Abstract
We report the development and characterization of a novel, injury-free rat model in which nociceptive sensitization following red light is observed in multiple body areas reminiscent of widespread pain in functional pain syndromes. Rats were exposed to red light emitting diodes (RLED) (LEDs, 660 nanometer) at an intensity of 50 Lux for 8 hours daily for 5 days resulting in time- and dose-dependent thermal hyperalgesia and mechanical allodynia in both male and female rats. Females showed earlier onset of mechanical allodynia than males. The pronociceptive effects of RLED were mediated through the visual system. RLED-induced thermal hyperalgesia and mechanical allodynia were reversed with medications commonly used for widespread pain including gabapentin, tricyclic antidepressants, serotonin/norepinephrine reuptake inhibitors, and NSAIDs. Acetaminophen failed to reverse the RLED induced hypersensitivity. The hyperalgesic effects of RLED were blocked when bicuculline, a GABA-A receptor antagonist, was administered into the rostral ventromedial medulla (RVM) suggesting a role for increased descending facilitation in the pain pathway. Key experiments were subjected to a replication study with randomization, investigator-blinding, inclusion of all data and high levels of statistical rigor. RLED induced thermal hyperalgesia and mechanical allodynia without injury offers a novel injury free rodent model useful for the study of functional pain syndromes with widespread pain. RLED exposure also emphasizes the different biological effects of different colors light exposure.
Keywords: Functional pain Syndromes, Wide spread pain, Diffuse hypersensitivity, Injury-free, Red Light Emitting Diode
Introduction
There are an increasing number of reports pointing to biological effects of exposure to different colors of light. For example, blue light has been associated with sleep and metabolic regulations 10, 15, 48 and light therapy has been used to control depression 20, 26. Exposing patients’ skin to light of 830 nanometer wavelength accelerated wound healing 52. Different wavelengths of light significantly reduced the release of pronociceptive interleukin-6 (405nm-blue) and interleukin-8 (405nm blue, 532nm green, and 650nm red) in a time-dependent manner 35. We previously showed that exposure to green light produced antinociception in rats 36. Therefore, there seems to be a potential to use certain wavelengths of light to produce biological effects. This approach may be desirable because of the affordability of using light as well as its relative ease of use.
While initially investigating the effects of green light emitting diode (LED) on rats 36, we investigated the behavioral responses to different wavelengths and observed increased sensitivity to thermal and mechanical cutaneous stimulus when we used red LED (RLED). This unexpected behavioral effect of RLED merited further in-depth investigation as it is a facile method to induce nociception without injury in rodents and may permit interrogation into mechanisms of functional pain syndromes.
Functional pain syndromes (FPS) are a group of medical conditions resulting in chronic pain without identifiable etiology or clear physical insults. For example, migraine, irritable bowel syndrome, fibromyalgia, interstitial cystitis and others are classified as FPS 8,13, 18, 44–46, 54, 56, 60, 67. Many of these functional pain syndromes are also associated with wide-spread pain with cutaneous hypersensitivity in numerous areas of the body. As the etiologies of the FPS remain unclear, the main course of treatment is usually symptom control and treating the associated pains with non-opioid analgesics such as tricyclic antidepressants, anticonvulsants, muscle relaxants and other modalities 11, 12, 17, 24, 27, 40, 42. Alternative therapies, such as massage, myofascial release, acupuncture, chiropractic, mindfulness, herbal supplements and yoga, have also been reported to be effective tools in managing some FPS conditions 25, 29, 63, 65. There are several existing models for injury free FPS. For example, consistent with clinical reports, priming of rats with triptans or opioids results in increased sensitivity to presumed migraine triggers, mimicking medication overuse headache 19, 55, 72.
Here, we characterize the thermal hyperalgesia and mechanical allodynia consequences of RLED exposure and explore possible mechanisms contributing to this phenomenon. We found that RLED exposure (660 nanometer wavelength) elicited multipoint cutaneous thermal hyperalgesia and mechanical allodynia and alterations in central nociceptive processing that could be ameliorated by clinically employed pharmacological therapies commonly used for FPS. Key experiments were subjected to a replication study with randomization, investigator-blinding, and high levels of statistical rigor.
Methods
Animals
Pathogen-free, adult male and female Sprague-Dawley (weight at testing 250–350 g; Harlan—Sprague—Dawley, Indianapolis, IN) were housed in climate-controlled rooms on a 12-h light/dark cycle and were allowed to have food and water ad libitum. All procedures were approved by the University of Arizona Animal Care and Use Committee and conform to the guidelines for the use of laboratory animals of the National Institutes of Health (publication no. 80–23, 1966). All behavioral experiments were conducted by the same experimenter. Key experiments were replicated using a randomized, double-blinded protocol wherein each step of the protocol: randomization, exposure of rats to RLED, behavioral testing and unblinding/data analysis was performed by different individuals.
Chemicals
All experimental compounds, doses, sources, and catalog numbers are described in Table 1. The doses of medications chosen were based on previously published data in animals and we elected to use the oral route of administration because it is the most common route used in clinical practice 7, 21, 34, 39, 51
Table 1.
Drugs used in this study.
| Drug | Source | Dose | Route |
|---|---|---|---|
| Acetaminophen | Sigma A3035 | 300 mg/kg | P.O. |
| Amitriptyline | Sigma A8404 | 100 mg/kg | P.O. |
| Duloxetine | Sigma SML0474 |
30 mg/kg | P.O. |
| Gabapentin | Sigma G154 | 100 mg/kg | P.O. |
| Ibuprofen | Sigma I4883 | 100 mg/kg | P.O. |
Light Emitting Diodes (LEDs)
All visible spectrum LED flex strips were purchased from ledsupply.com (VT, USA). The LED wavelengths used were: (i) #LS-AC60–66-RE, 620–630 nanometer wavelength (i.e., red), 8 watts, 120 Volts, 120 degree beam angle; (ii) #LS-AC60–66-WH, white, 9.6 watts, 120 Volts, 120 degree beam angle. : 390–700 nm (white, 20 Lux), 400 nm (purple, 4–5 Lux), 475–495 nm (blue, 4–5 Lux), 525–535 nm (green, 4–5 Lux), 575–590 nm (yellow, 4–5 Lux), 635 nm (orange, 4–5 Lux), 660 nm (red, 50 Lux), and 740–770 (infrared).
LED strips were affixed to the outside of clear plastic cages that housed the rats so as to avoid the strips from being chewed. Rats were exposed to the various LED in these cages with full access to food and water in a dark room devoid of any other source of light. Following behavioral assessment, the rats were returned to their cages for additional LED exposure. At the end of daily testing, the rats were returned to their regular animal room where they were exposed to room light illuminated with Sylvania Octron 3500K F032/835 model which is 48” in length and power output of 32-Watt florescent bulbs producing intensity of 750 Lux. A lux meter (Tondaj LX1010B, Amazon.com) was used to determine the illuminance and luminous emittance of the LED strips.
Thermal sensory thresholds
Paw withdrawal latencies were determined as described by Hargreaves et al. 31 was used. Rats were acclimated within Plexiglas enclosures on a clear glass plate maintained at 30°C. A radiant heat source (high-intensity projector lamp) was focused onto the plantar surface of the hind paw. When the paw was withdrawn, a motion detector halted the stimulus and a timer. A maximal cutoff of 33.5 sec was used to prevent tissue damage.
Tactile thresholds
The assessment of tactile sensory thresholds was determined by measuring the withdrawal response to probing the hindpaw with a series of calibrated fine (von Frey) filaments. Each filament was applied perpendicularly to the plantar surface of the paw of rats held in suspended wire mesh cages. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (the “up and down” method), and data were analyzed with the nonparametric method of Dixon, as described by Chaplan and colleagues 14 and expressed as the mean withdrawal threshold.
Testing sequence
Mechanical and thermal tests were performed on the same day. Rats were placed in the testing cages and were allowed 15–30 minutes to acclimate. Mechanical testing of the hind paws was performed first. While keeping the rats in the same cages, mechanical testing for the area between the scapula was done immediately after the mechanical testing of the hind paws. The rats were then transferred to different cages for thermal testing. The rats were allowed 15–30 minutes to acclimate to the thermal testing cages prior to thermal testing.
