Abstract
Background
We investigated role of coronary microvascular disease (CMD) in maladaptive LV remodeling and prognosis in patients with aortic sclerosis or stenosis and no overt CAD.
Methods
This was a retrospective cohort study of patients with aortic sclerosis or stenosis, normal myocardial perfusion and LV ejection fraction (EF) > 50% (n= 43) and matched controls without AS (n= 43). PET and echocardiograms were performed within 1 year of each other. Myocardial perfusion and myocardial flow reserve (MFR) were quantified using PET imaging. LV structure and function, including global longitudinal strain (GLS), were quantified by transthoracic echocardiography
Results
Global MFR declined with increasing AS severity (p=0.04). Probability of impaired MFR increased with severity of adverse LV remodeling (OR 1.88, CI 1.03–3.41, p= 0.04). Reduced MFR associated with impaired GLS (r= −0.29, p=0.002) and associated with reduced MACE-free survival at 7.27 years median follow-up. Adjusted annualized rate of MACE was highest in those with impaired GLS and MFR and lowest in those with normal GLS and MFR (30.99% vs 1.86%, p = 0.002).
Conclusion and relevance
In patients with AS and no overt CAD, impaired MFR associates with adverse LV remodeling and subclinical LV mechanical dysfunction, and is a marker increased clinical risk.
Introduction
Aortic stenosis (AS) is one of the most prevalent types of degenerative heart valve disease(1). Aortic valve replacement (AVR) is the only effective treatment, timing of which is predicated on AS severity, left ventricular ejection fraction (LVEF) and symptoms(2). However, despite improved outcomes with AVR (2), a significant proportion of patients continue to experience residual morbidity and mortality after valve replacement (3–5). This may be related to the fact that clinical factors used to define the timing of AVR are insensitive to the detection of the transition point from physiological to pathological LV remodeling, which ultimately leads to myocardial injury, altered LV mechanics and replacement fibrosis(6–9). Unfortunately, once interstitial replacement fibrosis is present, AVR is less effective due to limited post- procedural reversal of fibrotic changes(10, 11). This highlights the need to delineate the pathophysiology leading to adverse remodeling and to identify more sensitive markers of disease progression.
The coronary microvascular system, which is responsible for maintaining adequate blood supply and oxygen delivery to the thickened myocardium, is often insufficient or dysfunctional even in the absence of epicardial vessel atherosclerosis (12–14) and has been implicated as a key contributor to the transition from adaptive to maladaptive LV remodeling(15). Global longitudinal strain (GLS), a measure of subclinical LV systolic mechanics, has been shown to be impaired in patients with AS and to have prognostic significance(16–18). However, the relationship between coronary microvascular dysfunction (CMD) and altered LV systolic mechanics in AS has not been well defined, and there are limited data regarding their interaction with clinical outcomes in AS. Additionally, previous studies have shown that even aortic sclerosis confers increased risk of cardiovascular outcomes and death(19), indicating that aortic sclerosis may reflect an early stage in the natural history of AS and be a marker of overall vascular health. Accordingly, we sought to test the hypothesis that the severity of CMD worsens in parallel with the severity of AS and LV remodeling and that this type of vascular dysfunction associates with impaired cardiac mechanics and adverse outcomes.
Methods
Study population
In this retrospective cohort study, we evaluated men and women undergoing clinically indicated rest/stress myocardial perfusion PET imaging for investigation of chest pain and/or dyspnea between 1/1/2006 and 12/31/2014 and who also underwent transthoracic echocardiography (TTE) within 1 year of PET study date. Patients with a diagnosis of trileaflet aortic sclerosis or stenosis were included. Control patients without valvular disease were identified through propensity score matching in a 1:1 fashion using caliper width method based on age, sex, hypertension, diabetes mellitus and history of CAD (defined as prior MI, PCI, or CABG). The absolute standardized differences comparing baseline covariates between the control and AS groups in the matched sample was < 10%. Patients with LVEF < 50% and/or an abnormal myocardial perfusion study (summed stress score (SSS) ≥ 3) were excluded. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
PET imaging and myocardial flow quantitation
Myocardial perfusion imaging was performed on a whole-body PET- computed tomography (CT) scanner (Discovery RX or STE Lightspeed 64, GE Healthcare, Milwaukee, WI) in 2- dimensional mode using 82Rubidium or 13N-ammonia as previously described(20, 21). CT was used for attenuation correction. Coronary vasodilation was achieved using regadenoson or dipyridamole as per standard care. PET images were evaluated semi-quantitatively by a 17- segment visual assessment of gated myocardial perfusion images with a standard five-point scoring system. Rest LVEFs were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, MI). Summed stress score < 3 was considered to be normal and without evidence of obstructive CAD. Rest and stress myocardial blood flow (MBF in mL/min/g) was quantified using a validated tracer kinetic model as previously described (20, 21). To adjust for differences in baseline cardiac work, rest MBF was normalized by the rest pressure product [(MBF /(rest heart rate × rest systolic blood pressure))×10,000]. Corrected global myocardial flow reserve (MFR) was then calculated as stress MBF/corrected rest MBF(22). Quantitative measures of MBF and MFR were recorded by a single experienced operator blinded to patient data.
