Abstract
Standardized terminology is critical to providing consistent reports to referring clinicians. This lexicon aims to provide a reference for terminology frequently used in rectal cancer and reflects the consensus of the Society of Abdominal Radiology Disease Focused Panel in Rectal cancer. This lexicon divided the terms into the following categories: primary tumor staging, nodal staging, treatment response, anal canal anatomy, general anatomy, and treatments.
Introduction
Use of standardized terminology by radiologists is critical to providing relevant reports to referring clinicians. In rectal cancer, complex disease-specific terminology may cause confusion and lead to unclear descriptions in radiology reports. This guide aims to provide a reference for terminology/lexicon frequently used in rectal cancer, in order to encourage consistent reporting amongst radiologists. The document reflects the consensus of the Society of Abdominal Radiology Disease-Focused Panel in Rectal cancer. Commonly used lexicon has been divided into the following categories: 1) Primary tumor staging, 2) Nodal staging, 3) Treatment respons, 4) Anal canal anatomy, 5) General anatomy, and 6) Treatment.
Primary Tumor Staging
MRI is the preferred imaging modality for rectal cancer staging, primarily due to its ability to delineate the extent of the primary tumor. T-category depends on the presence of tumor extending beyond the muscularis propria and involvement of the circumferential resection margin (CRM) (Figure 1). Below we define terms used in the staging and description of the primary tumor:
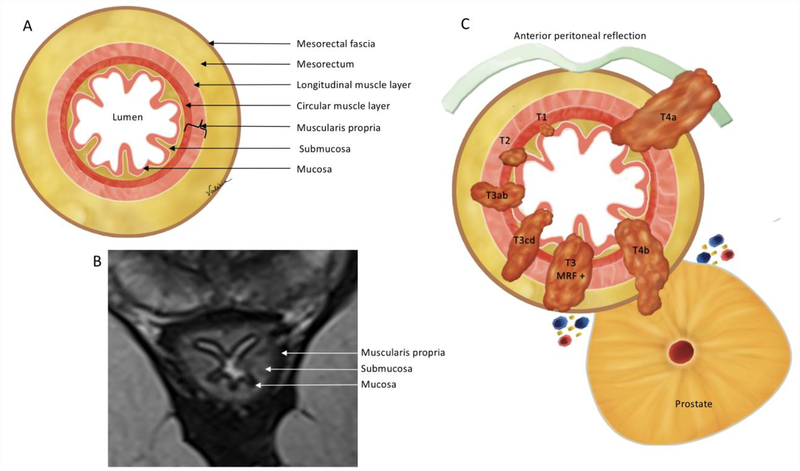
Figure 1:
Oblique axial view anatomy. (A) Illustration and (B) high-resolution axial oblique T2WI demonstrate the mesorectal fascia, mesorectum and rectal wall layers. On MRI (B) 3 layers are mostly visible: mucosa, as the innermost thin hypointense layer, submucosa, as a middle hyperintense layer and muscularis propria, as the outer hypointense layer. (C) Illustration demonstrating the T-category. T1 - tumor up to submucosa; T2 - tumor up to muscularis propria; T3 - tumor beyond muscularis propria (T3a < 1 mm, T3b: 1–5 mm, T3c: 5–15 mm, and T4d > 15 mm); T4 - tumor infiltrates the peritoneal reflection (T4a) or other pelvic organs and structures (T4b).
T-staging: For MRI, Tx - primary tumor cannot be assessed; T0 - no visible primary tumor; T1 - tumor extends to involve the submucosa; T2 - tumor extends to involve the muscularis propria; T3 - tumor extends beyond muscularis propria to involve the mesorectal fat (T3 tumors are substaged as follows: T3a < 1 mm; T3b: 1–5 mm; T3c: 5–15 mm; and T4d > 15 mm); T4 - tumor infiltrates/invades the peritoneal reflection (T4a) or other pelvic organs and structures (T4b) (1). MRI frequently cannot distinguish between T1 and T2 tumors.
Early rectal cancer: is defined as a primary tumor that is confined by the muscularis propria (Tis, T1 or T2) and without evidence of locoregional lymph nodes (N0) or metastatic disease (M0). These patients may be treated surgically without neoadjuvant chemoradiation. Some T1 lesions may be treated with transanal excision.
Circumferential resection margin (CRM): is the surgically dissected surface of the nonperitonealized rectum and in a properly performed total mesorectal excision (TME), is defined by the mesorectal fascia (2,3).
CRM status: depends on the shortest distance between the outermost part of the rectal tumor and the CRM, which is defined by the mesorectal fascia (4). The CRM is considered positive if the tumor is within 1 mm, and threatened if between 1–2 mm from the CRM. The levator ani defines the CRM inferiorly, therefore for low rectal tumors, tumors contacting or located within 1 mm of the levator ani are considered to involve the CRM.
