Abstract
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder in humans. Despite intense investigation, no effective therapy is available to stop the progression of this disease. It is becoming clear that both innate and adaptive immune responses are active in PD. Accordingly, we have reported marked increase in RANTES (regulated on activation, normal T cell expressed and secreted) and eotaxin, chemokines that are involved in T cell trafficking, in vivo in the substantia nigra (SN) and the serum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated hemiparkinsonian monkeys. Since RANTES and eotaxin share a common receptor CCR5, we examined the efficacy of maraviroc, an inhibitor of CCR5 and an FDA-approved drug against HIV infection, in hemiparkinsonian rhesus monkeys. First, we found glial limitans injury, loss of GFAP immunostaining and infiltration of T cells across the endothelial monolayer in SN of hemiparkinsonian monkeys. However, oral administration of low dose of maraviroc protected glia limitans partially, maintained the integrity of endothelial monolayer, reduced the infiltration of T cells, attenuated neuroinflammation, and decreased α-synucleinopathy in the SN. Accordingly, maraviroc treatment also protected both the nigrostriatal axis and neurotransmitters, and improved motor functions in hemiparkinsonian monkeys. These results suggest that low-dose maraviroc and other CCR5 antagonists may be helpful for PD patients.
Keywords: Parkinson’s disease, Monkey model, T cell infiltration, Maraviroc, TH neuron death, Dopamine loss
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder that is characterized by tremor, bradykinesia, rigidity, and postural instability (1, 2). Pathologically, it is characterized by gliosis and progressive degeneration of the DA neurons associated with the presence of intracytoplasmic inclusions (Lewy bodies) in the substantia nigra pars compacta (SNpc) (3).
Although the etiology of PD is poorly understood, recent studies indicate that PD is regulated by the adaptive arm of the immune system (4–11). According to Brochard et al (8), both CD8+ and CD4+ T cells significantly invade the SNpc of PD patients and MPTP-intoxicated mice. We have also found the infiltration of both CD8+ and CD4+ T cells into the SN of hemiparkinsonian monkeys (12). Although the contribution of each of these cell types in nigrostriatal degeneration is not yet known, removal of CD4+, but not of CD8+, T cells in mice greatly reduces MPTP-induced nigrostriatal dopamine cell death (8). While Th17 cells exacerbate nigrostriatal dopaminergic neurodegeneration, regulatory T cells have been shown to attenuate such neurodegeneration (9).
Mechanisms by which T cells infiltrate into the CNS under neurodegenerating conditions are poorly understood. Recently we have seen marked upregulation of RANTES and eotaxin, chemokines that are involved in the infiltration of T cells and other immune cells, in vivo in the SNpc and the serum of MPTP-intoxicated monkey (10). In another study, we have also demonstrated the upregulation of RANTES and eotaxin in the SNpc of postmortem PD brains as compared to age-matched controls (4). In MPTP mouse model as well, we have found that RANTES and eotaxin are rapidly upregulated in SN and serum and that functional blocking antibodies against RANTES and eotaxin protects mice against MPTP-induced nigrostriatal degeneration (4), suggesting that these chemokines play a role in dopamine neuron death.
While investigating the function of these chemokines, we find that a common receptor CCR5 is shared by both RANTES and eotaxin. Although different antagonists of CCR5 are available, maraviroc is the first member of the CCR5 antagonist class of antiretroviral medication, which has been approved for the treatment of HIV-1 infection by the FDA for use in combination with other antiretroviral agents. Here, we demonstrate that oral administration of low-dose maraviroc protects the integrity of endothelial monolayer, reduces the infiltration of T cells into the substantia nigra (SN), attenuates activation of glial cells, lowers α-syn pathology, protects dopaminergic neurons, normalizes striataSNl neurotransmitters, and improves motor functions in hemiparkinsonian monkeys. Therefore, low-dose maraviroc may be of therapeutic benefit for PD patients.
MATERIALS AND METHODS
Reagents:
Maraviroc was purchased from Selleck Chemicals (Houston, TX). Mouse anti-TH antibody (Immunostar), mouse anti-human laminin α5 antibody clone # 4B12 (Millipore), rabbit anti-α-syn antibody (clone: MJFR1) (Abcam), anti-CD4 antibody (Thermofisher), anti-CD8 antibody (Thermofisher), anti-Iba1 antibody (Abcam), anti-GFAP antibody (Agilent), and mouse anti-iNOS antibody (BD Bioscience) were purchased from different vendors. Cy2- and Cy5-conjugated secondary antibodies were obtained from Jackson Immuno Research Laboratories (West Grove, PA).
Subjects and MPTP intoxication:
Female rhesus monkeys (6–8 years old; 5–7 kg) were used in this study. All animals were singly housed with a 12-h light/dark cycle. Purine monkey chow and water were available ad libitum. Diets were supplemented with fruit during the testing sessions. The study was performed in accordance with federal guidelines of proper animal care and with the approval of the IACUC. Monkeys were intoxicated with MPTP according to protocol described previously (12–16). Briefly, animals were tranquilized with ketamine (10 mg/kg, i.m.) and then maintained on an anesthetic plane with isoflurane (1–2%). The animals were put in the supine position. For each injection, a right-sided incision was made along the medial edge of the sternocleidomastoid muscle. The carotid sheath was opened and the common carotid artery, internal jugular vein, and vagus nerves were identified. The common carotid was exposed below the carotid bifurcation. The external carotid artery was then ligated. A 27 gauge butterfly needle was inserted into the common carotid artery in a direction retrograde to blood flow; for each injection, 20 ml of saline containing 3 mg of MPTP-HCl (Sigma) was infused at a rate of 1.33 ml/min (15 min). After the infusion was completed, 3 ml of saline was delivered, and then the incision was closed.
Treatment of hemiparkinsonian monkeys by maraviroc:
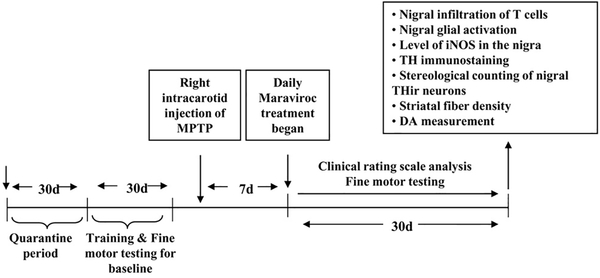
Monkeys displaying hemiparkinsonian symptoms 7 d after the first intracarotid injection were included in the study. Therefore, hemiparkinsonian monkeys were randomized and one group of hemiparkinsonian monkeys (n=4) were treated with maraviroc (1.0 mg/kg body wt/d) orally for 30 d through banana starting from 7 d of MPTP injection (Fig. 1). The control group of hemiparkinsonian monkeys (n=4) received only banana as vehicle.
Figure 1. Schematic presentation of experiments.