Replication study: reproducing key behavioral experimental findings with rigorous preclinical trial design
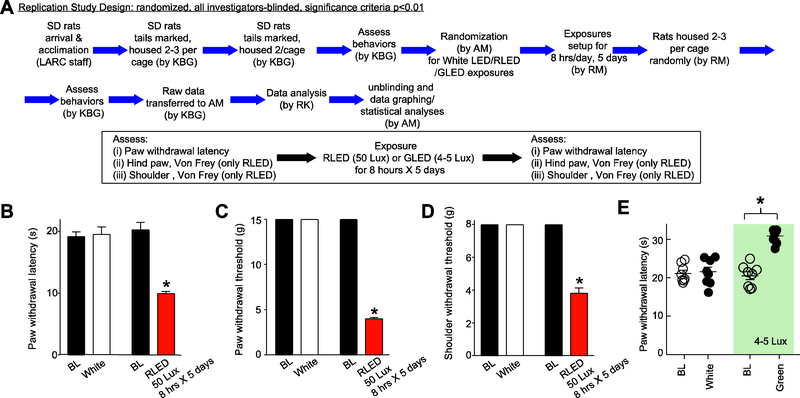
To address concerns about possible reliability and reproducibility of basic research that have been raised in the literature 53, we performed key experiments in a ‘replication study’ with randomization, investigator-blinding, analysis and reporting, matching the rigor of a clinical trial in male rats. We set a higher threshold of significance of P < 0.01 instead of the current standard of P < 0.05. No animals were excluded from the analysis. The detailed protocol is shown in Figure 10A and described as follows:
Figure 10. Replication study: reproducing key behavioral experimental findings with rigorous preclinical trial design.
(A) Study design. The initials of the four experimenters involved in this study are indicated in parentheses. (B-D) Rats were exposed to RLED for 8 hours daily for 5 days at 50 Lux and behaviors were assessed. Paw withdrawal latencies (PWLs) (B), Paw withdrawal thresholds (PWTs) (C), and shoulder withdrawal thresholds (D) were significantly decreased compared to pre-RLED exposures (*p<0.01, Kruskal-Wallis test, n=8 per group). Notably the levels of hypersensitivities were similar to those reported in panels A-C of Fig. 2. White light had no effect. (E) Scatter plots of PWLs of male rats (n=8 per group) exposed to white (20 Lux) or green light (525 nanometers, 4–5 Lux [46]9, 47) for 8 hours daily for five days (*p<0.01, compared to baseline (BL; pre-LED exposure), Kruskal-Wallis test. This experiment was done in a ‘replication’ style design with randomizing, investigator-blinded, all data included and analysis and reporting matching the rigor of a clinical trial.
Male, Sprague Dawley rats (300–350 grams) were allowed to acclimate in the animal care facility for a week after their arrival before they were baselined. Rats were marked on their tails with numbers and housed, two per cage, in cages coded with a letter. Rats had free access to food and water. All rats were baselined for sensory tactile and thermal thresholds of the body and hindpaws by experimenter 1 (KBG) as described in detail below between 8 AM and 10 AM. For the hindpaws, both the right and the left paws were tested, and readings were subsequently averaged for each rat when the data were analyzed. All baseline and test behavioral readings were done by experimenter 1 (KBG).
Rats were randomized to treatment groups ensuring that animals from different cages were assigned to different treatments by experimenter 2 (AM). Codes to the treatments were held by experimenter 2 (AM) and kept in a sealed envelope. The codes were not revealed until data analysis was completed. The following treatment groups were used: (a) white light emitting diode (LED) (20 Lux), (b) green LED (GLED) (4–5 Lux) 36, and (c) red LED (50 Lux).
Treatments were performed by experimenter 3 (RM). All behavioral testing was performed by experimenter 1 who did not wear any cosmetic products during the behavioral testing and always wore previously unworn personal protective equipment (gloves, disposable lab coat, hair covering, and facial mask). Experimenter 1 interacted with the rats by petting them and allowing them to smell her and walk over her hands to acclimate to her. Behavioral testing was performed in a quiet room illuminated with regular room light. The room was reserved for male rats. No mice or female rats were allowed in the room. The room and the testing apparatus were cleaned with soap and water and were allowed to air dry for 3–4 hours prior to testing.
For baselining shoulder hypersensitivity, rats were placed in a suspended wire mesh cage. Rats were allowed to acclimate for 45 minutes. The assessment of tactile sensory thresholds was determined by measuring the withdrawal response to probing the area between the scapulae with a series of calibrated fine (von Frey) filaments. Each filament was applied perpendicularly to the surface of the area between the scapulae of rats. Each filament was applied until it slightly bent. The filament was held in place for 2–3 seconds then removed. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (the “up and down” method), and data were analyzed with the nonparametric method of Dixon 14. A positive response was noted when the rat moved away from the probing filament.
For baselining paw withdrawal threshold (PWT), the assessment of tactile sensory thresholds was determined by measuring the withdrawal response to probing the hindpaw with a series of calibrated fine (von Frey) filaments. Initially, rats were allowed to acclimate in their new von Frey testing apparatus for 15–30 minutes. Each filament was applied perpendicularly to the plantar surface of the paw of rats held in suspended wire mesh cages. Each filament was applied until it slightly bent. The filament was held in place for 2–3 seconds then removed. A response was considered positive if the rat flicked its paw or flicked its paw and licked it. Simply walking away from the filament was not considered a positive response. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (the “up and down” method), and data were analyzed with the nonparametric method of Dixon 14.
Paw withdrawal latencies (PWLs) were determined as described by Hargreaves et al. 31. Rats were acclimated within Plexiglas enclosures on a clear glass plate maintained at 30°C for 15–30 minutes. A radiant heat source (high-intensity projector lamp) was focused onto the plantar surface of the hind paw. When the paw was withdrawn, a motion detector halted the stimulus and a timer. A maximal cutoff of 33.5 sec was used to prevent tissue damage. A positive response was considered if the rat flicked or flicked and licked its paw. Simply walking away from the source of heat did not represent a response.
At the end of the work day, experimenter 3 (RM) who was not involved in the behavioral testing, removed a designated rat from each housing cage and regrouped them in a new exposure cage. For example, Rat #1 from Cage A, Rat #1 from Cage B, and Rat #1 from Cage C were placed together in a new exposure cage. This method of rat randomization was kept throughout the whole experiment. Both the housing cages and the exposure cages contained 2–3 rats each. The exposure cages were pre-fitted with either white, red, or green LED strips on a timer. The timer was set to start at 7PM and end at 3AM for the red and white LED (8 hours), and at 3 AM ending at 6 AM for the green (3 hours) to allow testing in early morning. During the day, around 8AM, experimenter 3 returned the rats to their original housing cages. Rats housed in the same housing cage were exposed to the same light treatment in the exposure cages. Light exposure was done in a quiet laboratory. Rats were returned to the animal care facilities during the day after completion of the light exposure where they were exposed to ambient room light. This procedure was repeated for 5 consecutive days by experimenter 2.
On day 6 following the completion of the treatments, experimenter 1, who was completely unaware of the treatments received and had no knowledge of the experimental group to which an animal was randomized, performed sensory testing in the following order: (i) scapulae von Frey, (ii) hindpaw von Frey, (iii) hindpaw Hargreaves. Behavioral testing was again performed in a quiet room illuminated with regular room light. The room was reserved for male rats. No mice or female rats were allowed in the room. Additionally, no person who was handling female rats or mice were allowed into the testing room. The room and the testing apparatus were cleaned with soap and water and were allowed to air out for 3–4 hours prior to testing.
Rats had access to food and water at all times except during testing for behavior responses which lasted about 2 hours. Data was recorded in ink on a bound notebook and transferred to experimenter 2 who unsealed the randomization envelope. The data was analyzed and graphed by experimenter 4 (RK) who had no prior knowledge of the randomization or the assigned treatment groups. The replication experiments were performed ~16–18 months after completion of all the other experiments reported in this study and are shown in Fig. 10B-D for RLED and Fig. 10E for GLED.