Echocardiography imaging
All patients underwent clinically- indicated standard two- dimensional resting TTEs within 1 year of PET study date (iE33, Philips Medical Systems, Andover, MA). Conventional 2D echocardiography (Siemens, Tarrytown, NY) and speckle tracking 2D echocardiography analysis (TomTec Imaging Systems, Germany) were performed offline. All measurements were performed according to current American Society of Echocardiography (ASE) guideline recommendations(23) by a single experienced operator blinded to patient clinical data.
Conventional 2D echocardiography
The highest values of mean transaortic pressure gradient and maximum transaortic velocity were measured from all views. The aortic valve area (AVA) was calculated using the continuity equation with the time- velocity integral (TVI) at the LV outflow tract (LVOT) and aortic valve using pulse wave and continuous wave Doppler, respectively(24). Aortic valve severity was defined as sclerosis: leaflet thickening and calcification in the absence of significant ventricular obstruction (peak velocity ≤ 2.5 m/sec); mild: mean pressure < 20 mmHg or AVA > 1.5cm2; moderate: mean pressure 20–39 mmHg or AVA 1–1.5cm2; severe: mean pressure ≥ 40mmHg or AVA < 1cm2.(2, 24) All chamber size, wall thickness, relative wall thickness (RWT), LVEF, diastolic function (E- wave velocity, A- wave velocity, mitral E/e’ ratio, left atrium volume) measurements were made according to previously published guideline recommendations(23, 25). Using LV mass index (LVMI) and RWT, we categorized LV geometry as normal, concentric remodeling, concentric hypertrophy or eccentric hypertrophy according to guideline standards(23).
Speckle tracking 2D echocardiography
Global longitudinal strain (GLS) was derived from measurements made on 2D apical- four and apical- two chamber views and then averaged(26). End diastole and end systole were defined using ECG gating, ventricular cavity size and visualized aortic and mitral valve opening and closing(23). After end diastole and end systole was defined, GLS was derived by tracing endocardial borders followed by the software automatically tracking the speckles along the endocardial and epicardial borders through the cardiac cycle. Speckle tracking was then visually inspected for each tracing with the region of interest adjusted if tracking was not satisfactory. Views with >1 segment dropout or significant foreshortening of the LV were excluded. Because GLS measures the degree of myocardial deformation, more negative values represent greater degrees of contraction while less negative values represent lesser degrees of contraction.
Outcomes
Major adverse cardiac events (MACE) were defined as a composite of death, nonfatal myocardial infarction (MI), hospitalization for heart failure (HF) or aortic valve replacement (AVR). Adjudication of clinical endpoints was performed by a blinded expert adjudication committee using longitudinal medical record, Partners HealthCare Research Patient Data Registry, the National Death Index, mail survey, and telephone calls. The 2012 Third Universal Definition of Myocardial Infarction(27) and pre-defined clinical criteria for the presence of symptoms, signs and escalation of therapy for heart failure were used for these endpoints. All hospitalizations occurred > 30 days following PET imaging.
Statistical analysis
Patient baseline characteristics were reported as frequencies with percent and medians with interquartile range (IQR) where appropriate. To account for matching between groups, mixed- effects logistic regression was used to compare dichotomous variables and mixed- effects linear regression was used to compare continuous variables. PET MBF, MFR and echocardiographic systolic, diastolic parameters and GLS were reported as medians with IQR and compared between case and control groups using multilevel mixed-effects linear regression.
To assess the independent relationship between AS severity and MFR, mixed- effects linear regression accounting for matching and adjusted for age and sex was used. Paired t- tests were used to compare rest MBF to stress MBF in each category of AS severity. Analysis of variance was used to compare stress MBF in each coronary distribution for each category of AS severity. Corrected global MFR was categorized as normal (≥ 2) or abnormal (< 2) as MFR < 2 has been associated with adverse cardiovascular outcomes(28, 29). Multivariable logistic regression adjusted for age, sex and hypertension was used to assess the relationship between LV remodeling and abnormal MFR. Using this regression model, the probability of abnormal MFR for each category of LV remodeling was also calculated. To assess the relationship between global MFR and GLS, linear regression and correlation were used. Univariate associations were tested with the final multivariable model adjusted for age and sex.