Extramural depth of invasion: describes the depth of invasion of the tumor beyond the rectal wall and into the mesorectal fat, and is defined as the maximum distance from the outer edge of the muscularis propria to the outer edge of the primary tumor. EMD is used to determine the substage of T3 tumors (5).
Extramural vascular invasion (EMVI): is the extension of tumor within the vessels of the mesorectum (6,7). The MRI features are vessel wall irregularity, focal enlargement, and/or signal intensity of the tumor within the vessel. EMVI can be contiguous or discontiguous with the primary tumor. EMVI should be used to define the T-stage, including T3 substage, if contiguous with the primary tumor.
A number of anatomic structures are relevant for determining the T-stage of rectal cancer. An understanding of and the ability to recognize these structures is critical for accurately interpreting rectal MRI.
Anterior peritoneal reflection: is a thin layer of peritoneum attaching to the anterior wall of the middle rectum; it separates the intra- and extraperitoneal portions of the rectum (8). In the axial plane, the anterior peritoneal reflection typically has a V-shape or gull-wing appearance. In the sagittal plane, it extends from the top of the seminal vesicles in men, or from the level of uterocervical junction in women. Tumors above the peritoneal reflection drain superiorly through the superior rectal and inferior mesenteric nodes, while those below may drain through the internal iliac and obturator nodes (9).
Muscularis propria: is the T2 hypointense outer muscular layer of the rectum. Although it is comprised of both the inner circular and outer longitudinal muscular layers of the rectum, on MRI it is seen as a single layer outside of the more hyperintense submucosal layer.
Mesorectal fascia: is a thin T2 hypointense fascial layer that surrounds the extraperitoneal portion of the rectum and associated mesorectal fat and it defines the surgical plane of a total mesorectal excision. It fuses anteriorly with the Denonvilliers’ fascia and posteriorly with the presacral fascia. Inferiorly, the mesorectal fascia tapers into the intersphincteric space. Superiorly, it ends at the anterior peritoneal reflection. The mesorectal fascia envelops the lower rectum, covers the lateral and posterior aspects of the middle rectum, and surrounds the upper rectum posteriorly.
Additionally, there are a number of terms that are used to describe the appearance of the primary tumor. These findings do not directly impact the stage of the tumor, but are important to use consistently.
Annular / circumferential: is a morphological description of tumor, where the tumor extends around the entire circumference of the rectal lumen.
Mucinous rectal cancer: is a form of colorectal cancer characterized by abundant mucin within the stroma of the tumor. Mucinous tumors have fluid signal intensity on T2WI at initial staging (10). It is defined on pathology as having greater than 50% mucin within the tumoral tissue. Mucin must be visible within the tumoral tissue, and not simply in the lumen.
Nonmucinous rectal cancer: are rectal adenocarcinomas that demonstrate intermediate signal intensity on T2WI, and constitute the majority of rectal adenocarcinomas (11).
Polypoid: is a morphological description of tumor which may have a pedicle or stalk with obvious vessels. Tumor tissue extending from the stalk may protrude into the rectal lumen. Polyps may also be sessile in configuration.
Semiannular / semicircumferential: is a morphological description of tumor that only partially surrounds the rectal lumen.
Ulcerated: is an erosion frequently seen in the center of the tumor, and it is often the location of transmural extension. The ulceration may however be located anywhere in the lesion.
Nodal Staging
Nodal staging is one of the most important aspects of rectal cancer staging. Lymph nodes are divided into locoregional nodes and non-locoregional nodes as described below (Figure 2). In general, locoregional nodes are those that are routinely removed at time of surgical excision. The presence of suspicious locoregional nodes indicates that pre-operative chemoradiation should be performed. The presence of suspicious non-locoregional nodes impacts the decision of whether to do extended surgical resection.