Rostral ventromedial medulla (RVM) cannulation
Rats were first baselined for thermal and mechanical responses. Rats received a bilateral guide cannula (26GA, #C235–1.2mm, Plastics One Inc.) directed to the RVM. The cannula was placed at: −11.0 from bregma, −7.5mm from the dura and 0.6mm on either side of the midline. Rats were allowed one week to recover from surgery and were baselined again for thermal and mechanical responses. No significant changes in mechanical or thermal sensory thresholds were observed as a result of surgery: pre-surgery shoulder withdrawal thresholds were identical to post-surgery shoulder withdrawal thresholds (8 ± 0.0 (n=8)); pre-surgery PWTs were identical to post-surgery PWTs (15 ± 0.0 (n=8)); and pre-surgery PWL mean ± SEM = 20 ± 0.8 (n=8) vs. post-PWL mean = 19 ± 0.6 (n=8). No rats were excluded from our experiments. Injections were made by expelling 0.5 μl through an injection cannula protruding 1 mm beyond the tip of the guide. Cannula placement was confirmed with 0.5μl Evans Blue injected into both sides of the cannula and microscopic examination of medullary sections and data from rats with misplaced cannula were eliminated from the experiment. Acute single injections into the RVM were performed by inserting an injector (Plastics One Inc #C235I-SPC) attached to a 2μl Hamilton syringe and expelling 0.5μl at the same coordinates.
Fabricating rat contact lenses
All plastic materials were purchased from Evergreen Scale Models (Des Plaines, Illinois). We used the method developed by Levinson et al 66, with the following modifications. In brief, 0.25 mm sheets were cut into 2cm2 pieces held by forceps over a 6mm ball bearing, shaped when malleable with a copper pipe of 9 mm internal diameter and then trimmed in to a truncated hemisphere with iris scissors and sanded with a fine grit and emery cloth to a depth of 3.5 ± 0.2mm and a base diameter of 7.0 ± 0.2mm. We used a Wellar 1095–1000 watt dual temperature heat gun, instead of the Bunsen burner used by Levinson, as a heat source for the fabrication process. The rats where anesthetized using isoflurane just long enough to place the contact lens in their eyes and were allowed to recover from anesthesia. The rats were anesthetized again with isoflurane to remove the contact lens at the end of each exposure.
Corticosterone collections and measurements
Animals received 1ml/kg 10% Heparin, IP, 10 minutes before the RLED exposure ended. Upon completion of each animal’s RLED exposure each animal was taken to a laminar flow hood and induced on 4% Isoflurane in 2% O2 and maintained on 2% Isoflurane in 2% O2 during the tail vein blood collection. Warm water was applied to the tail and 300–400 μl of tail vein blood was collected and put into 1ml BD Microtainer® Green Topped Serum Separator Tubes with Lithium Heparin. Tail vein blood was collected within 5–7 minutes after the completion of the RLED exposure. Tubes were then spun down at 4 degrees Celsius at 3000 revolutions per minute for 10 minutes. Serum was transferred into microcentrifuge tubes on dry ice and transported to a −80 freezer and stored there until the time of the ELISA. Pre- and post-RLED exposure tail vein blood was collected between the hours of 9 am and 10 am for each of the animals. Animals were taken to a different room than the RLED exposure for the blood collection in the laminar flow hood and any blood was cleaned up from the hood surfaces between animals. The corticosterone ELISA kit was purchased from Assaypro, catalog #ABIN577656. The conjugate was stored separate from the rest of the kit at −20 degrees Celsius until the day the ELISA kit was run. All other components of the ELISA kit were stored at 4 degrees Celsius until the kit was run.
Data analysis
The statistical significance of differences between means was determined by parametric analysis of variance (ANOVA) followed by post hoc comparisons (Student-Newman-Keuls test) using GraphPad Software. Animal numbers required to achieve statistical power for each in vivo experiment were determined by G.Power3.1, using post-hoc variance estimates from pilot experiments. No outliers were removed. Differences were considered to be significant if p≤ 0.05, except for the replication studies where a difference were considered to be significant only if p≤ 0.01 was reached. All data were plotted in GraphPad Prism 7.
Results
Nociceptive effects of RLED and duplication of green LED (GLED) antinociceptive effects
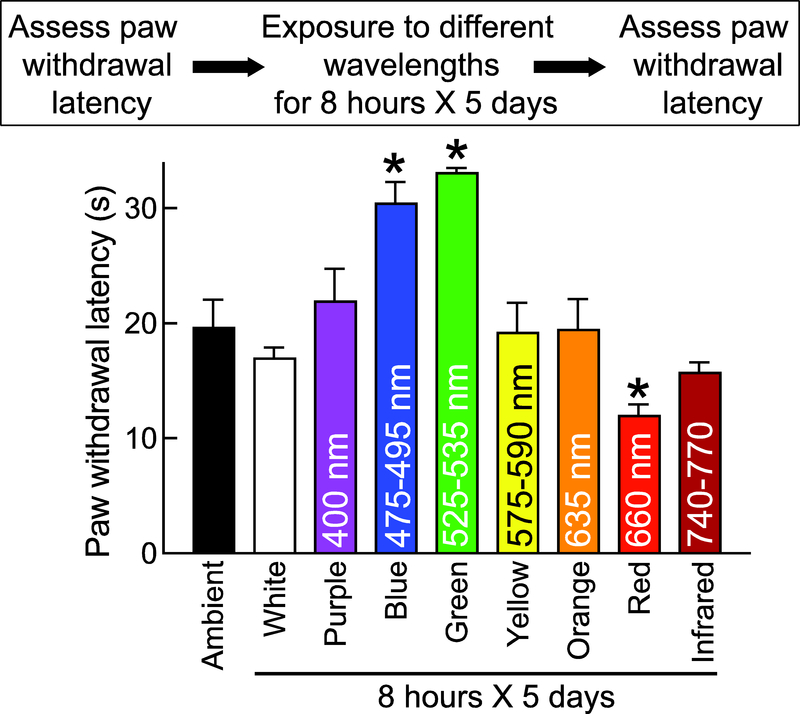
We previously reported that green LED (525–535 nanometers (nm) wavelength, 4 to 110 Lux intensity) for eight hours a day for five days demonstrated antinociception 36 (Fig. 1). In order to explore if other wavelengths in the color spectrum could elicit antinociception, rats were exposed to different color LEDs. Given that the 4–5 Lux was the least intensity exposure for both the red and green LED which resulted in biological responses, we decided to use 4–5 Lux intensity exposure for testing the biological effects of purple, blue, green, yellow and orange LED on rats. Therefore, we exposed rats (n=5–6) to the following wavelengths: 390–700 nm (white, 20 Lux), 400 nm (purple, 4–5 Lux), 475–495 nm (blue, 4–5 Lux), 525–535 nm (green, 4–5 Lux), 575–590 nm (yellow, 4–5 Lux), 635 nm (orange, 4–5 Lux), 660 nm (red, 50 Lux), and 740–770 (infrared). As a control, rats were exposed, for eight hours, to ambient room light that consisted of white fluorescent lights and ambient sunlight through glass windows (~750 Lux). Paw withdrawal latencies (PWLs) were measured immediately after termination of illumination. Consistent with our previous findings 36, naïve rats exposed to green LED (525 nm wavelength at 4–5 Lux) for eight hours daily for five days demonstrated an increase in PWL that was significantly higher than rats exposed to ambient room light (Fig. 1). No other colors of the spectrum elicited a behavioral response, except red (660 nm wavelength) and blue (475–495 nm wavelength). Red was pronociceptive in that it produced thermal hyperalgesia in naïve rats (Fig. 1). While blue light was also effective at eliciting antinociception (Fig. 1), it was not explored further. As pronociceptive effects of RLED have been previously observed in humans 33, 58, we sought to further explore the possibility of using RLED to induce thermal hyperalgesia and mechanical allodynia.
Figure 1. Effect of exposure to different colors on naïve rats’ paw withdrawal latency.