We performed additional analyses to investigate the effect of coronary microvascular dysfunction and LV systolic mechanics on clinical outcomes. GLS > −18% and global MFR < 2 were defined as abnormal. Univariable associations were tested for clinical and imaging markers on MACE with final multivariable models adjusted for confounders. Akaike information criterion was used to avoid model over-fitting. Cox proportional hazard ratio controlling for age, sex, AS, MFR < 2, GLS > −18% were calculated across categories of MFR and GLS. Event- free survival curves were plotted after adjusting for clinical factors and compared across dichotomous categories of MFR using the log- rank test. Poisson regression was performed to determine annualize rates of MACE. Model fit was assessed with the goodness of- fit chi- squared test, with a non- significant value indicating an adequately fitted model. For all analyses, unless otherwise indicated, α < 0.05 was considered statistically significant. Stata analysis system version 15 was used for all analyses (StataCorp, College Station, Tx).
Results
Baseline characteristics
We included 43 patients with trileaflet aortic sclerosis or stenosis (median age 72, IQR 67–81) and 43 control patients without valvular heart disease (median age 70, IQR 60–78). There were 9 patients with aortic sclerosis and 34 with AS including 25 with mild, 7 with moderate, and 2 with severe AS. Baseline characteristics were well balanced between the groups and described in Table 1.
Table 1:
Baseline characteristics of the study cohort
| No AS (n = 43) | Sclerosis (n = 9) | Mild (n= 25) | Moderate/severe (n = 9) | p value | |
|---|---|---|---|---|---|
| Age | 70 (60– 78) | 68 (59– 76) | 71 (67– 79) | 75 (72– 85) | 0.02 |
| Female | 26 (60) | 6 (67) | 13 (52) | 5 (56) | 0.49 |
| HTN | 41 (95) | 8 (89) | 24 (96) | 9 (100) | 1.00 |
| DM | 24 (56) | 3 (33) | 15 (60) | 5 (56) | 0.82 |
| CAD* | 20 (47) | 3 (33) | 10 (40) | 3 (33) | 0.21 |
| Medications | |||||
| BB | 34 (79) | 6 (67) | 20 (80) | 6 (67) | 0.59 |
| CCB | 14 (33) | 2 (22) | 10 (40) | 4 (44) | 0.81 |
| ACE inhibitor | 22 (51) | 4 (44) | 13 (52) | 3 (33) | 0.64 |
| Aspirin | 28 (65) | 5 (56) | 17 (68) | 4 (44) | 0.64 |
| Statin | 34 (79) | 7 (78) | 20 (80) | 7 (78) | 1.00 |
| Systemic hemodynamics | |||||
| Resting HR | 66 (60– 74) | 66 (59– 76) | 68 (59– 73) | 68 (60–80) | 0.77 |
| Resting SBP | 152 (127– 166) | 137 (126– 158) | 156 (132– 168) | 134 (129–145) | 0.93 |
| Resting DBP | 69 (61– 77) | 71 (62– 74) | 71 (62–81) | 67 (55–75) | 0.53 |
| Resting MAP | 101 (84– 104) | 93 (86– 108) | 96 (86– 109) | 91 (81–99) | 0.69 |
| Resting RPP | 9540 (8400– 11590) | 8800 (7906– 12008) | 9636 (8880– 11970) | 8700 (8305–9360) | 0.83 |
| Peak HR | 77 (68– 87) | 79 (63– 90) | 77 (66– 89) | 88 (70–120) | 0.63 |
| Peak SBP | 136 (117– 155) | 116 (114– 143) | 150 (136– 162) | 126 (124– 129) | 0.92 |
| Peak DBP | 63 (57– 71) | 61 (54– 65) | 67 (61–76) | 59 (56– 60) | 0.52 |
| Peak MAP | 87 (77– 103) | 81 (70–91) | 96 (82– 103) | 81 (80–83) | 0.42 |
| Peak RPP | 10413 (8540–12690) | 9120 (7268– 11880) | 11340 (9000– 13760) | 10472 (8308– 15120) | 0.67 |
Values are median with interquartile range or n (%).
CAD if history of MI, PCI or CABG.
BB= beta blocker; CBB= calcium channel blocker; ACE= angiotensin converting enzyme; HR= heart rate; SBP= systolic blood pressure; DBP= diastolic blood pressure; MAP= mean arterial pressure; RPP= rate pressure product defined as HR × SBP
HR in bpm; SBP, DBP, MAP in mmHg
Myocardial flow reserve associates with aortic stenosis severity
We first characterized MBF and MFR in our cohort across the spectrum of AS severity. At rest, MBF was globally homogeneous and similar across the four groups (Table 2). During hyperemia, MBF was regionally homogeneous within groups (Supplemental Figure 1) and global MBF increased significantly in all groups compared to baseline (Table 2, Supplemental Figure 2). However, there was a stepwise reduction in the magnitude of flow augmentation and resulting MFR with increasing severity of AS (Table 2, Supplemental Figures 2, 3).