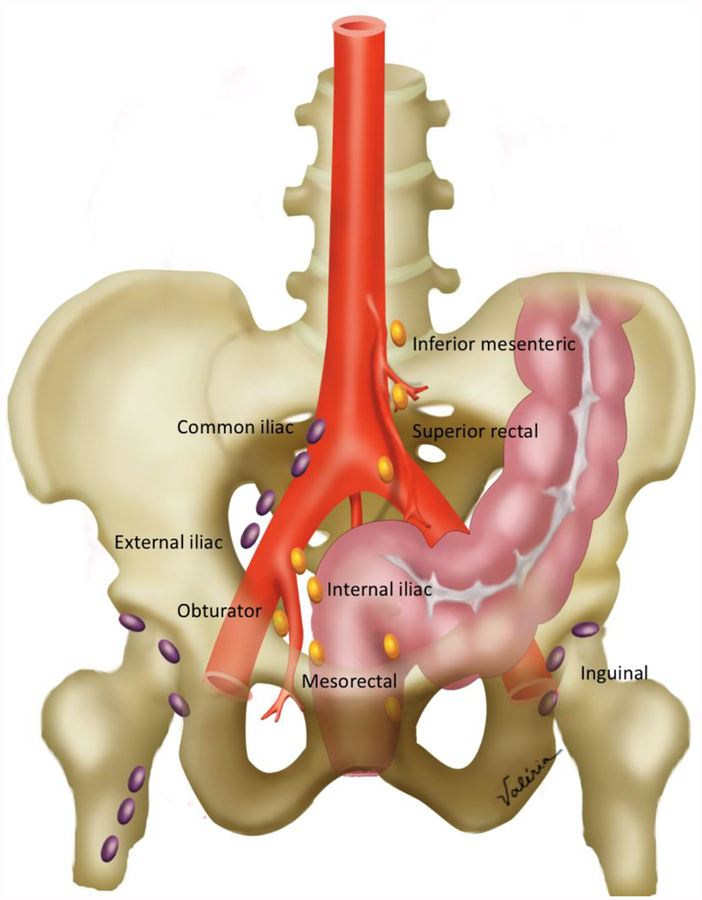
Figure 2:
Pelvic nodal anatomy. Locoregional nodes (colored in gold) include the inferior mesenteric, superior rectal, internal iliac, obturator and mesorectal nodes. Non-locoregional nodes (colored in purple) include common iliac, external iliac and inguinal nodes. Obturator nodes are lateral to the internal iliac artery, while internal iliac nodes are medial.
Locoregional lymph nodes: mesorectal, inferior mesenteric and superior rectal nodes, internal iliac and obturator lymph nodes are considered locoregional lymph nodes in the setting of rectal cancer (12). For locoregional lymph nodes at initial staging, the most common definition for suspicious nodes, uses both morphological and size (in the short-axis) criteria. These are the Dutch Criteria, which have been adopted by the SAR Rectal & Anal Cancer Disease-Focused Panel (13,14). The malignant morphological criteria are as following: (a) irregular borders, (b) heterogeneous signal intensity, and (c) round shape. Lymph nodes <5 mm are considered suspicious if the 3 morphological criteria are present. Lymph nodes between 5–9 mm need two morphological criteria to be suspicious. Finally, lymph nodes >9 mm are suspicious regardless the presence of morphological criteria. On restaging after neoadjuvant (chemo)radiation therapy, all nodes measuring greater than 5 mm should be mentioned.
Non-locoregional / distant lymph nodes: all other lymph nodes, including inguinal, external iliac, common iliac, and paraaortic nodes are considered non-locoregional / distant lymph nodes (metastatic disease, M1) in the setting of rectal cancer. Non-locoregional lymph nodes are considered suspicious if they measure more than 10 mm in the short-axis.
N-staging: N0 – no abnormal locoregional lymph nodes; N1 – between 1 and 3 abnormal locoregional lymph nodes (N1a – 1 lymph node, N1b – 2–3 lymph nodes, and N1c – tumor deposit); N2 – greater than 3 abnormal locoregional lymph nodes (N2a – 4–6 lymph nodes, and N2b – 7 or more lymph nodes). It is acceptable to use “N+” for abnormal locoregional lymph nodes on MRI regardless the number of them and “N-” for no abnormal locoregional lymph nodes on MRI.
Heterogeneous: used to describe lymph nodes with internal components or elements of varying signal intensities. Mesorectal lymph nodes with a heterogeneous pattern are considered suspicious for tumor involvement.
Irregular border: describes a lymph node with a spiculated margin as demonstrated by spikes or points on the surface (15). Mesorectal lymph nodes that demonstrate an irregular border are considered suspicious for tumor involvement.
Deposit: is used to describe extra-nodal sites of metastases. Tumor deposit is defined as a nodule with irregular contour within the mesorectum. Deposit is a pathologic term, and is defined as foci of tumor without evidence of residual lymph node tissue (16). The origins of tumor deposit may be due to discontinuous tumor spread, lymphatic spread, venous invasion, or a totally replaced lymph node, and may not be differentiated from a metastatic lymph node on imaging.
The location of lymph nodes is critically important in determining whether or not nodes are locoregional, but also in alerting the surgeon to the possible need for a lateral pelvic side-wall dissection in the case of suspicious obturator or internal iliac nodes. Below we define the common locations used in rectal cancer imaging reports:
External iliac lymph nodes: are considered non-locoregional lymph nodes in rectal cancer. They are situated along the external iliac artery and vein, caudal to the common iliac bifurcation and cranial to the inguinal ligament (1,12). Although not considered locoregional, they may be included in a lateral node dissection. Their involvement is extremely unusual.