Bar graph of PWL (in seconds) of male rats (n=5–6 per group) exposed to the indicated spectrum of light (wavelength in nanometers listed within bars) for 8 hours daily for 5 days. (*p<0.05, compared to baseline (BL; pre-LED exposure), Kruskal-Wallis test, n=6. In all figures, the experimental timeline and paradigm are shown above the graphs.
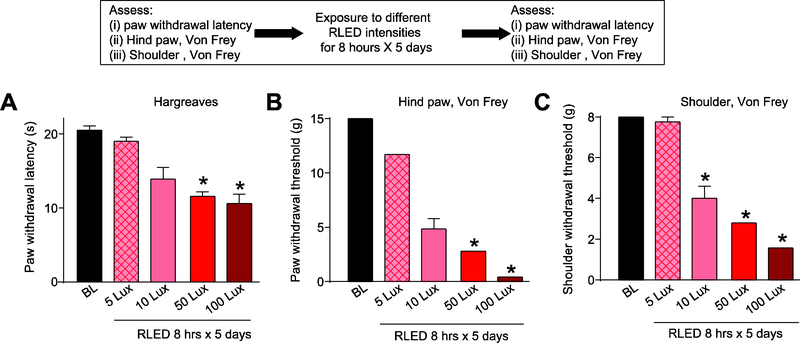
First, we performed a RLED intensity (in Lux) versus nociception analysis that revealed a Lux-dependent decrease in PWL (Fig. 2A). We also observed a lowering of the withdrawal thresholds, to mechanical stimulation with von Frey filaments, for the paw (Fig. 2B) as well as the shoulder (the upper back between the scapula) (n=6–8) (Fig. 2C).
Figure 2. RLED exposure induces thermal hyperalgesia and mechanical allodynia.
Rats were exposed to RLED for 8 hours daily for 5 days at variable lux intensities. (A) Paw withdrawal latencies (PWLs, measured via Hargreaves test) were significantly decreased compared to baseline (pre-RLED exposure) following exposure 50 and 100 Lux RLED. (*p<0.05, Kruskal-Wallis test, n=6–8). (B) Paw withdrawal thresholds (PWTs, measured via stimulation with von Frey filaments) and (C) withdrawal thresholds (measured via shoulder stimulation with von Frey filaments) were significantly decreased compared to baseline following exposure to 10, 50, and 100 Lux RLED. (*p < 0.05, Kruskal-Wallis test, n=6–8).
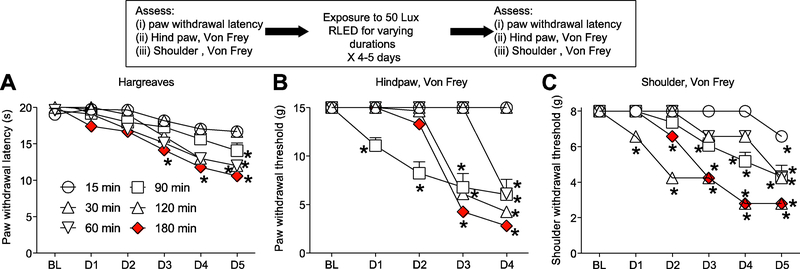
Since 50 Lux was sufficient to induce significant thermal hyperalgesia and mechanical allodynia (Fig. 2), we used 50 Lux for the next series of experiments. Using 50 Lux of RLED, we next varied the exposure duration, exposing rats for 15, 30, 60, 120, or 180 minutes to RLED for 5 days (n=6–8). Exposing rats for 15 minutes a day for 5 days to RLED failed to decrease PWL, and the hind paw or shoulder withdrawal thresholds (Fig. 3). Increasing the daily RLED exposure time to 180 minutes elicited increased the thermal hyperalgesia and mechanical allodynia in both the time of first onset and level of intensity achieved (Fig. 3). Exposing rats to room light or white LED for the same durations and intensities had no effect on thermal and mechanical testing (data not shown). Therefore, unless otherwise indicated, these experiments formed the basis of the RLED exposure paradigm used for the rest of these studies – Lux level (50), number of hours of exposure per day (3), and number of days of exposure (5).
Figure 3. Time dependency of RLED Lux intensity in induction of thermal hyperalgesia and mechanical allodynia.
Rats were exposed to 50 Lux of RLED for 15, 30, 60, 90, 120, and 180 min daily for 5 days. (A) Paw withdrawal latencies (measured via Hargreaves test) were significantly decreased in rats at points as indicated by asterisks compared to baseline (*p<0.05, Kruskal-Wallis test, n=6–8). (B) Paw withdrawal thresholds (measured via stimulation with von Frey filaments) and (C) withdrawal thresholds (measured via shoulder stimulation with von Frey filaments) were significantly decreased in rats at points as indicated by asterisks compared to baseline (*p < 0.05, Kruskal-Wallis test, n=6–8).
Characterization of the role of the visual system in the nociceptive effects of RLED
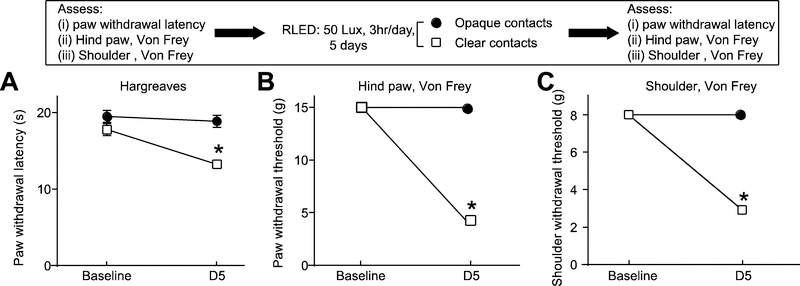
In our previous studies on the antinociceptive effects of green LED, we reported a key role for the visual system 36. Similarly, here we investigated a possible role of the visual system in mediating RLED-induced hypersensitivity. We fashioned dark opaque plastic contact lenses that permitted no light penetration (confirmed by measuring light intensity). The contacts were then fitted onto the rats’ eyes under anesthesia. As a control, transparent clear lenses were also installed onto control rats’ eyes (n=4). The application of contact lenses did not alter the baseline paw withdrawal latency. Both groups of rats were then exposed to RLED for three hours daily for five days and their PWLs and withdrawal thresholds were monitored. Following this exposure paradigm, rats fitted with the dark, opaque contact lenses failed to exhibit any thermal hyperalgesia or mechanical allodynia, whereas rats fitted with clear, transparent contact lenses developed thermal hyperalgesia and mechanical allodynia similar to rats with no contacts (Fig. 4). Thus, as with green LED induced antinociception, the visual system is required in mediating the pronociceptive effect of RLED.
Figure 4. RLED-induced thermal hyperalgesia and mechanical allodynia engages the visual system.
Rats were fitted with black or clear contact lenses, then exposed to RLED for 3 hours daily for 5 days at 50 Lux. (A) Paw withdrawal latencies (measured via Hargreaves test) were significantly decreased between the clear lens, but not opaque lens, condition and ambient light-exposed rats. (*p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=4). (B) Paw withdrawal thresholds (measured via stimulation with von Frey filaments) and (C) withdrawal thresholds (measured via shoulder stimulation with von Frey filaments) were significantly decreased between the clear lens, but not opaque lens, condition and ambient light-exposed rats. (*p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=4).
Effect of sex on RLED nociceptive thresholds
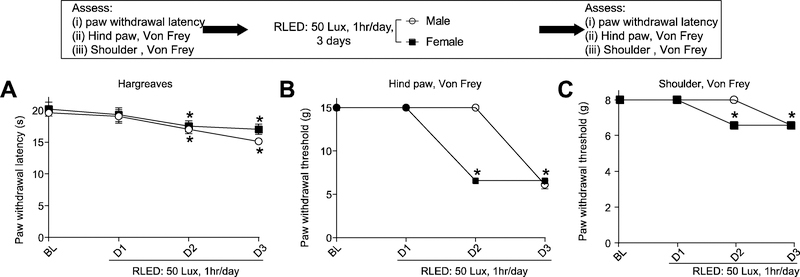
We tested male and female rats in order to determine if there were sex differences in the development of thermal hyperalgesia and mechanical allodynia to RLED exposure. We used 50 Lux intensity of RLED exposure for both male and female rats, however, we lowered the exposure time to one hour for both male and female rats in an attempt to identify potential subtle differences in biological responses. In these experiments, we purposely modified the exposure paradigm by shortening the time of RLED exposure (50 Lux RLED for 1 hour, instead of 3 hours, for three days) to maximize detectability of possible differences. With this protocol, female SD rats developed mechanical allodynia in both the hindpaw and shoulder a day earlier than male SD rats; importantly, there was no difference in the thermal hyperalgesia between males and females at this time point (n=5) (Fig. 5).