Table 2:
PET myocardial blood flow, myocardial flow reserve, and echocardiographic measures of myocardial structure and function in control patients and patients with aortic sclerosis or stenosis
| No AS (n = 43) | Sclerosis (n= 9) | Mild (n= 25) | Moderate/severe (n = 9) | p value | |
|---|---|---|---|---|---|
| PET quantitative myocardial blood flow | |||||
| Rest MBF | 0.91 (0.70– 1.05) | 0.97 (0.90– 1.04) | 0.94 (0.81– 1.03) | 0.88 (0.79–1.02) | 0.73 |
| Corrected rest MBF | 0.88 (0.71– 1.0) | 1.07 (0.92– 1.24) | 0.98 (0.70– 1.15) | 1.07 (0.78–1.14) | 0.52 |
| Stress MBF | 1.85 (1.53– 2.16) | 2.04 (1.58–2.17) | 1.61 (1.28–2.13) | 1.22 (1.07–1.81) | 0.11 |
| Rest CVR | 111.11 (88.32– 128.21) | 113.04 (96.54– 131.18) | 94.14 (82.69– 106.32) | 105.86 (95.51–124.89) | 0.26 |
| Stress CVR | 42.90 (34.62– 63.98) | 56.45 (45.79– 72.67) | 48.23 (39.87– 64.18) | 41.19 (36.56– 55.84) | 0.41 |
| Corrected MFR | 2.08 (1.73– 2.57) | 1.81 (1.67– 2.08) | 1.69 (1.38–2.31) | 1.30 * (1.04–1.79) | 0.04 |
| Echocardiography structure and function | |||||
| LVEF (%) | 62.5 (60–65) | 60 (60–65) | 65 (60–65) | 60 (55–60) | 0.68 |
| AVA (cm2) | NA | 1.80 (1.68– 2.20) | 1.69 (1.24– 1.82) | 1.08 (0.88–1.18) | <0.001 |
| Mean gradient (mmHg) | NA | 7.64 (6.19– 9.79) | 11.38 (9.54– 13.00) | 25.40 (11.00–28.89) | <0.001 |
| IVSd (cm) | 1.00 (0.90–1.16) | 0.95 (0.88– 1.02) | 1.20 (1.1– 1.23) | 1.19 (1.03– 1.23) | <0.001 |
| PWd (cm) | 1.00 (0.90–1.12) | 0.90 (.86– .99) | 1.18 (1.06– 1.30) | 1.10 (1.02–1.10) | 0.002 |
| LVEDs (cm) | 3.16 (2.70–3.40) | 3.70 (2.92– 3.9) | 2.90 (2.50– 3.40) | 2.90 (2.74–3.0) | 0.31 |
| LVEDd (cm) | 4.50 (4.10–4.9) | 4.73 (4.38– 5.10) | 4.40 (4.20– 4.80) | 4.00 (3.90–4.10) | 0.38 |
| RWT | 0.45 (0.36–0.54) | 0.39 (0.35– 0.45) | 0.54 (0.41–0.60) | 0.54 (0.50–0.58) | 0.01 |
| LV mass index (g/m2) | 75.5 (54–92) | 74 (69– 98) | 92 (76– 109) | 81 (75–93) | 0.05 |
| TR velocity (m/sec) | 2.55 (2.23– 3) | 2.87 (2.55– 3) | 2.70 (2.55– 2.92) | 2.66 (2.01–3.2) | 0.41 |
| E velocity (cm/s) | 0.75 (0.59-.94) | 0.88 (0.67– 0.93) | 0.91 (0.72–1.00) | 1.14 (0.74–1.26) | 0.02 |
| Average e’ (cm/s) | 0.07 (0.06-.09) | 0.08 (0.06– 0.09) | 0.08 (0.06– 0.09) | 0.07 (0.06–0.07) | 0.39 |
| LA volume (mL/m2) | 38.63 (29.39–49.87) | 43.35 (36.76– 48.38) | 41.41 (35.32– 46.11) | 44.11 (43.27 −60.32) | 0.08 |
| E/e’ | 10.57 (8.30–12.90) | 11.64 (8.67– 16.55) | 11.83 (8.73– 14.27) | 16.50 (9.78 –23.58) | 0.04 |
| Average GLS | −18.43 ᴪ (−20.35– −17.25) | −16.23 (−17.9– −14.33) | −17.54 (−19.33– −14.27) | −14.55 Ω (−15.49– −13.85) | <0.001 |
MBF= myocardial blood flow; CVR= coronary vascular resistance; MFR= myocardial flow reserve MBF in ml/min/g; CVR in mmHg/ml/min/g
Values are median with interquartile range.