Inferior mesenteric and superior rectal nodes: are considered locoregional nodes and are removed during a TME. Lymph nodes are defined as inferior mesenteric or superior rectal based upon the vessel next to which they lay. The inferior mesenteric artery becomes the superior rectal artery at the origin of the left colic artery. The radiologist should comment upon the superior most suspicious lymph node along these chains as it may alter the site of ligation during the TME (total mesorectal excision). Inferior mesenteric lymph nodes should not be mistaken for retroperitoneal (non-locoregional, M1) lymph nodes.
Inguinal lymph nodes: are considered non-locoregional nodes in rectal cancer and are situated in the groin below the inguinal ligament. They are more commonly involved in low rectal cancers. For anal cancers, inguinal lymph nodes are considered locoregional. They can be divided into: superficial (anterior to the saphenous vein and superficial femoral vessels) and deep (medial to the femoral vessels) (12,17,18).
Internal iliac lymph nodes: are considered locoregional and lateral pelvic sidewall nodes in rectal cancer, and are included in a lateral node dissection. They are situated along the course of the internal iliac vessels (12). At the level of the obturator muscle, nodes medial to the internal iliac artery are considered internal iliac nodes (14), while the ones lateral to the internal iliac artery are deemed obturator lymph nodes.
Mesorectal lymph nodes: are the lymph nodes located within the mesorectum and are considered locoregional nodes.
Obturator lymph nodes: are considered locoregional lymph nodes and lateral pelvic sidewall nodes. They are situated between the external and internal iliac arteries just medial to the pelvic side wall musculature (obturator internus). Obturator nodes occur along the course of the obturator artery, lateral to the internal iliac artery.
Pelvic side wall lymph nodes: are nodes located lateral to the mesorectal fascia including internal iliac and obturator nodes. Considering the anatomic overlap, it is preferable to use the more specific nodal station descriptor when feasible.
Evaluation of treatment response
There are a number of terms used in describing treatment response with rectal cancer. Currently MRI has poor sensitivity to detect remaining tumor after chemoradiation.
Complete response: is defined as no residual tumor left after neoadjuvant chemoradiotherapy. This may be seen as either fibrosis in the rectal wall at the site of the treated tumor with no residual tumor cells or as a return to normal rectal wall. Complete clinical response (cCR) refers to the absence of clinically detectable tumor, including on digital rectal exam, MRI, and endoscopic ultrasound, and is used as a surrogate for pathologic complete response (pCR). pCR is defined as the absence of viable tumor cells at pathology. On restaging MRI, very low signal intensity within the treated tumor correlates with the presence of post treatment fibrosis. Lack of restricted diffusion in the tumor bed and very low (fibrotic) signal within the tumor bed with a lack of residual intermediate tumor signal intensity or the return of the appearance of normal bowel wall layers are MRI findings that best correlate, albeit imperfectly, with a pCR.
Partial response: is defined as a decrease in the size of the primary tumor without complete resolution. The rectal mass is composed of both fibrosis and viable tumor.
Desmoplastic reaction: T2-hypointense fibroinflammatory strands or spiculations extending from the rectal tumor into the mesorectum (19,20). May be seen at initial staging or following neoadjuvant therapy, and can be difficult to distinguish from tumor infiltration in some cases.
Fibrosis / Scar: refers to the T2-hypointense appearance of the treated primary rectal tumor at restaging following neoadjuvant (chemo)radiation therapy (19,20). May also include fine hypointense spiculations into the perirectal fat.
Submucosal edema: is an area of high signal intensity on T2WI within the rectal wall adjacent to the treated tumor after chemoradiotherapyand should not be misinterpreted as tumor (21).
Acellular mucin: A pathological term indicating mucin pools lacking viable tumor cells. Acellular mucin is a type of treatment response seen in non-mucinous tumors. Mucinous tumors may also maintain their mucinous appearance after treatment but later be found to contain no malignant cells. MRI cannot distinguish acellular from cellular mucin (22).
Mucinous / colloid degeneration: describes T2 hyperintensity that develops within a nonmucinous tumor after neoadjuvant (chemo)radiation therapy (23). It is considered a form of treatment response after neoadjuvant (chemo)radiation therapy.
Anal canal terminology
For tumors that originate in the anal canal or extend inferiorly to involve the anal canal, describing the tumor’s relationship to the components of the anal canal is important for surgical planning (Figure 3).