Immunohistochemistry:
At the end point, monkeys were anesthetized with pentobarbital (25mg/kg intravenously) and killed via perfusion with 0.9% saline. The brain was removed, immersed in ice-cold saline for 10 min, and slabbed on a monkey brain slicer (12, 13, 15, 16). Slabs through the head of the caudate and putamen were punched bilaterally with a 1-mm brain punch. These punches were processed for HPLC analysis (for quantification of neurotransmitters). Tissue slabs were immersed in Zamboni’s fixative followed by immersion in 30% sucrose in PBS. Slabs were sectioned frozen (40 μm) on a sliding knife microtome. Tissue sections were stored in a cryoprotectant solution at 4°C before use. For TH staining, midbrain sections were immunostained with mouse monoclonal anti-TH antibody (Immunostar and visualized by using DAB/H2O2 (DAB kit, Vector Laboratories) as described (13, 15, 17, 18).
Estimates of dopaminergic nigral cell number were performed bilaterally by an investigator blind to the treatment groups using an unbiased design-based counting method (optical fractionator, StereoInvestigator; Microbrightfield, Williston, VT). All counts were performed by a single investigator blinded to the experimental conditions. Using a random start, we outlined the substantia nigra under low magnification (1.25× objective) and sampled 20% of the treated nigra or 5% of the intact nigra in a random but systematic manner. Quantitation of striatal TH immunostaining was performed as previously described (15, 17, 18). Optical density measurements were obtained by digital image analysis (Scion, Frederick, MD).
For immunofluorescence staining, anti-Iba-1 antibody (1:500), anti-iNOS antibody (1:1000), anti-human laminin α5 antibody (1:1000), and anti-GFAP antibody (1:1000) were used. The samples were mounted and observed under an Olympus IX81 fluorescence microscope as described before (17–19).
HPLC analysis:
Samples were analyzed for neurotransmitters by an investigator blind to the treatment groups. On the day of the analysis, striatal tissues were sonicated in 0.2M perchloric acid containing isoproterenol (internal standard) and resulting homogenates were centrifuged at 20,000 x g for 15 min at 4C. After pH adjusting and filtration, 10μl of supernatant was injected onto a Eicompak SC-3ODS column (HPLC-ECD System EiCOMHTEC-500 from JM Science Inc., Grand Island, NY) and levels of dopamine, 3, 4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were analyzed as described earlier (13, 17, 18, 20).
Behavioral analysis:
The animals were trained to a consistent level of performance before intracarotid injection of MPTP. Monkeys were rated thrice a week by an investigator blind to the treatment groups using a parkinsonian rating scale (PD scale) utilized to quantify the clinical status of the monkeys (13–16). The scale included ratings of 10 parkinsonian features (tremor, posture, locomotion, hypokinesia, bradykinesia, balance, fine and gross motor skills, startle response, and freezing). Each test consisted of twelve total trials alternating between arms, six per side. Delaying feeding time until after the task was completed ensured animals’ compliance with the test. Data collected included the time taken for the animal to move its hand into the chamber where the fruit was located (reaction time), the time taken to pick up the fruit while the hand was in the chamber (reception time) and the total time taken to move the hand into the chamber, retrieve the fruit and bring the hand back out of the panel and into the cage (total time).
Statistical analysis:
All values are expressed as means ± SEM. Differences among means were analyzed by one-way ANOVA or Kruskal-Wallis test (comparison among all groups) and post-hoc pair-wise comparison. In other cases, two sample t tests were also used to compare control vs MPTP and MPTP vs MPTP+maraviroc. For behavioral analysis, one way repeated measure ANOVA models were used. Pair-wise comparisons were conducted with Bonferroni adjustment.
RESULTS
Low dose of oral maraviroc maintains the integrity of endothelial monolayer and inhibits the infiltration of T cells into the substantia nigra (SN) of hemiparkinsonian monkeys:
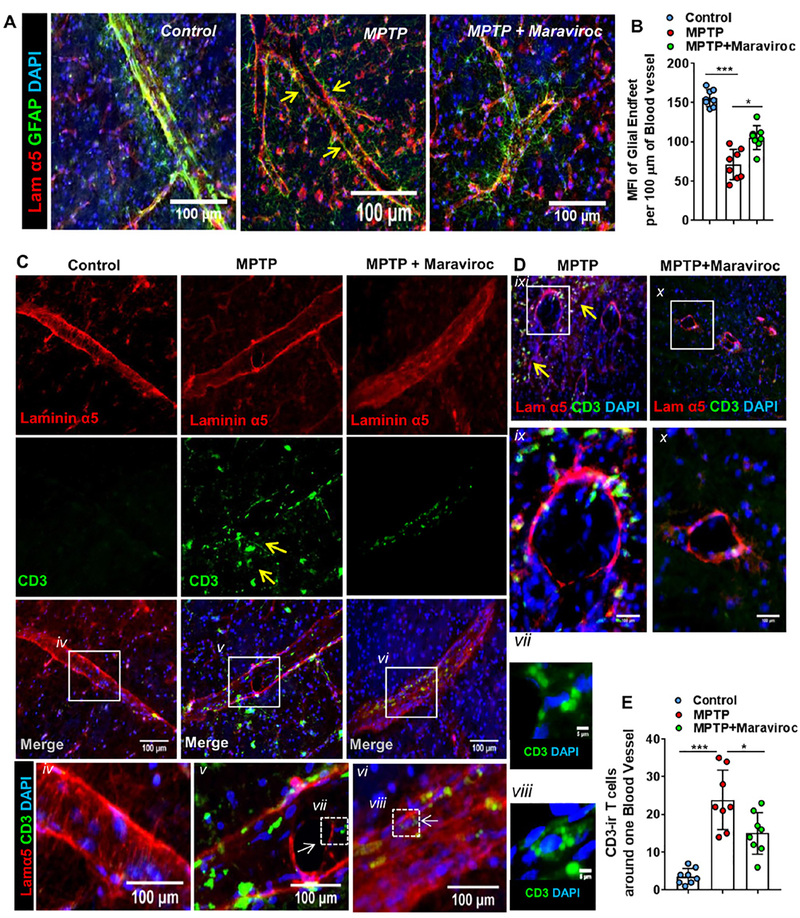
Since in contrast to AIDS, virus is not directly involved in the pathogenesis of PD and the degree of T cell infiltration is also less in the CNS of PD patients as compared to HIV-associated neurocognitive disorders, we used low dose of maraviroc for treating hemiparkinsonian monkeys. Moreover, in order to make our primate work relevant to the clinical scenario, monkeys with established hemiparkinsonian symptoms were treated with maraviroc orally (Fig. 1). At first, we examined whether T cells egressed from capillaries and/or also passed the perivascular glia limitans in the CNS of hemiparkinsonian monkeys. Since laminin α5 serves as a marker of endothelial basement membrane (BM) and astroglial endfeet could be monitored by GFAP staining, in order to stain pial blood vessels, nigral sections were also double-labeled for laminin α5 and GFAP. Figure 2A shows the integrity of glia limitans in control SN that was lost after MPTP intoxication. However, after maraviroc treatment, the integrity of glia limitans was restored partially (Fig. 2A–B). Next, we monitored infiltration of T cells. Double-labeling of laminin α5 and CD3 shows that T cells were almost absent around intact endothelial layer of control SN (Fig. 2C & 2E). However, significant increase in CD3+ T cells was seen around ruptured endothelial layer in the SN of MPTP-treated monkey brain (Fig. 2C, 2D & 2E). On the other hand, after maraviroc treatment, we found significant reduction in CD3+ T cells around endothelial layer (Fig. 2C, 2D & 2E).