p = 0.02 vs no AS
p < 0.05 vs sclerosis, mild, moderate/ severe
p = 0.004 vs mild
Relationship between LV remodeling, myocardial flow reserve and global longitudinal strain
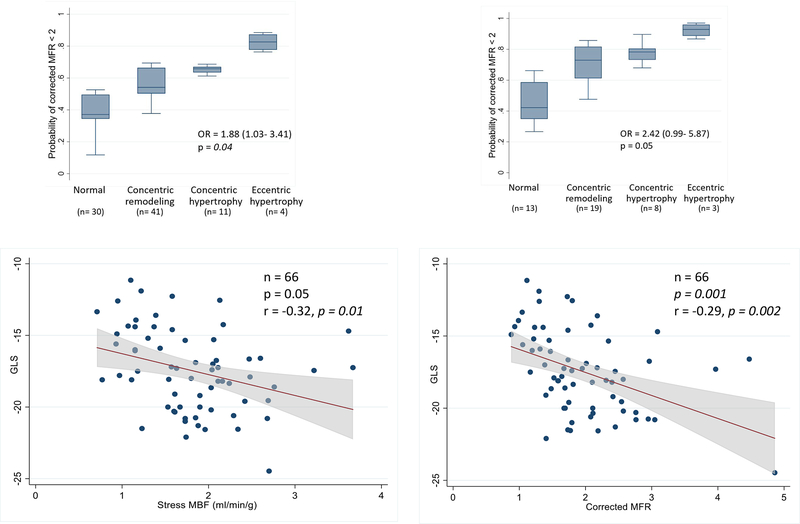
Next, we focused on the relationship between LV remodeling, MFR and GLS. Measures of LV remodeling and diastolic function worsened with increasing AS severity (Table 2). In multivariable modeling using the entire cohort, the odds of an impaired MFR (< 2) was nearly 2-fold higher with increasing degree of adverse LV remodeling(23) after adjusting for age, sex and hypertension (OR: 1.88, 95% CI:1.03–3.41, p = 0.04) (Figure 1A). The increased odds of impaired MFR was also found in a subgroup analysis of patients with aortic sclerosis or stenosis (OR: 2.42, 95% CI: 0.99– 5.87, p= 0.05) (Figure 1B). In addition, GLS worsened with increasing AS severity (p = <0.001) (Table 2). Finally, after controlling for age and sex there was a significant association between impaired microvascular perfusion and worsening GLS (Figure 1 C and D).
Figure 1:
Association between impaired myocardial flow reserve, LV remodeling and cardiac mechanics. There is an increased likelihood of impaired MFR (<2) with worsening LV remodeling observed in the entire study cohort (panel A) and in patients with aortic sclerosis or stenosis (panel B). There is also a significant association between impaired hyperemic myocardial blood flow (MBF) and flow reserve (MFR) and abnormal global longitudinal strain (GLS) (panels C and D, respectively). These findings suggest that coronary microcirculatory dysfunction is linked to subclinical mechanical systolic dysfunction and adverse remodeling in aortic stenosis.
Global longitudinal strain, myocardial flow reserve and adverse clinical outcomes
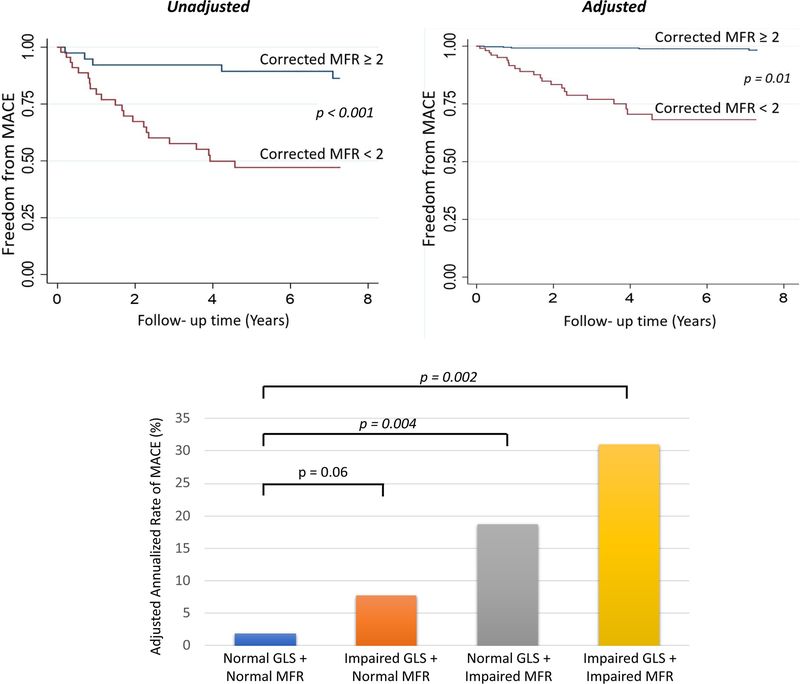
Over a median follow-up period of 7.27 years, 31 patients experienced a major adverse cardiovascular event (MACE; including death [n=18], non- fatal MI [n=5], heart failure hospitalization [n=6], or aortic valve replacement [n=2]). In univariable analysis, the cumulative incidence of MACE was significantly associated with older age (> 70 years), the presence of aortic sclerosis or stenosis, impaired GLS, and impaired global MFR. The association of MFR with MACE remained significant after the addition of clinically important covariates into a multivariable model that included age, sex, GLS, and presence of aortic sclerosis or stenosis (adjusted hazard ratio 6.18; 95% CI: 2.09–18.33, p= 0.001, Table 3, Figure 2).
