induce aversion resistance in cHAPs, even when given for the same duration that produced considerable quinine-resistant alcohol drinking. We opted to examine saccharin as it is also a reinforcing substance, and we have found that the HAP mice consume more saccharin solution than Low Alcohol Preferring (LAP) mice (Grahame et al. 1999; Oberlin et al. 2010), including at the 0.1% concentration used in the present experiments. These findings suggest that saccharin intake is genetically correlated with high alcohol consumption and would therefore be an interesting target for compulsivity studies, but QR saccharin drinking failed to develop. Thus, we can conclude that quinine resistant alcohol drinking does not simply reflect high avidity for a preferred substance. Instead it demonstrates an important difference between alcohol and a non-psychoactive substance like saccharin, validating the utility of the quinine adulteration model, as discussed by Hopf and Lesscher (2014). One caveat remains, however, in that alcohol has calories while saccharin does not. Thus, it is possible that continued consumption of alcohol, but not saccharin, in the presence of quinine, is driven by caloric, rather than pharmacological differences between these two compounds. Arguing against this possibility is the fact that the mice in these studies were never food deprived, and had continuous, simultaneous access to food in both alcohol and saccharin drinking studies. Moreover, there were no differences in body weight as a function of drinking history (p’s > 0.354, data not shown). Furthermore, previous work (Dess 2000) did not see quinine resistant sucrose drinking, although the author only assessed this behavior after a 3-day history. Future work could examine whether sucrose consumption over longer periods leads to quinine resistance in cHAP mice.
We are confident that the quinine resistance seen in the EtOH experiments is due to the effects of the varying alcohol history and not a change in taste sensitivity or indifference to the aversive taste, notwithstanding recent findings that brief exposure to the taste of quinine or alcohol decreases the aversiveness of alcohol’s taste (Loney and Meyer 2018). Four lines of evidence support this conclusion. First, previous work has demonstrated no differences in quinine detection and intake between our high and low alcohol preferring (HAP and LAP, respectively) mice (Grahame et al. 1999), indicating no baseline line differences in quinine detection when quinine is mixed in water. Second, the high quinine concentration (0.74 g/L) in Experiment 3 was sufficient to greatly reduce drinking in all groups, relative to their own baseline, although somewhat less so in the 5W animals. Third, when testing at 0.32 g/L, even long-term drinking mice in Experiment 3 initially showed sensitivity to quinine, as their intakes at the 9 am time point (Figure 2) were equivalent to the 0W animals. However, by the 11 am reading, animals with a drinking history seemed to overcome this and began drinking the adulterated alcohol, while relatively naïve animals did not. Finally, and most compellingly, we performed a taste test following Experiment 2 where subjects had access to 10% EtOH and 10% EtOH + 0.10 g/L quinine. There was an overwhelming preference for the unadulterated EtOH (p < 0.001), indicating that the animals were able to detect the 0.10 g/L quinine in alcohol solution, but nonetheless preferred an alcohol solution without quinine. This means that consumption of quinine-adulterated alcohol during the QR tests cannot be attributed to the alcohol experience reducing the ability of these mice to detect and avoid quinine in alcohol, or to some kind of reduction in the aversiveness of quinine, but must instead rely on the motivational properties of alcohol being sufficient for mice to overcome their consistent natural aversion to quinine’s bitter flavor.
An interesting pattern emerges from examining these findings as a whole: the link between intake escalation and development of compulsive drinking. As we have observed previously (O’Tousa and Grahame 2016; Oberlin et al. 2010), alcohol intake escalates in the high-drinking lines over time. Prior work links this escalation to behavioral tolerance (Matson et al. 2014), metabolic tolerance (Matson and Grahame 2013), and acquired aversion resistance (O’Tousa and Grahame 2016). That escalation of alcohol intake appears to be associated with the development of compulsivity, which is consistent with recent theories giving escalation of drug intake a central role in the development of pathological self-administration, both with alcohol (Edwards and Koob 2013) and cocaine (Kippin et al. 2006; Lesscher and Vanderschuren 2012). Consistent with this idea is the fact that saccharin intake demonstrated the opposite of escalation over time–saccharin intake declined with longer term access – which associated with a lack of aversion resistance to saccharin. Although the reasons that these animals would show a decline in avidity for a highly preferred solution are unclear, these findings indicate that escalation and aversion resistance aren’t a simple function of preference. Instead, they point to alcohol’s pharmacological effects as a potential mediator in the development of compulsive behavior. Previous work using alcohol has observed both escalation and QR drinking (Lesscher et al. 2010; Wolffgramm 1991) and even related the two mechanistically (Warnault et al., 2016), but other work has not reported whether or not escalation in alcohol drinking occurs prior to the QR test (Fachin-Scheit et al. 2006; Hopf et al. 2010; Seif et al. 2013). In principle, the same mechanisms that lead to escalation of unadulterated alcohol drinking may also cause an increase in drinking during the quinine test. In clinical populations, consumption of aversive alcohol solutions by alcoholics (such as mouthwash or other unpleasant tasting and potentially harmful sources of alcohol) would similarly be accompanied by a personal history of escalating alcohol consumption during the course of development of this disease. Considering the risk associated with drinking alcohol substitutes, it stands to reason that individuals likely consume lower amounts of potentially hazardous substances, relative to their normal alcohol use. Thus, we do not believe that escalation invalidates this observation of QR drinking, but it is possible, and perhaps likely, that both emerge from some common underlying process.