Figure 2. Infiltration of T cells and their localization vis-à-vis endothelial membrane of blood vessels and glial limitans.
A) Pial blood vessels located in the nigral tissue of control, MPTP and (MPTP+Maraviroc)-treated monkey brains were stained with Laminin alpha 5 (Lam α5; red) [Mouse anti-human Lam α5; Clone # 4B12; diln =1:1000] in combination with GFAP (green) [Rabbit anti-GFAP; diln = 1:2000; DAKO] as a marker of glial limitans. Representative images are sagittal view of blood vessels. Arrows indicate reduction of GFAP signal around blood vessel. B) Mean fluorescence intensity (MFI) of glial endfeet was measured per 100 μm of blood vessel. For each vessel, three 100 μm segments were randomly selected, analyzed for MFI and then averaged. Eight blood vessels (two vessels from each of four monkeys per group) were included in counting. One-way ANOVA with treatment as a single factor was adopted to test the significance of mean between groups and the resultant descriptive statistics was F2,21= 60.93 (>Fc =3.46; ***p<0.00001 (=7.93*10–8) vs. Control and *p<0.0001 (=8.42*10–5) vs. MPTP. C) Sagittal view of blood vessels immunostained with Lam α5 (red) and CD3 (green) in control, MPTP and (MPTP+Maraviroc)-treated monkey brain SN. Arrows indicate T cell entry into the parenchyma. Regions enclosed in squares were magnified and then displayed underneath for (iv) control, (v) MPTP and (vi) MPTP+Maraviroc. High resolution images (160 X) of individual T cells in blood vessels of (vii) MPTP- (viii) and (MPTP+Maraviroc)-treated monkey brain nigra were shown in side panes. D) Coronal view of blood vessel immunostained with Lam α5 (red) and CD3 (green) in nigral sections of MPTP and (MPTP+Maraviroc)-treated monkey brains. (ix-x) Individual blood vessel enclosed in squares shown in figure D was magnified and displayed underneath. E) CD3-ir cells were counted around blood vessels in nigral tissue of control, MPTP and (MPTP+Maraviroc)-treated monkey brain and then plotted as a histogram. Eight blood vessels (two vessels from each of four monkeys per group) were included in counting. Significance of mean was tested with one-way ANOVA considering treatment as a single factor with F2,21= 25.57 (>Fc =3.46; ***p<0.001 (=0.000154) vs. control and *p<0.05 (=0.017313) vs. MPTP.
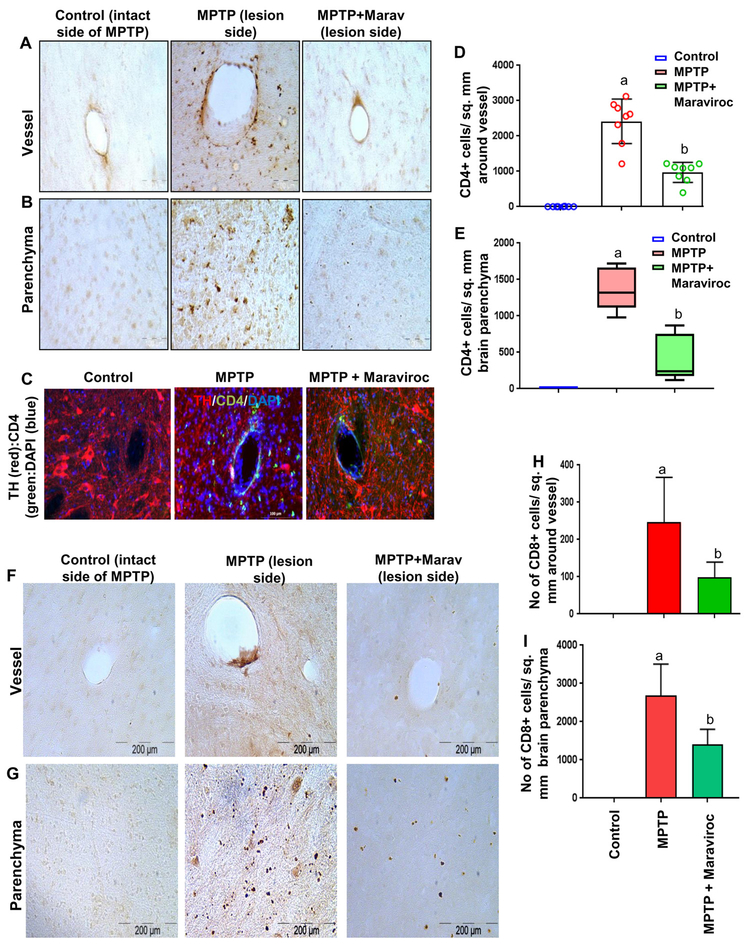
Next, we tried to characterize these T cells by immunostaining of nigral sections. CD4 immunostaining (Fig. 3A–B, DAB staining; Fig. 3C, TH & CD4 double-labeling) clearly shows a typical CD4-immunoreactive inflammatory cuffing in the SN of hemiparkinsonian, but not control, monkeys. Similarly, we also observed infiltration of CD8+ T cells into the SN of hemiparkinsonian, but not control, monkeys (Fig. 3F–G). Although, CD4+ T cells were found to be more abundant than CD8+ T cells near blood vessel (Fig. 3D & 3H), more CD8+ T cells were detected in the deep parenchyma far from the lumen of blood vessels (Fig. 3E & 3I) in the SN of MPTP-treated monkey brain. However, oral treatment with maraviroc significantly attenuated the infiltration of both CD4+ and CD8+ T cells near the blood vessel as well as in deep parenchyma (Figs. 3). These results suggest maraviroc treatment is capable of suppressing the infiltration of T cells.
Figure 3. Oral maraviroc treatment inhibits the infiltration of CD4+ and CD8+ T cells into the SN of hemiparkinsonian monkeys.
Naïve female rhesus monkeys received a right intracarotid injection of MPTP. After 7 d of injection, monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. MPTP group of monkeys also received normal banana as control. After 30 d of treatment, ventral midbrain sections were DAB-stained for CD4 (A, vessel; B, parenchyma). C) Sections were double-labeled for CD4 and TH. CD4-positive cells were counted in two nigral sections (D, within 100 μm around vessel; E, parenchyma within 100 to 200 μm around vessel) of each of four monkeys (n=4) per group. Similarly, ventral midbrain sections were DAB-stained for CD8 (F, vessel; G, parenchyma). CD8-positive cells were counted in two nigral sections (H, within 100 μm around vessel; I, parenchyma within 100 to 200 μm around vessel) of each of four monkeys (n=4) per group. ap<0.001 vs. control; bp<0.01 vs. MPTP. Control represents the intact side of MPTP-intoxicated monkeys.