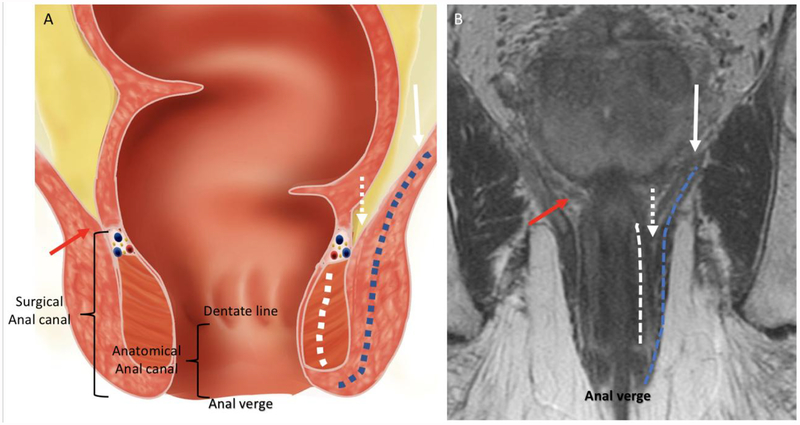
Figure 3:
Coronal anatomy of the anal canal. (A) Illustration and (B) coronal T2WI showing the levator ani (white arrow), internal sphincter (dashed white line), external sphincter complex (dashed blue line), intersphincteric plane (dashed white arrows), anorectal junction (red arrows), anal verge, dentate line, anatomical anal canal and surgical anal canal.
Anal canal: is the caudal part of the gastrointestinal tract and measures between 3–5 cm. There are 2 definitions of anal canal, anatomical and surgical. The anatomical anal canal extends from the dentate line to the anal verge, while the surgical anal canal extends from the anorectal ring to the anal verge (24).
Anal verge: is the distal end of the anal canal, where the squamous mucosa of the anal canal combines with the perianal skin, without hair follicles (25). On MRI, it is the distal part of the sphincter complex easier assessed in the sagittal and coronal planes.
Anal margin: is the skin within a radius of 5 cm from the anal verge, with hair follicles (24).
Anorectal junction: is the junction between the anal canal and the rectum. The top of the puborectalis muscle corresponds to the anorectal ring, and defines the level of the anorectal junction (26).
Anorectal ring: is a muscular ring formed by the upper margin of the puborectalis muscle and upper border of the internal anal sphincter (27). It is the ring that the surgeon feels when doing a rectal exam. It is synonymous with anorectal junction.
Dentate / pectinate line: divides the upper two thirds (lined by columnar epithelium) and lower third (lined by squamous epithelium) of the anal canal (28,29). It is the upper limit of the anatomical anal canal and it is not visible on MRI. Tumors above dentate line drain through mesorectal nodes, while the ones below this line drain to inguinal and external iliac nodes.
External anal sphincter: is composed predominantly by the levator ani and external sphincter muscle (skeletal muscle) and it is located lateral to the internal sphincter (30). It is responsible for voluntary continence.
Internal anal sphincter: is a continuation of the inner circular muscular layer of the rectum (smooth muscle) in the anus (24). It is responsible for the resting tone. It has slightly higher signal on T2WI than the external sphincter and enhances more avidly.
Intersphincteric space: is a fatty space separating the internal and external anal sphincters, and is hyperintense on non-fat saturated T2 weighted images (31).
Levator ani: is a complex pelvic floor muscle which contributes to the pelvic diaphragm. It is composed of three parts including iliococcygeus, pubococcygeus, and puborectalis muscles (32). Tumors involving the levator ani are generally considered T4b disease, albeit this is not specifically stated by AJCC.
General anatomy terms
There are a number of terms that are relevant to rectal cancer (Figures 1 and 4). It is important to understand their definitions in or order to accurately describe the location and extent of tumors.
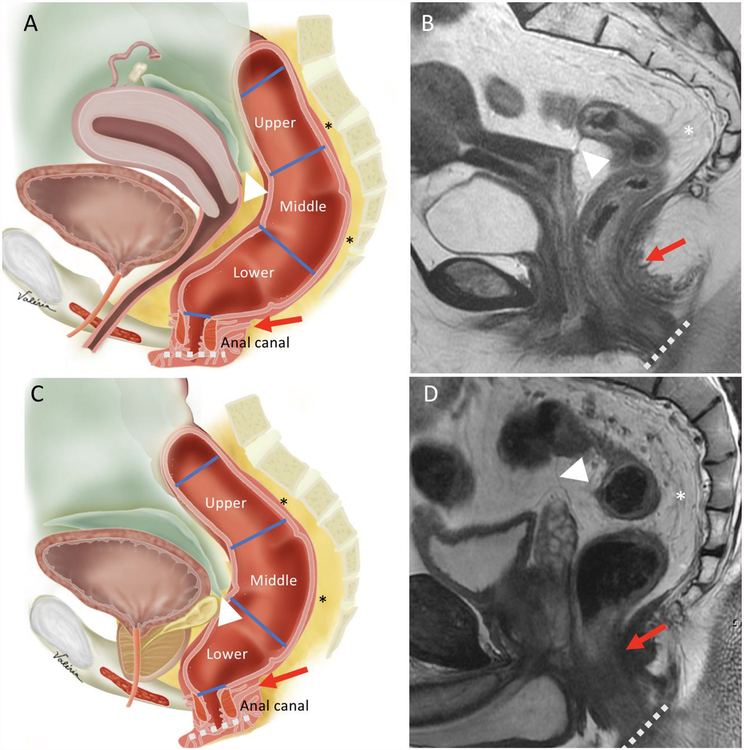
Figure 4:
Sagittal view anatomy. Illustrations and sagittal T2WI sequences in female (A-B) and male (C-D) demonstrate the upper, middle and lower rectum, mesorectum (asterisks), anal canal, anterior peritoneal reflection (white arrowheads), anorectal junction (red arrows) and anal verge (dashed lines).