Table 3:
Association between impaired myocardial flow reserve or GLS and clinical outcomes
| Outcome | Univariable Model Hazard Ratio (95% CI, p value) |
Multivariable Model*
Hazard Ratio (95% CI, p value ) |
||
|---|---|---|---|---|
| MFR < 2 | GLS > −18 | MFR < 2 | GLS > −18 | |
| Death, myocardial infarction, heart failure, or AVR | 6.71 (2.56–17.6, p< 0.01) | 4.15 (1.78–9.7, p= < 0.01) | 6.18 (2.09–18.3, p= 0.01) | 2.71 (1.07–6.89, p= 0.04) |
Adjusted for age, sex, aortic sclerosis/stenosis, GLS > −18, and MFR < 2.
Figure 2:
Association between impaired myocardial flow reserve, global longitudinal strain, and MACE. The probability of survival free from MACE was lower among patients with impaired myocardial flow reserve, even after adjusting for age, sex, aortic stenosis, and GLS (top panel). The adjusted annualized rate of MACE was highest in patients with abnormal MFR and GLS. Among patients with normal GLS, a reduced MFR identified a higher risk of MACE (lower panel). These findings suggest that functional and structural abnormalities are interconnected and affect clinical outcomes in aortic stenosis.
In exploratory stratified analysis of impaired MFR, GLS and MACE, the annualized incidence of MACE was highest among patients with abnormal GLS and MFR (30.99%) and lowest for patients with normal MFR and GLS (1.86%) (Figure 2). In patients with normal GLS, MFR modified the effect of GLS on MACE such that those with abnormal MFR experienced a higher incidence of MACE after controlling for age, sex, and AS (18.73% vs 1.86%, respectively, p= 0.004).
Discussion
We tested the hypothesis that in patients with aortic sclerosis/stenosis and no apparent epicardial CAD, reduced MFR, herein reflecting insufficient coronary microcirculatory reserve, associates with impaired cardiac mechanics and is a marker of prognosis. We found a significant association between reduced MFR and impaired GLS, a marker of subclinical systolic LV dysfunction. Moreover, we demonstrate that reductions in MFR were more pronounced with worsening degrees of LV remodeling. Importantly, reduced MFR was also strongly associated with MACE even after adjusting for other important clinical and imaging markers of increased risk. Indeed, we observed that patients with abnormal MFR and GLS had the highest incidence of MACE, and that an abnormal MFR modified the effect of GLS on clinical outcomes such that the incidence of MACE was significantly higher in the setting of reduced MFR even among those with preserved GLS. Our findings provide new mechanistic insights linking coronary microcirculatory dysfunction, subclinical mechanical systolic dysfunction, adverse LV remodeling and outcomes in aortic stenosis. These data further suggest that these common and almost always coexisting functional and structural abnormalities are interconnected and conspire to promote and accelerate the progression from adaptive to maladaptive LV remodeling and affect clinical outcomes in aortic stenosis.
The ability of the coronary microvasculature to dilate and augment flow during stress is summarized by the concept of MFR(22, 30). During hyperemia, augmentation in coronary flow is linearly related to aortic valve area and coronary perfusion pressure(14). Our results demonstrate a stepwise reduction in hyperemic myocardial blood flow and flow reserve with increasing severity of AS, which is consistent with prior studies (14, 31, 32). The lack of regional differences in hyperemic myocardial blood flow suggests that the observed decrease in global myocardial flow reserve is unlikely related to concurrent obstructive epicardial coronary disease in these patients with normal myocardial perfusion PET images. Rather, AS and subsequent myocardial remodeling in response to altered ventricular hemodynamics and other mechanisms including adverse arteriolar remodeling, perivascular fibrosis, and possibly capillary rarefaction likely contribute to the observation of reduced myocardial flow reserve. The pathophysiology of impaired coronary vascular reserve in AS is multifactorial and the result of a complex interplay between reduced diastolic coronary filling time, delayed end-systolic myocardial relaxation, low coronary perfusion pressure, increased intramyocardial systolic pressure from extravascular compression, and impaired vasomotor responsiveness and/or reduced capillary density (14, 30, 33, 34).
Although progressive LV remodeling has been linked to poor prognosis, the mechanisms underlying this observations are poorly understood(35). Previous studies have shown that AS severity does not always correlate with the degree of LVH(6) and that those with inappropriate LVH experience worse outcomes(36). Our observations of an increased prevalence of impaired MFR with worsening LV remodeling and the association between impaired coronary vascular reserve and subclinical mechanical dysfunction support the notion that subendocardial ischemia is likely an important determinant in the transition to malaptive LV remodeling. This suggests that careful assessment of MFR may provide an opportunity to non-invasively identify functional abnormalities that may help refine disease severity earlier in the natural history of AS.