Low dose of oral maraviroc suppresses inflammation in vivo in the SN of hemiparkinsonian monkeys:
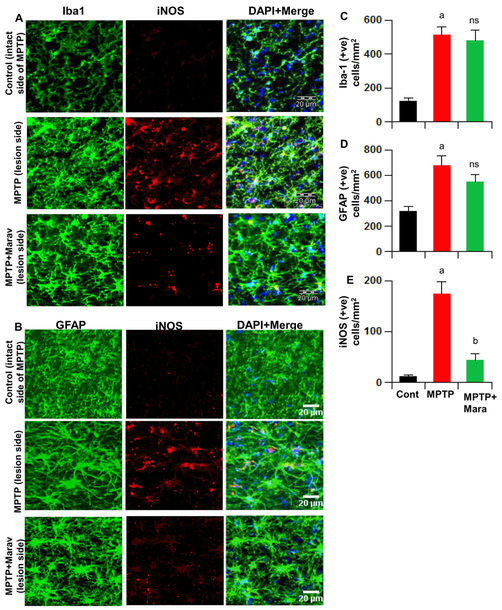
It is becoming clear that neuroinflammation driven by glial cells (astrocytes and microglia) plays an important role in the loss of dopaminergic neurons in PD and its animal model (17, 18, 21–25). As reported earlier (13), MPTP intoxication led to marked increase in nigral iNOS protein expression in hemiparkinsonian monkeys. Consistent to the involvement of glial cells, this iNOS was mainly expressed by either Iba1-positive microglia (Fig. 4A) or GFAP-positive astrocytes (Fig. 4B). However, oral maraviroc treatment reduced the expression of iNOS protein in the SN of hemiparkinsonian monkeys (Fig. 4A, B & E). Similar to the increase in iNOS, we also observed increase in microglia and astrocytes in the SN of hemiparkinsonian monkeys (Fig. 4A–D). Interestingly, maraviroc treatment did not significantly reduce the number of astrocytes and microglia in the SN of hemiparkinsonian monkeys (Fig. 4A–D).
Figure 4. Oral maraviroc treatment inhibits glial inflammation in the SNpc of hemiparkinsonian monkeys.
Monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. After 30 d of treatment, nigral sections were double-labeled for Iba1 and iNOS (A) and GFAP and iNOS (B). Cells positive for Iba1 (C), GFAP (D) and iNOS (E) were counted using the olympus microsuite V software in two nigral sections (two images per slide) of each of four monkeys (n=4) per group. ap<0.001 vs. control; bp < 0.001 vs MPTP; ns, not significant vs MPTP.
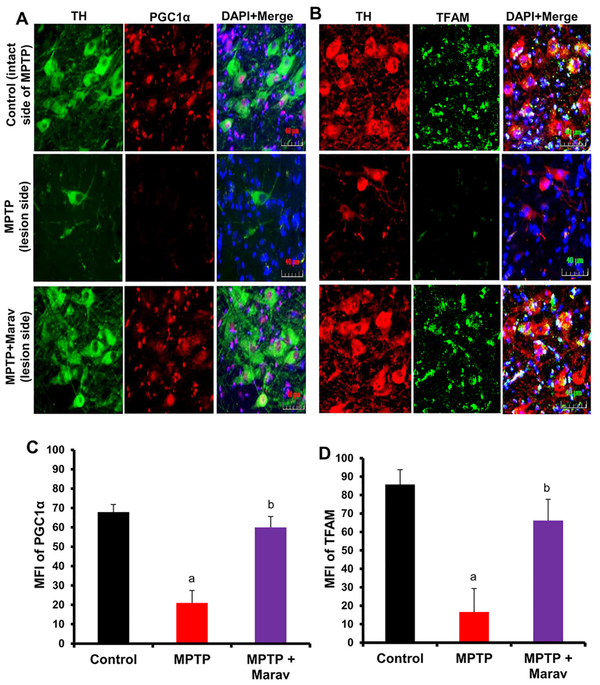
Low-dose maraviroc treatment restores mitochondrial biogenesis in the SN of hemiparkinsonian monkeys:
Recent studies demonstrate alteration in mitochondrial homeostasis in the CNS of patients with different neurodegenerative diseases including PD (26–30). We have also described decreased mitochondrial biogenesis in the SN of MPTP mouse model of PD (31). Therefore, here, we examined the effect of maraviroc on mitochondrial biogenesis in the SN of hemiparkinsonian monkeys. While PPARγ coactivator 1α (PGC1α) is considered as the master regulator of mitochondrial biogenesis (32), mitochondrial transcription factor A (TFAM) is known to control the transcription of mitochondrial genes (33). Mitochondrial biogenesis was monitored in nigral sections by double-labeling of TH & PGC1α (Fig. 5A & 5C) and TH & TFAM (Fig. 5B & 5D). In control SN, both PGC1α and TFAM were present in TH-positive neurons as well as TH-negative cells (Fig. 5A–B). MPTP challenge resulted in marked decrease in mitochondrial content in the SN as compared to control, which is evident from decrease in PGC1α (Fig. 5A & 5C) and TFAM (Fig. 5B & 5D). However, low-dose maraviroc treatment led to significant protection of both PGC1α (Fig. 5A & 5C) and TFAM (Fig. 5B & 5D) in the SN of hemiparkinsonian monkeys.
Figure 5. Oral maraviroc treatment restores mitochondrial biogenesis in the SNpc of hemiparkinsonian monkeys.
Monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. After 30 d of treatment, nigral sections were double-labeled for TH & PGC1α (A) and TH & TFAM (B). Mean fluorescence intensities (MFI) of PGC1α (C) and TFAM (D) were calculated in two nigral sections (two images per slide) of each of four monkeys (n=4) per group. ap<0.001 vs. control; bp < 0.001 vs MPTP.
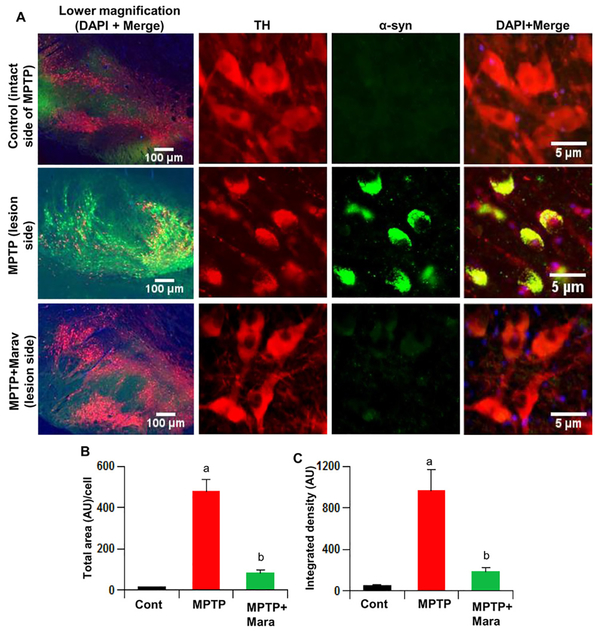
Low-dose maraviroc inhibits α-synucleinopathy in vivo in the SN of hemiparkinsonian monkeys:
Neuropathological hallmarks of PD are the presence of intracytoplasmic inclusions containing α-synuclein (α-syn) and the demise of dopaminergic neurons in the SNpc. In addition to PD, accumulation of α-syn is also an important pathological hallmark of dementia with Lewy bodies (DLB) and multiple system atrophy (MSA) (34–36). Many in vitro and in vivo studies have shown that post-translational modifications of α-syn and associated protein aggregation are intimately coupled with neurotoxicity. Therefore, decreasing α-synucleinopathy may have therapeutic importance in PD and other Lewy body diseases. Accordingly, we observed widespread α-syn pathology in the SN of monkeys after 37 d of MPTP intoxication (Fig. 6A–C). However, α-syn pathology was almost missing in the SN of hemiparkinsonian monkeys after oral maraviroc treatment (Fig. 6A–C).