Figure 5. RLED exposure induces sex-independent thermal hyperalgesia and mechanical allodynia.
Male and female rats were exposed to RLED for 1 hr daily for 3 days at 50 Lux. (A) Paw withdrawal latencies (measured via Hargreaves test) were significantly decreased in both male and female rats compared to baseline (pre-RLED exposure) (*p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=5) (B) Paw withdrawal thresholds (measured via stimulation with von Frey filaments) and (C) withdrawal thresholds (measured via shoulder stimulation with von Frey filaments) were significantly decreased in both male and female rats compared to baseline (pre-RLED exposure). Notably, females developed mechanical allodynia more rapidly than males. (*p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=5)
Reversibility of the nociceptive effects of RLED and characterization of repetitive exposure to RLED
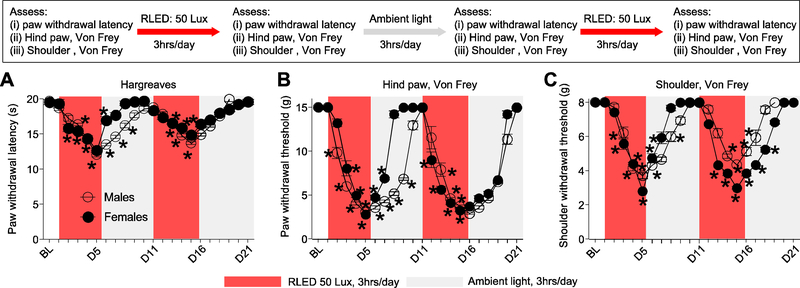
We next tested if the RLED-induced thermal hyperalgesia and mechanical allodynia was reversible and altered by repetitive exposure. Male and female rats exposed to 50 Lux RLED for 3 hours a day for five days exhibited robust thermal hyperalgesia and mechanical allodynia that reversed to baseline levels within 4 days after cessation of RLED exposure (Fig. 6). A re-exposure of these rats to a second round of exposure of 50 Lux RLED for 3 hours a day for five days returned the animals to a hyperalgesic state similar to the first exposure (Fig. 6). Female rats recovered to baseline levels in response to thermal stimulation or mechanical stimulation of the hind paw faster than male rats in the first RLED exposure, although no difference in time to develop thermal hyperalgesia or mechanical allodynia was observed upon re-exposure to a second round of RLED (n=9) (Fig. 6A, B).
Figure 6. RLED-induced thermal hyperalgesia and mechanical allodynia are reversible even with a repeat exposure.
Male (open circles) and female (closed circles) rats were exposed to RLED for 3 hrs daily for 5 days at 50 Lux (red shaded boxes), followed by a period of 5 days with ambient light exposure (gray shaded boxes), and a second 5-day period of RLED exposure for 3 hrs daily, and another 5-day period with ambient light exposure. (A) Paw withdrawal latencies (PWLs) during the initial period of RLED exposure (measured via Hargreaves test) were significantly decreased in both male and female rats. Following cessation of RLED, the decrease in PWLs progressively normalized to baseline (pre-RLED exposure) during the five-day ambient light exposure. PWLs significantly decreased following a second round of RLED exposure and reversed to baseline levels about 4 to 5 days after termination of RLE. *p<0.05, two-way ANOVA with Tukey’s post hoc analysis. (B) Paw withdrawal thresholds (PWTs, measured via stimulation by Von Frey filaments) during the initial period of RLED exposure and (C) shoulder withdrawal thresholds (SWTs, measured via shoulder stimulation by Von Frey filaments) were significantly decreased in both male and female rats. During the next 5-day period with ambient light exposure, PWTs and WTs gradually increased until neither was significantly different from baseline (pre-RLED exposure). PWTs and SWTs significantly decreased following a second round of RLED exposure. PWTs and SWTs gradually increased until there was no significant difference from baseline about 4 to 5 days after termination of RLE. *p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=9.
Pharmacological characterization of the RLED induced thermal hyperalgesia and mechanical allodynia
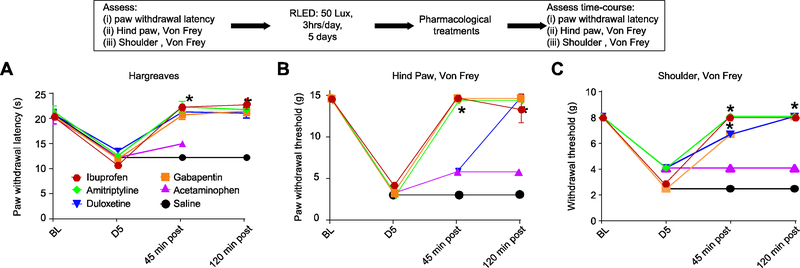
There are several classes of medications commonly used in the clinical management of diffuse pain such as that seen in FPS. These include non-opioid analgesics, tricyclic antidepressants, anticonvulsants, and muscle relaxants. Therefore, here we tested if some of these drugs could alleviate the thermal hyperalgesia and mechanical allodynia induced by RLED. The doses of the medications used were chosen based on previously published data in animals and the oral route of administration was selected because it is the most common route used for these medications in clinical practice 7, 21, 34, 39, 51. The anticonvulsant/analgesic gabapentin (100 mg/kg given orally), completely reversed thermal hyperalgesia and mechanical allodynia within 45 min post administration, an effect that lasted for 2 hours (Fig. 7). The tricyclic antidepressants amitriptyline (100 mg/kg given orally), also completely reversed thermal hyperalgesia and mechanical allodynia within 45 min post administration, an effect that lasted for 2 hours (Fig. 7). The serotonin/norepinephrine reuptake inhibitor duloxetine (30 mg/ kg given orally) reversed thermal hyperalgesia at 45 minutes after administration but full reversal for mechanical allodynia to the paw and shoulder was observed only at 2 hours post administration (Fig. 7). Acetaminophen (300 mg/kg given orally) failed to reverse the nociceptive effects of RLED (Fig. 7). All vehicle controls had no effect on RLED-induced thermal and mechanical hypersensitivities (n=5–8).
Figure 7. RLED exposure-induced thermal hyperalgesia and mechanical allodynia can be reversed by typical treatments for widespread pain.
Rats were exposed to RLED for 3 hrs daily for 5 days at 50 Lux and received oral administration of gabapentin (100 mg/kg), amitriptyline (100 mg/kg), duloxetine (30 mg/kg), acetaminophen (300 mg/kg), or control. (A) Paw withdrawal latencies (measured via Hargreaves test) were significantly increased in rats that received gabapentin, amitriptyline, and duloxetine, but not acetaminophen or control (*p<0.05 versus control, two-way ANOVA with Tukey’s post hoc analysis, n=5–8). (B) Paw withdrawal thresholds (measured via stimulation with von Frey filaments) and (C) withdrawal thresholds (measured via shoulder stimulation with von Frey filaments) were significantly increased in rats that received gabapentin, amitriptyline, and duloxetine, but not acetaminophen or control (*p<0.05 versus control, two-way ANOVA with Tukey’s post hoc analysis, n=5–8).