Denonvilliers fascia / Rectoprostatic fascia / Rectovaginal fascia: is the anteroinferior component of the mesorectal fascia inferior to the anterior peritoneal reflection (11,33). In men, it lies between the mesorectum and prostate. In women, it is termed the rectovaginal fascia and lies between the vagina and the rectum. Injuries to nerves running in this fascia may lead to impotence. Preservation is surgically important.
Inguinal ligament: is the aponeurosis of the external oblique muscle and joins the anterior superior iliac spine to the pubic tubercle.
Lower rectum: is the caudal 5 cm of the rectum starting inferiorly from the anal verge. Lower rectal cancers require a detailed evaluation of the anal canal and sphincter complex.
Mesorectum: contains the perirectal tissue composed of fat, lymphatics, and blood vessels, and is enclosed by the mesorectal fascia (34). The mesorectum tapers caudally, being thinner in the lower rectum. Superiorly it ends at the peritoneal reflection (well-seen anteriorly, but inconspicuous posteriorly) and inferiorly tapers into the intersphincteric space.
Middle rectum: is the rectum between 5–10 cm superior to the anal verge.
Mucosa layer: is the innermost thin rectal layer, and is hypointense on T2WI when visualized (11).
Pre-sacral space: refers to space between the pre-sacral fascia and the sacrum (35).
Pre-sacral fascia: is the fascial layer that defines the posterior mesorectal fascia and divides the mesorectum from the pre-sacral space (36).
Puborectalis: is a U-shaped muscular sling that is part of the levator ani and contributes to the formation of external anal sphincter complex (9). The upper border of the puborectalis sling forms the upper edge of the surgical anal canal.
Rectovaginal fascia: is an anteroinferior component of the mesorectal fascia. It is located between the rectum and vagina.
Rectum: is defined for imaging purposes as the distal 15 cm of large bowel immediately superior the anal verge, based on the surgical definition (3). Anatomically the rectum extends only to the dentate line.
Rectosigmoid junction: is the transition between the rectum and the sigmoid, and is defined as being 15 cm above the anal verge (3). The exact location is debated, but can be considered at the sacral promontory (37).
Submucosa layer: is the middle rectal layer and is hyperintense on T2WI.
Upper rectum: is the rectum between 10–15 cm proximal to the anal verge, usually, superior to the anterior peritoneal reflection.
Miscellaneous terms
High resolution T2WI: is the cornerstone of rectal MRI. T2WI should be 3 mm or less thick and should be triplanar oriented along the axis of the rectum where the tumor is located (38). For example, “oblique axial” is placed perpendicular to the rectum where the tumor is located.
Parallel plane: refers to a plane that is along the axis of the rectal lumen. In the setting of rectal MRI, parallel plane is typically used to described an oblique coronal T2WI that is acquired parallel to the axis of the rectum at the level of the tumor.
Abut: is used to describe tumor that extends to and may contact a structure, without clear invasion/involvement.
M-staging: M0 – no distant metastasis, M1 – distant metastasis (M1a – confined to one organ, M1b – more than one organ, and M1c – peritoneum regardless of organ involvement). Involved non-locoregional lymph nodes (e.g. common iliac) are included as metastatic disease but still described in the nodal category as well.
Treatments for Rectal Cance
There are a number of treatments for rectal cancer, which go from minimally invasive such as transanal excision to extensive surgeries such as an abdominoperineal resection. It is important to understand the various surgical approaches in order to understand how the extent of the tumor impacts the selection of the surgical approach. Below we define a number of the existing surgical approaches for rectal cancer:
Abdominoperineal resection (APR) / abdominoperineal excision (APE): includes resection of the entire sphincter complex and requires a permanent colostomy. It is indicated for tumors that infiltrate the anal canal, levator ani / external sphincter or intersphincteric space, or in cases with poor preoperative continence or diarrheal disorders, which can result in postoperative incontinence.
Extralevator abdominoperineal excision (ELAPE): is a variation of the Standard APR with a broader dissection of the sphincter complex and levator ani resulting in a more cylindrical resection without the characteristic “waisting” that occurs at the level of the anorectal junction with standard APR (39).
Neoadjuvant chemoradiation: is a combination of chemotherapy and radiation therapy that is administered prior to definitive resection of the primary rectal cancer. The chemotherapy here is considered radiosensitizing (dose and schedule) rather than “systemic” (to address distant disease).