Figure 6. Oral maraviroc treatment decreases α-synucleinapathy in the SN of hemiparkinsonian monkeys.
Naïve female rhesus monkeys received a right intracarotid injection of MPTP. After 7 d of injection, monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. MPTP group of monkeys also received normal banana as control. After 30 d of treatment, nigral (A) sections were double-labeled for TH and α-syn. Total area (B) and integrated density (C) of α-syn bodies were quantified in two sections (two images per section) of each of four monkeys per group. ap < 0.001 vs control (MPTP-intact); bp < 0.001 vs MPTP-lesion.
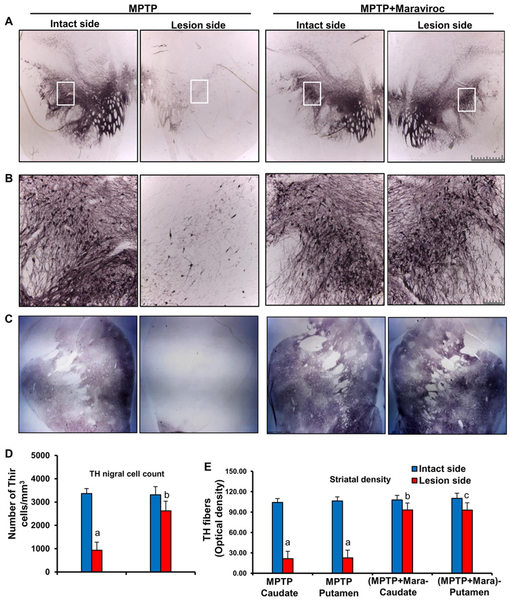
Low-dose maraviroc protects dopamine neurons and fibers in hemiparkinsonian monkeys:
Since low-dose maraviroc treatment suppressed T cell infiltration into the SN and inhibited glial activation as well as α-synucleinopathy in the SN of MPTP-intoxicated mice, we examined the effect of maraviroc on nigrostriatal pathology. Therefore, after 30 d of treatment, animals were processed for quantification of dopaminergic cell bodies in the SNpc and of projecting dopaminergic fibers in the striatum using TH immunostaining. As expected, MPTP intoxication led to marked loss of nigral TH-positive neurons on 37 d of MPTP intoxication (Fig. 7A–B) compared with the intact side (control). Stereological counting of TH-ir neurons in nigral sections showed that MPTP-intoxication led to almost 80% loss of SNpc TH-positive neurons (Fig. 7D) in the lesion side compared with the control intact side. Similarly, quantitative measurements of optical density of the TH staining in caudate nucleus and putamen exhibited marked loss of striatal TH fibers (Fig. 7C & 7E) in the lesion side compared with the intact side. However, oral maraviroc treatment significantly restored SNpc TH-positive neurons (Fig. 7A, 7B & 7D) and striatal TH fibers (Fig. 7C & 7E) in hemiparkinsonian monkeys.
Figure 7. Oral maraviroc treatment protects dopaminergic neurons in the SNpc and THir fibers in the striatum of hemiparkinsonian monkeys.
Naïve female rhesus monkeys received a right intracarotid injection of MPTP. After 7 d of injection, monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. MPTP group of monkeys also received normal banana as control. A) After 30 d of treatment, nigral sections were immunostained for TH. B) A magnified image of TH-stained SNpc is shown. C) Striatal sections were also immunostained for TH. D) Estimates of dopaminergic nigral cell number [MPTP, n=4; (MPTP+maraviroc), n=4] were performed bilaterally using an unbiased design-based counting method (optical fractionator, StereoInvestigator; Microbrightfield, Williston, VT). All counting were performed by a single investigator blinded to the experimental conditions. ap < 0.001 vs MPTP-intact; bp < 0.01 vs MPTP-lesion. E) Optical density of TH fibers in caudate and putamen was calculated by digital image analysis. Six different striatal sections of each monkey were analyzed. ap < 0.001 vs MPTP-intact; bp < 0.01 vs MPTP-Caudate-lesion; cp < 0.01 vs MPTP-Putamen-lesion.
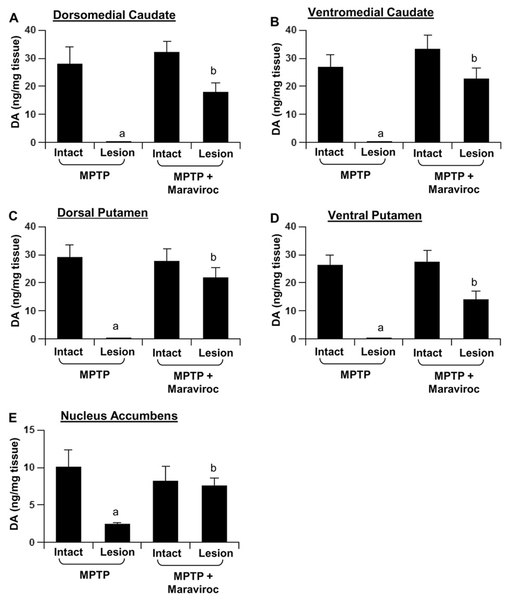
Low-dose maraviroc protects striatal neurotransmitters in hemiparkinsonian monkeys:
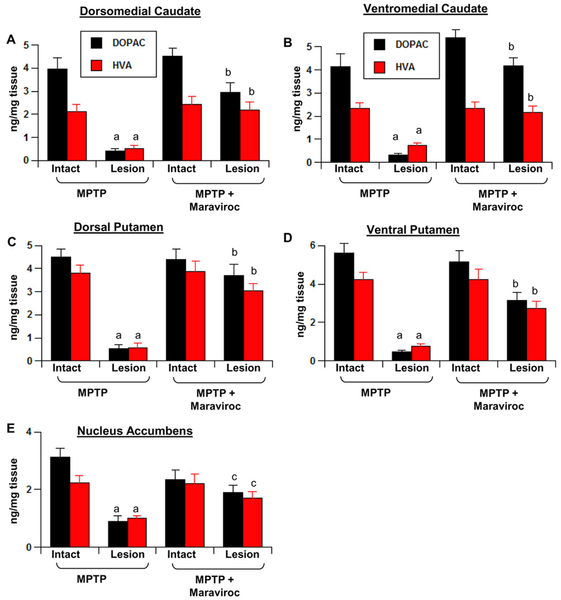
Since maraviroc protected dopaminergic neurons and fibers, next we determined if maraviroc also protected against biochemical deficits caused in hemiparkinsonian monkeys. Therefore, we quantified levels of dopamine (DA), 3,4-Dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in different parts of striatum. MPTP intoxication led to marked loss of DA in dorsomedial (Fig. 8A) and ventromedial caudate (Fig. 8B), the dorsal (Fig. 8C) and ventral putamen (Fig. 8D), and the nucleus accumbens (Fig. 8E) of the lesion side compared to intact control side. In contrast, hemiparkinsonian monkeys that were treated with low-dose maraviroc exhibited pronounced preservation of DA in different parts of striatum (Fig. 8A–E). Similarly, maraviroc treatment also restored the levels of DOPAC and HVA in different parts of striatum of hemiparkinsonian monkeys (Fig. 9A–E).