Current guidelines use depressed LVEF as an imaging-based discriminator to indicate myocardial stress(2) although it may overestimate LV function due to the disproportional increase in myocardial thickness to cavity volume(37). GLS has recently been identified as an imaging marker that has additional prognostic value across the spectrum of AS severity(17, 38, 39). However, in one series, almost 10% of patients with normal LVEF and GLS died before AVR(38), highlighting the need for refinement of risk assessment strategies. Our observation that the risk of MACE is significantly higher in the setting of reduced MFR even among patients with preserved GLS suggests that abnormalities in MFR represent an upstream event and may be a sensitive early marker of risk. Improvement in disease phenotyping and patient selection for intervention is important. Our study suggests that assessment using MFR may have a role as part of a comprehensive approach to identify disturbances in microvascular perfusion, diastolic function, and subclinical systolic function to elucidate early stages of vascular- ventricular- valvular dysfunction which may trigger earlier intervention than currently recommended.
Study limitations
This study is a relatively small, single center observational analysis in which patients were referred for clinically indicated PET studies. Although multivariable adjustments of association were performed, residual and unmeasured confounding may still exist. Due to our relatively small sample size, mediation analysis could not be performed in our regression analysis to determine whether MFR is a mediator variable in the relationship between AS severity and GLS. Coronary angiography was not available in the majority of our patients. However, we only included patients without evidence of regional perfusion abnormalities on PET scanning, which has high sensitivity and negative predictive value for excluding flow-limiting CAD. Recognizing these important limitations, this hypothesis-generating work attempts to mechanistically link the associations of coronary microvascular dysfunction, impaired cardiac mechanics and adverse LV remodeling with cardiovascular outcomes in patients with aortic stenosis without overt flow-limiting CAD. Prospective studies are needed to investigate the role of impaired MFR as a functional marker of coronary microvascular disease for evaluation of the severity of aortic stenosis and/or selecting patients with asymptomatic severe AS for early intervention.
Conclusion
In patients with AS and no apparent epicardial CAD, impaired MFR was associated with AS severity, the degree of adverse LV remodeling and impaired myocardial systolic mechanics. Importantly, reduced MFR was strongly associated with adverse cardiovascular events independent of other clinical and imaging markers of risk. The identification of increased risk even among patients with preserved LV mechanics suggests that MFR may be a sensitive early marker of pathologic remodeling that may play an important role in the evaluation of patients with AS.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant numbers T32 HL094301 to Drs. Zhou, Bajaj, Gupta, Divakaran; K23HL135438 to Dr. Taqueti; and R01HL132021 to Dr. Di Carli.
Disclosures
This was supported by National Institutes of Health grant numbers 5T32HL094301 to Drs. Zhou, Bajaj, Gupta, Divakaran; K23HL135438 to Dr. Taqueti; and R01HL132021 to Dr. Di Carli.
Dr. Dorbala is a member of an advisory board for General Electric Health Care.
Dr. Di Carli has received consulting fees from Sanofi and General Electric.
Dr. Kaneko has served as a proctor and speaker for Edwards Lifesciences, Abbot and Medtronic.
Dr. Shah has served as a proctor and educator for Edwards Lifesciences and educator for St. Jude Medical.
Acronyms and abbreviations
- AS
aortic stenosis
- AVA
aortic valve area
- AVR
aortic valve replacement
- CABG
coronary artery bypass surgery
- CAD
coronary artery disease
- CMD
coronary microvascular dysfunction
- CT
computed tomography
- ECG
electrocardiogram
- GLS
global longitudinal strain
- HF
heart failure
- IQR
interquartile range
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- LVH
left ventricular hypertrophy
- LVMI
left ventricular mass index
- LVOT
left ventricular outflow tract
- MACE
major adverse cardiac events
- MBF
myocardial blood flow
- MFR
myocardial flow reserve
- MI
myocardial infarction
- PET
positron emission tomography
- PCI
percutaneous coronary intervention
- RWT
relative wall thickness
- SSS
summed stress score
- TTE
transthoracic echocardiogram
- TVI
time- velocity integral
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. The Lancet. 2006;368(9540):1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. July 11;70(2):252–89. Epub 2017/03/21. [DOI] [PubMed] [Google Scholar]
- 3.Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005. June 21;111(24):3290–5. Epub 2005/06/16. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia SR, Tuzcu EM, Makkar RR, Svensson LG, Agarwal S, Kodali S, et al. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation. 2014. October 21;130(17):1483–92. Epub 2014/09/11. [DOI] [PubMed] [Google Scholar]
- 5.Arnold SV, Spertus JA, Vemulapalli S, Li Z, Matsouaka RA, Baron SJ, et al. Quality-of-Life Outcomes After Transcatheter Aortic Valve Replacement in an Unselected Population: A Report From the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017. April 1;2(4):409–16. Epub 2017/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012. November 6;60(19):1854–63. Epub 2012/10/16. [DOI] [PubMed] [Google Scholar]