Role of the rostral ventromedial medulla in mediating RLED nociceptive response
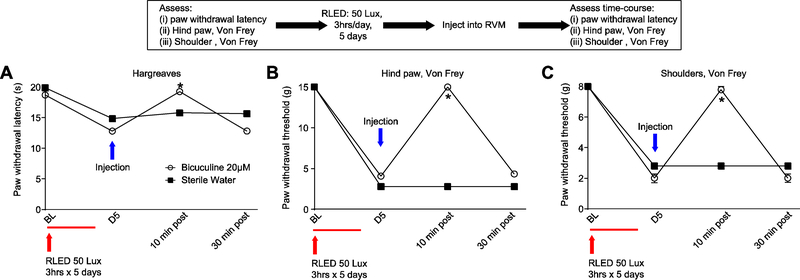
It is unclear how RLED can elicit a wide-spread nociceptive response. A possible mechanism might require engagement of descending pain modulatory pathways. Neurons within the rostral ventromedial medulla (RVM) are known to project to the spinal or medullary dorsal horns to directly or indirectly enhance or diminish nociceptive traffic 73. We examined the possible contribution of the RVM in the RLED-induced nociceptive response by microinjection of 20 μM bicuculline, a GABA A receptor antagonist 32. Rats were exposed to RLED for 3 hours daily for 5 days at 50 Lux. Following RLED exposure, one cohort of rats received injections of bicuculine 20 μM in a volume of 0.5 μL to the RVM, while another cohort received injections of sterile water at 0.5 μL to the RVM. RVM bicuculline reversed the RLED-induced thermal hyperalgesia and mechanical allodynia in rats at 10 minutes post administration (Fig. 8). The antinociceptive effect of bicuculline was not observed at 30 minutes post administration (n=8) (Fig. 8). All injection sites were verified post-hoc.
Figure 8. GABAergic signaling in the rostral ventromedial medulla (RVM) underlies RLED-induced thermal hyperalgesia and mechanical allodynia.
Rats were exposed to RLED for 3 hrs daily for 5 days at 50 Lux. Following RLED exposure, one cohort of rats received injections of bicuculine 20 μM at 0.5 μL to the RVM, while another cohort received injections of sterile water at 0.5 μL to the RVM. (A) Paw withdrawal latencies (measured via Hargreaves test) were significantly increased in bicuculine-injected rats compared to sterile water-injected counterparts (*p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=4–8) (B) Paw withdrawal thresholds (measured via stimulation with von Frey filaments) and (C) withdrawal thresholds (measured via shoulder stimulation with von Frey filaments) were significantly increased in bicuculine-injected rats compared to sterile water-injected counterparts (*p<0.05, two-way ANOVA with Tukey’s post hoc analysis, n=4–8).
Measuring corticosterone concentration in the plasma following RLED exposure
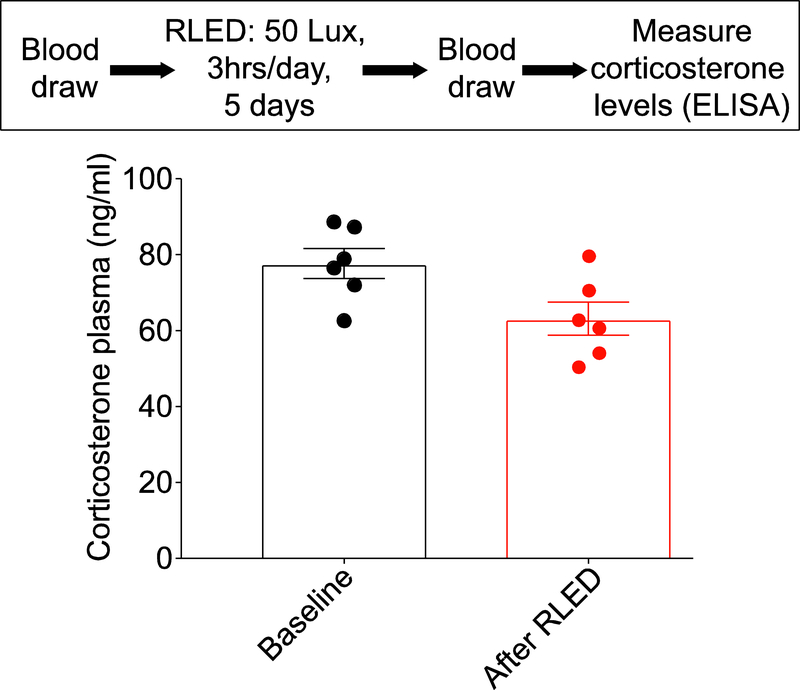
Rats may experience stress following exposure to certain stimuli. Stress may result in increased sensitivity to mechanical and thermal stimuli 43 Plasma corticosterone is considered a marker for stress. We measured the amount of serum corticosterone in male rats (n=6) before and after exposure to 50 Lux RLED for 3 hours a day for five days. There was no significant difference in the concentration of corticosterone. The baseline serum corticosterone was 78 ± 4 ng/ml and following exposure to RLED was 63 ± 4 ng/ml (Fig. 9).
Figure 9. Nociceptive effects of RLED are not stress induced.
Rats were exposed to RLED for 3 hrs daily for 5 days at 50 Lux. Following verification of thermal hyperalgesia and mechanical allodynia, blood samples were collected and analyzed for corticosterone using an ELISA. Plasma corticosterone levels were unchanged following RLED exposure (p>0.05, non-parametric Mann-Whitney test; n=6 per condition).
Replication study: reproducing key behavioral experimental findings with rigorous preclinical trial design
We performed a replication study as described above (see Methods and Fig. 10A) with RLED. This experiment was performed in accordance with the randomization, investigator-blinding, analysis and reporting matching the rigor of a clinical trial and with a higher threshold of significance of P < 0.01 as described for the confirmation study (see Methods). No data was excluded in the analysis. We exposed rats (n=8) to 50 Lux of RLED for 8 hours daily for five days and observed hypersensitivities (Fig. 10B, C, D) that matched those from experiments performed 16–18 months earlier (Fig. 2A, B, C). Rats randomly assigned to the white group (20 Lux, 8 hours a day exposure for 5 days) had no observed change to their baseline.
We also performed a separate replication study for green light (GLED). We tested the effects of GLED exposure in rats (n=8) for eight hours daily for five days at 4–5 Lux intensity. Notably, the antinociception induced by GLED exposure (Fig. 10E) in this experiment almost exactly matched the level of GLED-induced antinociception performed more than 3 years ago and published previously 36. Rats that were randomized to the white group (20 Lux, 8 hours a day exposure for 5 days) had no observed change to their baseline (Fig. 10E).
Discussion
There is an increased appreciation for the biological effects of different color of lights. We have developed and performed an initial characterization of the consequences of RLED exposure on sensory thresholds in male and female rats. We found that RLED produces thermal hyperalgesia and mechanical allodynia, reminiscent of generalized allodynia observed in patients with FPS. Our findings show that RLED-induced thermal hypersensitivity and mechanical allodynia requires input through the visual system and likely engages descending pain modulatory mechanisms. We noticed it takes several days to develop the thermal hyperalgesia and mechanical allodynia after RLED exposure. The RLED induced thermal hyperalgesia and mechanical allodynia could be reversed by medications used for managing medical conditions with widespread and localized pain syndromes suggesting potential applicability for evaluation of new molecular pain targets. Additionally, key behavioral findings were reproduced in a replication design with randomization, investigator-blinding, inclusion of all data and analysis and reporting matching the rigor of clinical trials.
Exposure to RLED was found to produce wide-spread allodynia that could be detected at multiple areas of the body in the absence of injury. Most patients with FPS do not report traumas prior to the development of their conditions 22. The injury-free model proposed here may have high translational relevance because the animals are not subjected to physical injuries. Some of the FPS conditions have no available laboratory tests to diagnose and are considered “diagnosis of exclusion”. Our RLED experiments captures multi-point pain in the absence of physical injury. The thermal hyperalgesia and mechanical allodynia associated with the RLED exposure were completely reversed when commonly used medications for FPS such as ibuprofen, amitriptyline, gabapentin, duloxetine were administered. It is worth noting that most patients with FPS experience partial, but not complete relief of pain with these medications. This may be due to other existing comorbidities. It may also be a limitation in the RLED model. At the dose tested, acetaminophen failed to reverse the thermal hyperalgesia, or the mechanical allodynia associated with RLED exposure. This is in agreement with clinical observations that acetaminophen has limited efficacy in the management of FPS 3, 6, 47, 50. Amitriptyline, gabapentin, and duloxetine, but not NSAIDs, are typically used clinically for managing FPS 16 and neuropathic pain 23.