Figure 8. Maraviroc protects striatal dopamine in hemiparkinsonian monkeys.
Naïve female rhesus monkeys received a right intracarotid injection of MPTP. After 7 d of injection, monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. MPTP group of monkeys also received normal banana as control. After 30 d of treatment, dopamine levels were measured in dorsomedial caudate (A), ventromedial caudate (B), dorsal putamen (C), ventral putamen (D), and nucleus accumbens (E). HPLC quantification of dopamine was performed by a single investigator blinded to the experimental conditions. Data are means ± SEM of four monkeys per group. ap < 0.001 vs MPTP-intact; bp < 0.001 vs MPTP-lesion.
Figure 9. Maraviroc protects striatal DOPAC and HVA in hemiparkinsonian monkeys.
Naïve female rhesus monkeys received a right intracarotid injection of MPTP. After 7 d of injection, monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. MPTP group of monkeys also received normal banana as control. After 30 d of treatment, levels of DOPAC and HVA were measured in dorsomedial caudate (A), ventromedial caudate (B), dorsal putamen (C), ventral putamen (D), and nucleus accumbens (E). HPLC quantification of DOPAC and HVA was performed by a single investigator blinded to the experimental conditions. Data are means ± SEM of four monkeys per group. ap < 0.001 vs MPTP-intact; bp < 0.001 vs MPTP-lesion; cp < 0.05 vs MPTP-lesion.
Although maraviroc treatment displayed beneficial effects in hemiparkinsonian monkeys, it was important to know if this antiretroviral drug might evoke adverse effects on dopaminergic neurons in normal monkeys. However, in our unilateral model, we did not see any significant change in nigral dopaminergic neurons, striatal TH fiber density and striatal neurotransmitters in the intact side even after 30 d of maraviroc treatment, suggesting that maraviroc does not have detrimental effect on the normal nigrostriatum.
Low-dose maraviroc leads to functional improvement in hemiparkinsonian monkeys:
The eventual objective of any neuroprotective therapy is to decrease functional impairments. Therefore, monkeys were examined for performance on a hand reaching task, general activity, and clinical dysfunction (tremor, posture, locomotion, bradykinesia, balance, fine and gross motor skills, startle response, and freezing) based on a clinical rating scale modeled after the Unified Parkinson’s Disease Rating Scale. As reported earlier (13–16), MPTP injection caused a marked decrease in performance in the left hand (contralateral to the MPTP infusion) while maintaining relatively normal function with the right hand (Table-1). However, oral maraviroc treatment significantly improved functional ability as observed on both a subjective clinical rating scale and an objective operant motor test (Table-1). Importantly, during maraviroc treatment, we did not observe any drug related side effect (e.g. hair loss, untoward infection, hyperkinesia, psychological disturbance, vomiting, diarrhea, etc.) in any of these monkeys.
Table 1:
Oral Maraviroc treatment led to functional improvements in hemiparkinsonian monkeys.
| Motor parameters | Before MPTP infusion | After MPTP infusion | After treatment | |
|---|---|---|---|---|
| MPTP + banana | MPTP + Maraviroc-banana | |||
| General (Gross) motor skills (0–3) | ||||
| RHand | 0.00 ± 0.00 | 0.12 ± 0.01 | 0.07 ± 0.04 | 0.01 ±0.00 |
| LHand | 0.00 ± 0.00 | 1.93 ± 0.12 | 1.57 ± 0.88 | 0.89 ± 0.05 |
| Tremor (0–3) | ||||
| RHand | 0.00 ± 0.00 | 0.21 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| LHand | 0.00 ± 0.00 | 1.24 ± 0.32 | 1.07 ± 0.70 | 0.42 ± 0.03a |
| Balance (0–3) | 0.00 ± 0.00 | 1.75 ± 0.28 | 1.59 ± 0.50 | 0.57 ± 0.04a |
| Posture (0–3) | 0.00 ± 0.00 | 1.50 ± 0.43 | 1.35 ± 0.50 | 0.39 ± 0.04a |
| Bradykinesia (0–5) | 0.00 ± 0.00 | 2.12 ± 0.32 | 1.97 ± 0.42 | 0.85 ± 0.28a |
| Gait (0–5) | 0.00 ± 0.00 | 1.75 ± 0.05 | 1.63 ± 0.83 | 0.25 ± 0.03a |
| Defense Reaction (0–2) | 0.96 ±0.71 | 0.87 ± 0.14 | 0.75 ± 0.08 | 0.24±0.07a |
| Freezing (0–2) | 0.00 ± 0.00 | 0.92 ± 0.14 | 0.81 ± 0.23 | 0.39±0.03a |
| Total (Sum mean) | 0.96 ± 0.07 | 12.41 ± 1.23 | 10.81 ± 0.32 | 4.01±0.40a |
Naïve female rhesus monkeys received a right intracarotid injection of MPTP. After 7 d of injection, monkeys displaying classical parkinsonian postures received maraviroc (1 mg/kg body wt/d) orally mixed with banana. MPTP group of monkeys also received normal banana as control. Monkeys were rated thrice a week using a parkinsonian rating scale (PD scale) utilized to quantify the clinical status of the monkeys. The scale included ratings of 10 parkinsonian features (tremor, posture, locomotion, hypokinesia, bradykinesia, balance, fine and gross motor skills, startle response, and freezing). Monkeys were not tested on these tasks during the week between MPTP intoxication and initiation of maraviroc treatment. Results are mean ± SEM of four monkeys per group.
p < 0.001 vs MPTP-banana.
DISCUSSION
At present, no effective therapy is available to halt the progression of PD. Carbidopa-Levodopa (Sinemet®) and/or a dopamine agonist has been the standard treatment for PD. However, it is often associated with a number of side effects and unsatisfactory outcomes. For example, after 2 years of therapy with Sinemet®, 30–50% of patients begin to experience dopaminergic-related complications, including dyskinesis, wearing off, or on-off motor fluctuations. These motor complications eventually influence the clinical picture. Therefore, developing an effective therapeutic approach to halt the progression of PD is of paramount importance. Numerous studies have been carried out in rodents on the role of innate and adaptive immune responses in PD (4, 37–42). However, there is a wide immunological gap between inbred rodent strains and heterogeneous human population. As a result, sometimes rodent results are not translated to humans. On the other hand, rhesus monkeys are old world monkeys and both rhesus monkeys and human are thought to have diverged from a common ancestor, a primitive anthropoid, approximately 25 million years ago. Interestingly, despite 25 million years of evolutionary separation, humans and macaques apparently share about 93% of their DNA sequence (43). It is also important to note that the immune systems of rhesus monkeys and humans are very similar, suggesting that in situations, where it is desirable, but impossible, to study human neuroimmune responses in vivo, rhesus monkey provides the best available alternative.