- 7.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010. July 20;56(4):278–87. Epub 2010/07/17. [DOI] [PubMed] [Google Scholar]
- 8.Galiuto L, Lotrionte M, Crea F, Anselmi A, Biondi-Zoccai GG, De Giorgio F, et al. Impaired coronary and myocardial flow in severe aortic stenosis is associated with increased apoptosis: a transthoracic Doppler and myocardial contrast echocardiography study. Heart. 2006. February;92(2):208–12. Epub 2005/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barone-Rochette G, Pierard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014. July 15;64(2):144–54. Epub 2014/07/12. [DOI] [PubMed] [Google Scholar]
- 10.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009. August 18;120(7):577–84. Epub 2009/08/05. [DOI] [PubMed] [Google Scholar]
- 11.Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients With Aortic Stenosis. J Am Coll Cardiol. 2018. February 27;71(8):860–71. Epub 2018/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meimoun P, Germain AL, Elmkies F, Benali T, Boulanger J, Espanel C, et al. Factors associated with noninvasive coronary flow reserve in severe aortic stenosis. J Am Soc Echocardiogr. 2012. August;25(8):835–41. Epub 2012/06/30. [DOI] [PubMed] [Google Scholar]
- 13.Nemes A, Forster T, Varga A, Vass A, Borthaiser A, Pálinkás A, et al. How can coronary flow reserve be altered by severe aortic stenosis? Echocardiography. 2002;19(8). [DOI] [PubMed] [Google Scholar]
- 14.Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, et al. Mechanisms of Coronary Microcirculatory Dysfunction in Patients With Aortic Stenosis and Angiographically Normal Coronary Arteries. Circulation. 2002;105(4):470 LP-6. [DOI] [PubMed] [Google Scholar]
- 15.Marcus ML. Importance of abnormalities in coronary flow reserve to the pathophysiology of left ventricular hypertrophy secondary to hypertension. (0160–9289 (Print)). eng. [DOI] [PubMed] [Google Scholar]
- 16.Kearney LG, Lu K Fau - Ord M, Ord M Fau - Patel SK, Patel Sk Fau - Profitis K, Profitis K Fau - Matalanis G, Matalanis G Fau - Burrell LM, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. (2047–2412 (Electronic)). eng. [DOI] [PubMed] [Google Scholar]
- 17.Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. (1942–0080 (Electronic)). eng. [DOI] [PubMed] [Google Scholar]
- 18.Vollema E, Sugimoto T, Shen M, et al. Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis: Natural course and prognostic value. JAMA Cardiology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto CM, Lind Bk Fau - Kitzman DW, Kitzman Dw Fau - Gersh BJ, Gersh Bj Fau - Siscovick DS, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. (0028–4793 (Print)). eng. [DOI] [PubMed] [Google Scholar]
- 20.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)Nammonia PET. J Nucl Med. 2009. July;50(7):1062–71. Epub 2009/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007. November;34(11):1765–74. Epub 2007/07/10. [DOI] [PubMed] [Google Scholar]
- 22.Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015. January;12(1):48–62. Epub 2014/10/15. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. January;28(1):1–39 e14. Epub 2015/01/07. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017. April;30(4):372–92. Epub 2017/04/08. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016. April;29(4):277–314. Epub 2016/04/03. [DOI] [PubMed] [Google Scholar]
- 26.Shah AM, Cheng S Fau - Skali H, Skali H Fau - Wu J, Wu J Fau - Mangion JR, Mangion Jr Fau - Kitzman D, Kitzman D Fau - Matsushita K, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. (19420080 (Electronic)). eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. 2012. October 16;126(16):2020–35. Epub 2012/08/28. [DOI] [PubMed] [Google Scholar]
- 28.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009. July 7;54(2):150–6. Epub 2009/07/04. [DOI] [PubMed] [Google Scholar]
- 29.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011. November 15;124(20):2215–24. Epub 2011/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014. May;35(17):1101–11. Epub 2013/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemes A, Forster T, Varga A, Vass A, Borthaiser A, Palinkas A, et al. How can coronary flow reserve be altered by severe aortic stenosis? Echocardiography. 2002. November;19(8):655–9. Epub 2002/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 32.Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, et al. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985). 2009. January;106(1):113–21. Epub 2008/11/01. [DOI] [PubMed] [Google Scholar]
- 33.Camici PG, Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356(8):830–40. eng. [DOI] [PubMed] [Google Scholar]
- 34.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010. June;3(6):623–40. Epub 2010/06/15. [DOI] [PubMed] [Google Scholar]
- 35.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Journal of the American College of Cardiology. 2000;35(3):569–82. [DOI] [PubMed] [Google Scholar]
- 36.Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011. February;97(4):301–7. Epub 2010/08/20. [DOI] [PubMed] [Google Scholar]
- 37.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? (1522–9645 (Electronic)). eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng ACT, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Ajmone Marsan N, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging. 2018. August 1;19(8):859–67. Epub 2017/09/28. [DOI] [PubMed] [Google Scholar]
- 39.Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012. October;13(10):827–33. Epub 2012/06/28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.