Intersphincteric abdominoperineal resection: is a sphincter sparing APR for low rectal cancer where only a portion of the internal anal sphincter is removed and the external anal sphincter is preserved (40,41). It is considered in selected patients when the intersphincteric space is not infiltrated by the tumor.
Locally advanced rectal cancer (LARC): is defined as a primary tumor that extends beyond the muscularis propria (T3/T4) and/or has locoregional lymph nodes (N1/2), i.e. stage II or stage III. There should be no evidence of metastatic disease (M0). In this setting, neoadjuvant (chemo)radiation therapy followed by surgical resection is the standard therapy in the United States.
Low anterior resection (LAR): is the most common transabdominal (can be laparoscopic or robotic) resection indicated for tumors located in the middle or upper rectum. This technique is characterized by a TME with resection of the sigmoid. There is a possibility of re-anastomosis and continence after surgery.
Pelvic exenteration: includes the radical en-bloc resection of all pelvic organs affected by cancer followed by pelvic reconstruction (42). It may be total or partial. Total pelvic exenteration involves resection of the reproductive organs, lower urinary tract, rectosigmoid colon, anal canal, and surrounding soft tissues. In anterior exenteration, the rectum and anal canal are spared from resection, while in posterior exenteration the urinary bladder and urethra are preserved.
Total mesorectal excision (TME): is characterized by an en bloc resection of the mesorectum through a dissection along the mesorectal fascia. This allows the surgeon to excise the entire rectum with surrounding vessels and lymph nodes. It is considered the standard transabdominal surgery indicated for curative treatment of rectal cancer.
Transanal excision (TAE): is a local excision surgery characterized by full thickness excision of the tumor down to the mesorectal fat, using direct view (43). There is a potential risk of residual tumor due to the limited degree of view of the rectum. Lymph nodes are not removed and are not pathologically staged. It may be considered for selected patients with early rectal cancer but lesions in the mid and upper rectum are usually inaccessible with this technique because of their distance from the anal verge.
Transanal endoscopic microsurgery (TEMS): is a local excision surgery characterized by full thickness excision of the tumor down to the mesorectal fat, using a rectoscope (43). This technique provides a higher degree of view of the rectal wall. It may be considered for selected patients with early rectal cancer.
Conclusion
We have developed a lexicon to use when reporting rectal cancer imaging studies. These definitions should increase the consistency of reports across readers and institutions. In particular, definitions for primary tumor staging and nodal staging are critical in surgical planning and consistent terminology is required for therapeutic planning.
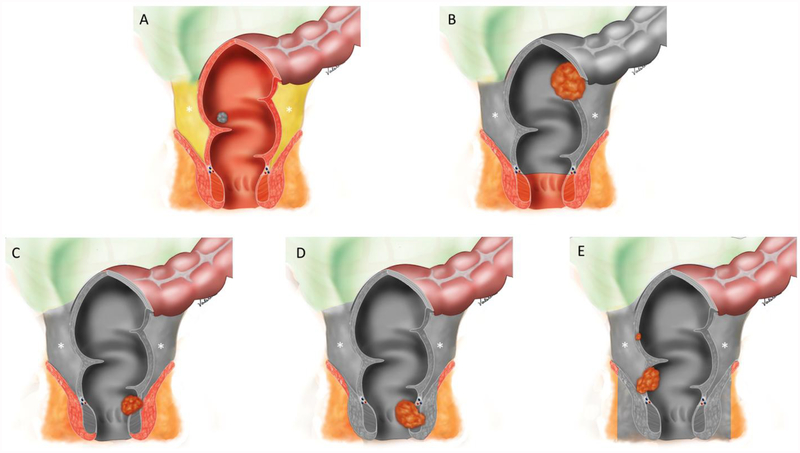
Figure 5:
Illustrations demonstrating surgical techniques for rectal cancer: (A) Transanal excision (TAE) / transanal endoscopic microsurgery (TEMS); (B) Low anterior resection (LAR); (C) Intersphincteric abdominoperineal resection; (D) Abdominoperineal resection (APR), and (E) Extralevator abdominoperineal excision (ELAPE). Gray coverage demonstrate the structures that are resected. Note that B-E include total mesorectal excision (TME) with an en bloc resection of the mesorectum (asterisks) through a dissection along the mesorectal fascia.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
References
- 1.Weiser MR. AJCC 8th Edition: Colorectal Cancer. Annals of surgical oncology. 2018;25:1454–1455. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Annals of surgical oncology. Springer-Verlag. 2012;19:2212–2223. [DOI] [PubMed] [Google Scholar]
- 3.Horvat N, Petkovska I, Gollub MJ. MR Imaging of Rectal Cancer. Radiol Clin North Am. 2018;56:751–774. [DOI] [PubMed] [Google Scholar]
- 4.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting Eur Radiol. Springer Berlin Heidelberg; 2013. pp. 2522–2531. [DOI] [PubMed] [Google Scholar]
- 5.MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132–139. [DOI] [PubMed] [Google Scholar]