Maraviroc (Selzentry®) is an FDA-approved drug for treating HIV infection. This drug is also currently being explored for treating different cancer (44, 45). Recent studies have shown that maraviroc suppresses the metastasis of breast cancer (46) in different models. One clinical trial is also underway to study the efficacy of maraviroc in metastatic colorectal cancer. Several lines of evidence presented in this manuscript clearly demonstrate that oral maraviroc treatment results in protection of hemiparkinsonian monkeys both clinically and pathologically. Our conclusions are based on the following. First, only the monkeys that exhibited parkinsonian posture, tremor and left hand freezing after intracarotid injection of MPTP were selected for maraviroc treatment. Second, chronic inflammation is a hallmark of PD pathology. Similarly, even after 37 d of single MPTP injection, levels of GFAP, Iba-1 and iNOS remained markedly higher in the lesioned SN than non-lesioned SN. However, oral maraviroc markedly reduced nigral expression of iNOS. This result was specific as we did not notice any significant inhibition in the number of GFAP-positive astroglia and Iba-1-positive microglia after maraviroc treatment. Third, mitochondrial dysfunction is known to contribute to nigrostriatal pathology in PD patients and in animal models of PD (27, 28). We also observed decrease in mitochondrial biogenesis in the SN of hemiparkinsonian monkeys. However, marked restoration of mitochondrial biogenesis was observed in the SN after maraviroc treatment. Fourth, α-syn accumulation is one of the hallmarks of both sporadic and familial PD (34–36), which is usually observed in hemiparkinsonian monkeys. However, maraviroc treatment markedly decreased α-syn pathology in the SN of hemiparkinsonian monkeys. Fifth, as observed in PD, nigral dopaminergic neurons disappeared and the level of dopamine (DA) decreased in hemiparkinsonian monkeys. But treatment with maraviroc protected TH-positive dopaminergic neurons and restored the level of DA. Sixth, oral maraviroc also reversed motor deficits in a hand-reach task. Seventh, daily FDA-approved doses of maraviroc for adult HIV patients are 300 and 600 mg BID per patient. It is prescribed at a dose of 150 mg/d for baby HIV patients. However, in hemiparkinsonian monkeys, maraviroc at a dose of 1 mg/kg body wt/d markedly protected the nigrostriatum and improved locomotor activities at a much lower dose. Interestingly, a dose of 1 mg/kg body wt/d is equivalent to 70–80 mg QD per patient that is lower than even the baby dose of maraviroc. These results suggest that maraviroc may be effective in PD patients at very low doses. Moreover, at regular doses (300 and 600 mg BID per patient), maraviroc exhibits side effects related to some kind of infection (e.g. upper respiratory infections, Herpes infection, etc.) in less than 10% HIV patients. Although maraviroc itself does not cause hyperkinesia in HIV patients, concomitant use of maraviroc and ritonavir may lead to hyperkinesia. However, at very low doses (70–80 mg QD per patient), we should not expect such side effects from maraviroc.
Mode of action of maraviroc is becoming clear. Recently our lab has demonstrated that RANTES and eotaxin, chemokines that are involved in the infiltration of T cells and other immune cells, are upregulated in the SNpc and the serum of MPTP-intoxicated monkey (10). These two chemokines are also upregulated in vivo in the SNpc of postmortem PD brains as compared to age-matched controls (4). According to Tang et al (47), serum RANTES levels strongly correlated with Hoehn-Yahr score and disease duration in PD patients. Interestingly, both RANTES and eotaxin share the same receptor CCR5, which is inhibited by maraviroc. CCR5-mediated T cell infiltration into the target organ occurs during many pathological conditions including HIV infection and cancer (48, 49). By antagonizing CCR5, maraviroc inhibits the infiltration of T cells T cells in HIV infection and cancer. T cells enter into brain parenchyma through a bilayer of endothelial basement membrane and glial limitans made of astrocytic end feet. Accordingly, we found loss of glia limitans and infiltration of both CD8+ and CD4+ T cells into ventral midbrain of hemiparkinsonian monkeys. Although we did not characterize circulating T cell subsets in the blood on hemiparkinsonian monkeys before and after maraviroc treatment, CD3 immunostaining showed decrease in overall T cell infiltration into the SN by maraviroc. There are several direct and indirect pathways by which T cell infiltration could influence dopaminergic neurodegeneration. For example, it has been reported that the migration of antigen-specific CD4+ T cells from the periphery to the CNS generates immunocyte-microglial activities that perpetuate neuroinflammation and affect neuronal survival (50). Earlier we have shown that effector T cells are capable of activating microglia for the production of various proinflammatory molecules via cell-to-cell contact (51, 52). This contact process involves VLA4 (α4β1) integrin of T cells and VCAM1 of microglia (52, 53). Furthermore, activated T cells may also activate microglia via CD40-CD40 ligation (7, 9, 11). According to Nitsch et al (10), cytotoxic T cell-mediated lethal increase in neuronal calcium could be prevented by blocking both perforin and glutamate receptors. Interestingly, oral administration of maraviroc restored the integrity of endothelial monolayer and strikingly suppressed the infiltration of CD8+ and CD4+ T cells into the SN. Adaptive immune response can be driven by a number of mechanisms (54, 55). Here, the beauty of our finding is that antagonism of a particular component of the adaptive immune arm (CCR5) is sufficient to reinstate glia limitans, protect the loss of dopaminergic neurons, improve striatal DA, and reverse motor deficits in hemiparkinsonian monkeys.
Key points:
Loss of glia limitans and infiltration of T cells in SN of monkey model of PD
Oral maraviroc protected endothelial monolayer and reduced T cell infiltration
Oral maraviroc protected the nigrostriatum and improved locomotor activities
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NS083054).