Our findings suggest that long-term alcohol consumption at high levels can induce quinine-resistant alcohol consumption after only two weeks. These results indicate that long-term alcohol exposure can lead to maladaptive behavior, such as compulsive intake. Using animal models of family history positive individuals, we can conclude that having a genetic predisposition for high alcohol consumption can increase vulnerability to compulsive drinking.
Acknowledgements
This work was supported by the Indiana Alcohol Research Center, AA07611 to David Kareken, and by the IUPUI School of Science.
Funding: AA07611 to David Crabb; AA07462 to Cristine Czachowski
Footnotes
No conflict of interest
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Dess NK (2000) Responses to basic taste qualities in rats selectively bred for high versus low saccharin intake. Physiology & behavior 69:247–257 [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability. Behavioural pharmacology 24:356–362 doi: 10.1097/FBP.0b013e3283644d15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachin-Scheit DJ, Frozino Ribeiro A, Pigatto G, Oliveira Goeldner F, Boerngen de Lacerda R (2006) Development of a mouse model of ethanol addiction: naltrexone efficacy in reducing consumption but not craving. Journal of neural transmission (Vienna, Austria: 1996) 113:1305–1321 doi: 10.1007/s00702-005-0416-z [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L (1999) Selective breeding for high and low alcohol preference in mice. Behavior genetics 29:47–57 [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A (2010) Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res 34:1565–1573 doi: 10.10111/j.1530-0277.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM (2014) Rodent models for compulsive alcohol intake. Alcohol 48:253–264 doi: 10.1016/j.alcohol.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE (2006) Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 187:60–67 doi: 10.1007/s00213-006-0386-3 [DOI] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Simms JA, Hopf FW (2016) A single alcohol drinking session is sufficient to enable subsequent aversion-resistant consumption in mice. Alcohol 55:9–16 doi: 10.1016/j.alcohol.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, van Kerkhof LW, Vanderschuren LJ (2010) Inflexible and indifferent alcohol drinking in male mice. Alcohol Clin Exp Res 34:1219–1225 doi: 10.10111/j.1530-0277.2010.01199.x [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Vanderschuren LJ (2012) Compulsive drug use and its neural substrates. Reviews in the neurosciences 23:731–745 doi: 10.10515/revneuro-2012-0066 [DOI] [PubMed] [Google Scholar]
- Loney GC, Meyer PJ (2018) Brief Exposures to the Taste of Ethanol (EtOH) and Quinine Promote Subsequent Acceptance of EtOH in a Paradigm that Minimizes Postingestive Consequences. Alcohol Clin Exp Res 42:589–602 doi: 10.10111/acer.13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ (2013) Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol 18:921–929 doi: 10.10111/j.1369-1600.2011.00412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Kasten CR, Boehm SL 2nd, Grahame NJ (2014) Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res 38:267–274 doi: 10.10111/acer.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Liangponsakul S, Crabb D, Buckingham A, Ross RA, Halcomb M, Grahame N (2013). Chronic free-choice drinking in crossed High Alcohol Preferring mice leads to sustained blood ethanol levels and metabolic tolerance without evidence of liver damage. Alcohol Clin Exp Res 37: 194–201 doi: 10.1111/j.1530-0277.2012.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH (1970) Experimentally induced intoxication in alcoholics: a comparison between programed and spontaneous drinking. J Pharmacol Exp Ther 173:101–116 [PubMed] [Google Scholar]
- O’Tousa DS, Grahame NJ (2016) Long-Term Alcohol Drinking Reduces the Efficacy of Forced Abstinence and Conditioned Taste Aversion in Crossed High-Alcohol-Preferring Mice. Alcohol Clin Exp Res 40:1577–1585 doi: 10.10111/acer.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N (2011) Derivation and Characterization of Replicate High- and Low-Alcohol Preferring Lines of Mice and a High-Drinking Crossed HAP Line. Behavior genetics 41:288–302 doi: 10.1007/s10519-010-9394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ (2010) Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res 34:1363–1375 doi: 10.10111/j.1530-0277.2010.01220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Stewart RT, Besheer J (2017) Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacology, biochemistry, and behavior 156:1–9 doi: 10.1016/j.pbb.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature neuroscience 16:1094–1100 doi: 10.1038/nn.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, Longo FM, Ron D (2016) The BDNF valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. Biological psychiatry, 79(6), 463–473. doi: 10.1016/j.biopsych.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J (1991) An ethopharmacological approach to the development of drug addiction. Neuroscience and biobehavioral reviews 15:515–519 [DOI] [PubMed] [Google Scholar]