As previous studies have demonstrated that stress may elicit thermal hyperalgesia and mechanical allodynia in rats under some conditions 38, 43, we measured serum corticosterone in rats before and after exposure to RLED. Plasma levels of corticosterone have been shown to correlate with stress in animals 37. We found no difference in serum corticosterone after exposure to RLED in male rats.
Our studies do not directly address the mechanism by which RLED induces thermal hyperalgesia and mechanical allodynia. However, we demonstrated the need for visual input for RLED to produce thermal hyperalgesia and mechanical allodynia. This was demonstrated when RLED exposure failed to produce nociception in the presence of dark contact lenses that prevented the RLED from interacting with the visual system. We previously conducted similar studies using GLED light that induced antinociception in rats. The effect of the GLED was also dependent upon activation of the visual system 36. While both mechanisms of RLED and GLED therapy involve the visual system, exactly how this occurs remains unknown. Neuronal connections exist between the visual system and areas of the nervous system that influence pain behavior. For example, retrograde labeling from the PAG demonstrated glutamate-like immunoreactivity projections from several regions of the brain including the occipital cortices 5. It is not clear what rats “see” when they are exposed to RLED. Rodents have different composition of rods and cones than humans 41. Color discrimination experiments in the 1930s established that albino rats showed a preference for greens and blues and manifested ability to discriminate between red and blue, red and green, blue and yellow, and red and yellow 28, 69, 70. Walton performed color discrimination in 18 children and noted that color preference was largely the same as in rats 69 A recent study identified a possible neuroanatomical substrate showing that, in rats, axons of retinal ganglion cells converge on hypothalamic neurons that project directly to nuclei in the brainstem and spinal cord that regulate parasympathetic and sympathetic functions 59. Therefore, it may be possible that activation of the retinal ganglion cells is responsible for the observed effects of RLED. Thus, rats may not need visual representation in the cortex to experience diffuse hypersensitivity from exposure to RLED. These findings provide a framework for conceptualizing how different wavelengths may have different biological responses mediated by the visual system.
Descending facilitatory pathways from the RVM are required for the development and maintenance of widespread muscle pain induced by acetic saline in rats suggesting central sensitization 68. Here, we administered bicuculline, a GABA A receptor antagonist, into the RVM and demonstrated a reversal of the thermal and mechanical hypersensitivity produced by RLED exposure in rats. The possible effects of RLED on pain facilitation and pain inhibition cells within the RVM remains to be determined, but these findings are suggestive of a shift in the balance towards net facilitation to promote the thermal hyperalgesia and mechanical allodynia observed. Bicuculline may have disinhibited pain inhibitory (presumably OFF cells) resulting in an analgesic effect. The engagement of descending facilitation is consistent with RLED promoting a sustained state of central sensitization. Our data demonstrated that the descending facilitatory pathways from the RVM are required for the maintenance of the RLED induced thermal hyperalgesia and mechanical allodynia; tonically active descending facilitation from the RVM may help drive signs of neuropathic pain 62.
Women are more afflicted by FBS than men 30, 49, 64, 71. We demonstrated that male and female rats exposed to RLED developed nociception. However, while no difference was observed in the magnitude of the thermal hyperalgesia or mechanical allodynia between male and female rats, female rats developed mechanical allodynia faster than males.
Our findings are notable in highlighting the effects of light on sensory thresholds. Together with our previous report demonstrating antinociceptive effect of green light exposure, we now show that red light produces thermal hyperalgesia and mechanical allodynia. Both of these observations were confirmed in a rigorous replication trial confirming the main biological observation. How light exposure influences sensory thresholds remains unknown but our observations are consistent with other reports that exposure to light produces changes in neurobiology. For example, blue light has been associated with sleep and metabolic regulations 10, 15, 48; light therapy has been used to control depression 20, 26, while bright light improved the mood of adolescents taking antidepressants compared to those without light therapy 57. Kilgore and colleagues also reported that blue light modulated the activation of the anterior cingulate gyrus, may facilitate structural and functional recovery following mild traumatic brain injury, and may improve memory functions in humans 1, 2, 4 Recently, a study comparing the treatment of actinic keratosis (AK) using red vs green light demonstrated that while both red and green light managed AK, green light exposure resulted in significantly less pain than red 61.
We note that our study has some limitations. First, it is possible that our randomization protocol induced social stress in the tested rats secondary to changing cagemates which may have influenced the results. However, the results obtained from the randomization experiment were very similar to the non-randomized experiments suggesting that social stress did not play a significant role in the observed behavior. Second, in the initial scouting experiments testing the effects of other colors (purple, yellow, and orange) we used only 4–5 Lux as this light intensity was proven to be antinociceptive for GLED and nociceptive for RLED. As we did not observe any effects of purple, yellow, and orange LED at 4–5 Lux on behaviors we did not study these colors any further. It remains a possibility that, at higher Lux intensities, these colors may have some biological effects. Overall, these studies provide inroads to further investigations of the biological consequences of exposure to light at different intensities and wavelengths in rodents and exploration of underlying mechanisms that may eventually allow translation into human therapies. The injury-free thermal hyperalgesia and mechanical allodynia resulting from RLED exposure may also offer an additional tool permitting evaluation of potential therapies relevant to FPS. An unexpected implication of our results is that the red light intensity commonly used in vivariums during the “dark cycle” studies intended to keep rats in “dark” conditions might be an experimental confound. These studies contribute to the increased appreciation for the biological effects of different colors of light in different fields of medicine.
Perspective.
This study demonstrates the effect of light exposure on nociceptive thresholds. These biological effects of red LED adds evidence to the emerging understanding of biological effects of light of different colors in animals and humans. Understanding the underlying biology of red light-induced wide spread pain may offer insights into functional pain states.
Highlights.
Different wavelengths of light elicit different biological responses
Rats exposed to red light develop diffuse nociception, an injury-free pain model
Nociception was reversed by medications commonly used clinically for chronic pain
Acknowledgments
Research funding
This work was supported, in part, by the National Center for Complementary and Alternative Health (R01AT009716,) (M.I. and R.K.), by the National Institute for Neurological Disorders and Stroke (1R01NS098772 to RK), a Career Development Award from the NINDS (A.P.), and a Children’s Tumor Foundation NF1 Synodos grant (to R.K.), and start-up seed funds (to M.I. and R.K.). A.M. was partially supported by a Young Investigator Award from the Children’s Tumor Foundation.