References
- 1.Vila M, and Przedborski S. 2004. Genetic clues to the pathogenesis of Parkinson’s disease. Nat Med 10 Suppl: S58–62. [DOI] [PubMed] [Google Scholar]
- 2.Olanow CW, and Tatton WG. 1999. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22: 123–144. [DOI] [PubMed] [Google Scholar]
- 3.Dauer W, and Przedborski S. 2003. Parkinson’s disease: mechanisms and models. Neuron 39: 889–909. [DOI] [PubMed] [Google Scholar]
- 4.Chandra G, Rangasamy SB, Roy A, Kordower JH, and Pahan K. 2016. Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease. J Biol Chem 291: 15267–15281. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Chandra G, Roy A, Rangasamy SB, and Pahan K. 2017. Induction of Adaptive Immunity Leads to Nigrostriatal Disease Progression in MPTP Mouse Model of Parkinson’s Disease. J Immunol 198: 4312–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, and Sette A. 2017. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 546: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jana M, Dasgupta S, Liu X, and Pahan K. 2002. Regulation of tumor necrosis factor-alpha expression by CD40 ligation in BV-2 microglial cells. J Neurochem 80: 197–206. [DOI] [PubMed] [Google Scholar]
- 8.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, and Hunot S. 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabot S, Charlet D, Wilson TL, and Yong VW. 2001. Cytokine production consequent to T cell--microglia interaction: the PMA/IFN gamma-treated U937 cells display similarities to human microglia. J Neurosci Methods 105: 111–120. [DOI] [PubMed] [Google Scholar]
- 10.Nitsch R, Pohl EE, Smorodchenko A, Infante-Duarte C, Aktas O, and Zipp F. 2004. Direct impact of T cells on neurons revealed by two-photon microscopy in living brain tissue. J Neurosci 24: 2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jana M, Liu X, Koka S, Ghosh S, Petro TM, and Pahan K. 2001. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem 276: 44527–44533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy A, Mondal S, Kordower JH, and Pahan K. 2015. Attenuation of microglial RANTES by NEMO-binding domain peptide inhibits the infiltration of CD8(+) T cells in the nigra of hemiparkinsonian monkey. Neuroscience 302: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, and Pahan K. 2012. Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol 7: 544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emborg ME, Shin P, Roitberg B, Sramek JG, Chu Y, Stebbins GT, Hamilton JS, Suzdak PD, Steiner JP, and Kordower JH. 2001. Systemic administration of the immunophilin ligand GPI 1046 in MPTP-treated monkeys. Exp Neurol 168: 171–182. [DOI] [PubMed] [Google Scholar]
- 15.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, and Aebischer P. 2000. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290: 767–773. [DOI] [PubMed] [Google Scholar]
- 16.Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J 3rd, Gasmi M, and Bartus RT. 2006. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 60: 706–715. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, and Pahan K. 2007. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A 104: 18754–18759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, and Pahan K. 2009. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci 29: 13543–13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khasnavis S, and Pahan K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Neuroprotective Parkinson Disease Protein DJ-1 in Astrocytes and Neurons. J Neuroimmune Pharmacol 7: 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy A, and Pahan K. Prospects of statins in Parkinson disease. Neuroscientist 17: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, and Nagatsu T. 1996. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211: 13–16. [DOI] [PubMed] [Google Scholar]
- 22.Nagatsu T, Mogi M, Ichinose H, and Togari A. 2000. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl: 277–290. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi GA, Baig S, Bednar I, Sodersten P, Forsberg G, and Siden A. 1995. Increased cerebrospinal fluid concentration of nitrite in Parkinson’s disease. Neuroreport 6: 1642–1644. [DOI] [PubMed] [Google Scholar]
- 24.Dehmer T, Lindenau J, Haid S, Dichgans J, and Schulz JB. 2000. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem 74: 2213–2216. [DOI] [PubMed] [Google Scholar]
- 25.Gao HM, Liu B, Zhang W, and Hong JS. 2003. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci 24: 395–401. [DOI] [PubMed] [Google Scholar]
- 26.Guedes-Dias P, Pinho BR, Soares TR, de Proenca J, Duchen MR, and Oliveira JM. 2015. Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiol Dis. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno Y, Ikebe S, Hattori N, Nakagawa-Hattori Y, Mochizuki H, Tanaka M, and Ozawa T. 1995. Role of mitochondria in the etiology and pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1271: 265–274. [DOI] [PubMed] [Google Scholar]
- 28.Surmeier DJ, and Sulzer D. 2013. The pathology roadmap in Parkinson disease. Prion 7: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baloyannis SJ 2006. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 9: 119–126. [DOI] [PubMed] [Google Scholar]
- 30.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, and Smith MA. 2001. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21: 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandra G, Kundu M, Rangasamy SB, Dasarathy S, Ghosh S, Watson R, and Pahan K. 2018. Increase in Mitochondrial Biogenesis in Neuronal Cells by RNS60, a Physically-Modified Saline, via Phosphatidylinositol 3-Kinase-Mediated Upregulation of PGC1alpha. J Neuroimmune Pharmacol 13: 143–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, and Kaasik A. 2009. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem 284: 21379–21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukat C, and Larsson NG. 2013. mtDNA makes a U-turn for the mitochondrial nucleoid. Trends Cell Biol 23: 457–463. [DOI] [PubMed] [Google Scholar]
- 34.Volpicelli-Daley LA, Luk KC, and Lee VM. 2014. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 9: 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goedert M 2001. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2: 492–501. [DOI] [PubMed] [Google Scholar]
- 36.Lashuel HA, Overk CR, Oueslati A, and Masliah E. 2013. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannarkat GT, Boss JM, and Tansey MG. 2014. The role of innate and adaptive immunity in Parkinson’s disease. J Parkinsons Dis 3: 493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunot S, and Hirsch EC. 2003. Neuroinflammatory processes in Parkinson’s disease. Ann Neurol 53 Suppl 3: S49–58; discussion S58–60. [DOI] [PubMed] [Google Scholar]
- 39.Khasnavis S, Roy A, Ghosh S, Watson R, and Pahan K. 2014. Protection of dopaminergic neurons in a mouse model of Parkinson’s disease by a physically-modified saline containing charge-stabilized nanobubbles. J Neuroimmune Pharmacol 9: 218–232. [DOI] [PubMed] [Google Scholar]
- 40.Laurie C, Reynolds A, Coskun O, Bowman E, Gendelman HE, and Mosley RL. 2007. CD4+ T cells from Copolymer-1 immunized mice protect dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neuroimmunol 183: 60–68. [DOI] [PubMed] [Google Scholar]
- 41.Roy A, Ghosh A, Jana A, Liu X, Brahmachari S, Gendelman HE, and Pahan K. 2012. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson’s disease. PLoS One 7: e38113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, and Przedborski S. 2002. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22: 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien W E, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, and Zwieg AS. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316: 222–234. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe Y, Sasaki S, Mukaida N, and Baba T. 2016. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget 7: 48335–48345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zi J, Yuan S, Qiao J, Zhao K, Xu L, Qi K, Xu K, and Zeng L. 2017. Treatment with the C-C chemokine receptor type 5 (CCR5)-inhibitor maraviroc suppresses growth and induces apoptosis of acute lymphoblastic leukemia cells. Am J Cancer Res 7: 869–880. [PMC free article] [PubMed] [Google Scholar]
- 46.Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, and Pestell RG. 2012. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 72: 3839–3850. [DOI] [PubMed] [Google Scholar]
- 47.Tang P, Chong L, Li X, Liu Y, Liu P, Hou C, and Li R. 2014. Correlation between serum RANTES levels and the severity of Parkinson’s disease. Oxid Med Cell Longev 2014: 208408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velasco-Velazquez M, Xolalpa W, and Pestell RG. 2014. The potential to target CCL5/CCR5 in breast cancer. Expert Opin Ther Targets 18: 1265–1275. [DOI] [PubMed] [Google Scholar]
- 49.Gilliam BL, Riedel DJ, and Redfield RR. 2011. Clinical use of CCR5 inhibitors in HIV and beyond. J Transl Med 9 Suppl 1: S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gendelman HE, and Mosley RL. 2015. A Perspective on Roles Played by Innate and Adaptive Immunity in the Pathobiology of Neurodegenerative Disorders. J Neuroimmune Pharmacol 10: 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasgupta S, Jana M, Liu X, and Pahan K. 2002. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis. J Biol Chem 277: 39327–39333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dasgupta S, Jana M, Liu X, and Pahan K. 2003. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem 278: 22424–22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brahmachari S, and Pahan K. 2010. Gender-specific expression of beta1 integrin of VLA-4 in myelin basic protein-primed T cells: implications for gender bias in multiple sclerosis. J Immunol 184: 6103–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwasaki A, and Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16: 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki A, and Medzhitov R. 2010. Regulation of adaptive immunity by the innate immune system. Science 327: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]