Footnotes
Conflicts of interest
None of the authors declares any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alkozei A, Smith R, Dailey NS, Bajaj S, Killgore WDS. Acute exposure to blue wavelength light during memory consolidation improves verbal memory performance. PLoS One. 12:e0184884, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkozei A, Smith R, Killgore WD. Exposure to blue wavelength light modulates anterior cingulate cortex activation in response to ‘uncertain’ versus ‘certain’ anticipation of positive stimuli. Neurosci Lett. 616:5–10, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Arreola Ornelas H, Rosado Buzzo A, Garcia L, Dorantes Aguilar J, Contreras Hernandez I, Mould Quevedo JF. Cost-effectiveness analysis of pharmacologic treatment of fibromyalgia in Mexico. Reumatol Clin. 8:120–127, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Bajaj S, Vanuk JR, Smith R, Dailey NS, Killgore WDS. Blue-Light Therapy following Mild Traumatic Brain Injury: Effects on White Matter Water Diffusion in the Brain. Front Neurol. 8:616, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beitz AJ. Possible origin of glutamatergic projections to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain research bulletin. 23:25–35, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 8:27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 48:252–263, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bourke JH, Langford RM, White PD. The common link between functional somatic syndromes may be central sensitisation. J Psychosom Res. 78:228–236, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 289:G42–53, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 21:6405–6412, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carette S, Bell MJ, Reynolds WJ, Haraoui B, McCain GA, Bykerk VP, Edworthy SM, Baron M, Koehler BE, Fam AG, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia. A randomized, double-blind clinical trial. Arthritis Rheum. 37:32–40, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Carette S, McCain GA, Bell DA, Fam AG. Evaluation of amitriptyline in primary fibrositis. A doubleblind, placebo-controlled study. Arthritis Rheum. 29:655–659, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Chang L Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol Clin North Am. 34:271–279, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Cheung IN, Zee PC, Shalman D, Malkani RG, Kang J, Reid KJ. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLoS One. 11:e0155601,2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 311:1547–1555, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Clouse RE, Lustman PJ, Geisman RA, Alpers DH. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 8:409–416, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Correa JB, Costa LO, de Oliveira NT, Sluka KA, Liebano RE. Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: a case-control study. Experimental brain research. Experimented Hirnforschung. Experimentation cerebrale 233:2391–2399,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, Dodick DW, Vanderah TW, Dussor G, Porreca F. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Annals of neurology. 67:325–337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 55:883–889, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 112:83–93, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ferrari R A prospective study of the 1-year incidence of fibromyalgia after acute whiplash injury. RMD Open. 1:e000007, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet neurology. 14:162–173, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giusto LL, Zahner PM, Shoskes DA. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother. 19:1097–1108, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Glickman-Simon R, Ehrlich A. Omega-3 supplementation and cardiovascular disease, acupuncture and chronic obstructive pulmonary disease (COPD), myofascial physical therapy and interstitial cystitis, and yoga and chronic pain. Explore (NY). 9:54–57, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. The American journal of psychiatry. 162:656–662, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg DL, Felson DT, Dinerman H. A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis Rheum. 29:1371–1377, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Graham CH, Riggs LA. The Visibility Curve of the White Rat as Determined by the Electrical Retinal Response to Lights of Different Wave-Lengths. Journal of General Psychology. 12:275–295, 1935 [Google Scholar]
- 29.Gur A Physical therapy modalities in management of fibromyalgia. CurrPharm Des. 12:29–35, 2006 [PubMed] [Google Scholar]
- 30.Hanno P, Nordling J, van Ophoven A. What is new in bladder pain syndrome/interstitial cystitis? Curr Opin Urol. 18:353–358, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 32:77–88, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Heinricher MM, Haws CM, Fields HL. Evidence for GABA-mediated control of putative nociceptive modulating neurons in the rostral ventromedial medulla: iontophoresis of bicuculline eliminates the off-cell pause. Somatosens Mot Res. 8:215–225, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Hoggan RN, Subhash A, Blair S, Digre KB, Baggaley SK, Gordon J, Brennan KC, Warner JE, Crum AV, Katz BJ. Thin-film optical notch filter spectacle coatings for the treatment of migraine and photophobia. J Clin Neurosci. 28:71–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, Fontana DJ. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol. 324:153–160, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Hwang MH, Shin JH, Kim KS, Yoo CM, Jo GE, Kim JH, Choi H. Low Level Light Therapy Modulates Inflammatory Mediators Secreted by Human Annulus Fibrosus Cells during Intervertebral Disc Degeneration In Vitro. Photochemistry and photobiology. 91:403–410, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim MM, Patwardhan A, Gilbraith KB, Moutal A, Yang X, Chew LA, Largent-Milnes T, Malan TP, Vanderah TW, Porreca F, Khanna R. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 158:347–360, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa M, Hara C, Ohdo S, Ogawa N. Plasma corticosterone response of rats with sociopsychological stress in the communication box. Physiology & behavior. 52:475–480, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Itoga CA, Roltsch Hellard EA, Whitaker AM, Lu YL, Schreiber AL, Baynes BB, Baiamonte BA, Richardson HN, Gilpin NW. Traumatic Stress Promotes Hyperalgesia via Corticotropin-Releasing Factor-1 Receptor (CRFR1) Signaling in Central Amygdala. Neuropsychopharmacology. 41:2463–2472, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 311:576–584, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 108:65–72, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Jacobs GH. Evolution of colour vision in mammals. Philos Trans R Soc Lond B Biol Sci. 364:2957–2967, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 133:136–147, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Korczeniewska OA, Khan J, Tao Y, Eliav E, Benoliel R. Effects of Sex and Stress on Trigeminal Neuropathic Pain-Like Behavior in Rats. J Oral Facial Pain Headache. 31:381–397, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 70:41–51, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 13:189–196, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 13:936–944, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Locke GR 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol. 95:157–165, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 88:4502–4505, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 107:991–1000, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Meeus M, Ickmans K, Struyf F, Hermans L, Van Noesel K, Oderkerk J, Declerck LS, Moorkens G, Hans G, Grosemans S, Nijs J. Does acetaminophen activate endogenous pain inhibition in chronic fatigue syndrome/fibromyalgia and rheumatoid arthritis? A double-blind randomized controlled cross-over trial. Pain Physician. 16:E61–70, 2013 [PubMed] [Google Scholar]
- 51.Millecamps M, Etienne M, Jourdan D, Eschalier A, Ardid D. Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain. 109:214–224, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Min PK, Goo BL. 830 nm light-emitting diode low level light therapy (LED-LLLT) enhances wound healing: a preliminary study. Laser therapy. 22:43–49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mogil JS, Macleod MR. No publication without confirmation. Nature. 542:409–411, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E, Weissman-Fogel I. Waning of “conditioned pain modulation”: a novel expression of subtle pronociception in migraine. Headache. 53:1104–1115, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Nation KM, Dodick DW, Navratilova E, Porreca F. Sustained exposure to acute migraine medications combined with repeated noxious stimulation dysregulates descending pain modulatory circuits: Relevance to medication overuse headache. Cephalalgia.333102418804157, 2018 [DOI] [PubMed] [Google Scholar]
- 56.Ness TJ, Lloyd LK, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol. 191:364–370, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niederhofer H, von Klitzing K. Bright light treatment as add-on therapy for depression in 28 adolescents: a randomized trial. Prim Care Companion CNS Disord. 13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, Hovaguimian A, Cestari DM, Saavedra-Walker R, Borsook D, Doran BL, Buettner C, Burstein R. Migraine photophobia originating in cone-driven retinal pathways. Brain. 139:1971–1986, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noseda R, Lee AJ, Nir RR, Bernstein CA, Kainz VM, Bertisch SM, Buettner C, Borsook D, Burstein R. Neural mechanism for hypothalamic-mediated autonomic responses to light during migraine. Proceedings of the National Academy of Sciences of the United States of America. 114:E5683–E5692, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oono Y, Wang K, Baad-Hansen L, Futarmal S, Kohase H, Svensson P, Arendt-Nielsen L. Conditioned pain modulation in temporomandibular disorders (TMD) pain patients. Exp Brain Res. 232:3111–3119, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Osiecka BJ, Nockowski P, Szepietowski JC. Treatment of Actinic Keratosis with Photodynamic Therapy Using Red or Green Light: A Comparative Study. Acta Derm Venereol. 98:689–693, 2018 [DOI] [PubMed] [Google Scholar]
- 62.Ossipov MH, Lai J, Malan TP Jr., Porreca F Spinal and supraspinal mechanisms of neuropathic pain. Annals of the New York Academy of Sciences. 909:12–24, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Pang R, Ali A. The Chinese approach to complementary and alternative medicine treatment for interstitial cystitis/bladder pain syndrome. Transl Androl Urol. 4:653–661, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratner V, Perilli L. Interstitial cystitis: an updated overview. Urol Nurs. 23:107–110; quiz 111,2003 [PubMed] [Google Scholar]
- 65.Ripoll E, Mahowald D. Hatha Yoga therapy management of urologic disorders. World J Urol. 20:306–309, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Sheridan DMLaCL. Assessment of the contact eye cover as an effective method of restricting visual input. Bahavior Research Methods & Instrumentation. 10:376–388, 1978 [Google Scholar]
- 67.Staud R Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 12:577–585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 136:331–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walton WE. Color vision and color preference in the albino rat. II. The experiments and Results. Comparative Psychology. 15:373–394, 1933 [Google Scholar]
- 70.Walton WE, Bornemeier RW. Further evidence of color discrimination in rodents. Journal of Genetic Psychology. 52:165–181, 1938 [Google Scholar]
- 71.Wolfe F, Walitt B, Perrot S, Rasker JJ, Hauser W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS One. 13:e0203755, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F. Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia. 37:780–794, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han BX, Zhou X, Wang F. Identifying local and descending inputs for primary sensory neurons. The Journal of clinical investigation. 125:3782–3794, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]