- 6.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clinical Radiology. 2014;69:619–623. [DOI] [PubMed] [Google Scholar]
- 7.HORN A, DAHL O, MORILD I. Venous and Neural Invasion as Predictors of Recurrence in Rectal Adenocarcinoma. Dis Colon Rectum. 1991;34:798–804. [DOI] [PubMed] [Google Scholar]
- 8.Gollub MJ, Maas M, Weiser M, et al. Recognition of the anterior peritoneal reflection at rectal MRI. American Journal of Roentgenology. 2013;200:97–101. [DOI] [PubMed] [Google Scholar]
- 9.Kaur H, Choi H, You YN, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2012;32:389–409. [DOI] [PubMed] [Google Scholar]
- 10.Hugen N, Brown G, Glynne-Jones R, de Wilt JHW, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol. Nature Publishing Group. 2016;13:361–369. [DOI] [PubMed] [Google Scholar]
- 11.Brown G, Kirkham A, Williams GT, et al. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol. 2004;182:431–439. [DOI] [PubMed] [Google Scholar]
- 12.McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors:anatomic classification, characterization, and staging. Radiology. 2010;254:31–46. [DOI] [PubMed] [Google Scholar]
- 13.Kusters M, Marijnen CAM, van de Velde CJH, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–476. [DOI] [PubMed] [Google Scholar]
- 14.Ogura A, Konishi T, Cunningham C, et al. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019;37:33–43 http://ascopubs.org/doi/10.1200/JCO.18.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Beets GL, Kim M-J, Kessels AGH, Beets-Tan RGH. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78–83. [DOI] [PubMed] [Google Scholar]
- 16.Taylor FGM, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. American Journal of Roentgenology. 2008;191:1827–1835. [DOI] [PubMed] [Google Scholar]
- 17.Bontumasi N, Jacobson JA, Caoili E, Brandon C, Kim SM, Jamadar D. Inguinal lymph nodes: size, number, and other characteristics in asymptomatic patients by CT. Surg Radiol Anat. Springer Paris. 2014;36:1051–1055. [DOI] [PubMed] [Google Scholar]
- 18.Bhosale PR, Patnana M, Viswanathan C, Szklaruk J. The inguinal canal: anatomy and imaging features of common and uncommon masses. Radiographics. 2008;28:819–35–quiz913. [DOI] [PubMed] [Google Scholar]
- 19.Barbaro B, Vitale R, Leccisotti L, et al. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics. 2010;30:699–716. [DOI] [PubMed] [Google Scholar]
- 20.Klessen C, Rogalla P, Taupitz M. Local staging of rectal cancer: the current role of MRI. European radiology. Springer-Verlag. 2007;17:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aker M, Boone D, Chandramohan A, Sizer B, Motson R, Arulampalam T. Diagnostic accuracy of MRI in assessing tumor regression and identifying complete response in patients with locally advanced rectal cancer after neoadjuvant treatment. Abdominal Radiology. Springer US. 2018;43:3213–3219. [DOI] [PubMed] [Google Scholar]
- 22.Shia J, McManus M, Guillem JG, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2011;35:127–134. [DOI] [PubMed] [Google Scholar]
- 23.Rullier A, Laurent C, Vendrely V, Le Bail B, Bioulac-Sage P, Rullier E. Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma. Am J Surg Pathol. 2005;29:602–606. [DOI] [PubMed] [Google Scholar]
- 24.MORGAN CN, THOMPSON HR. Surgical anatomy of the anal canal with special reference to the surgical importance of the internal sphincter and conjoint longitudinal muscle. Ann R Coll Surg Engl. Royal College of Surgeons of England. 1956;19:88–114. [PMC free article] [PubMed] [Google Scholar]
- 25.Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? 2013;268:330–344. [DOI] [PubMed] [Google Scholar]
- 26.Shank B, Enker WE, Flam MS. Gross and Microscopic Anatomy Holland-Frei Cancer Medicine 6th edition Hamilton, ON: BC Decker; 2003https://www.ncbi.nlm.nih.gov/books/NBK13638/. [Google Scholar]
- 27.MILLIGAN E, MORGAN CN, JONES LE, OFFICER R. Classic Articles in Colonic Rectal Surgery - Surgical Anatomy of the Anal-Canal, and the Operative Treatment of Hemorrhoids (Reprinted From Lancet, Vol 2, Pg 1150–1213, 1934). Dis Colon Rectum. 1985;28:620–628. [PubMed] [Google Scholar]
- 28.Steele SR, Hull TL, Read TE, Saclarides TJ, Senagore AJ, Whitlow CB, editors. The ASCRS Textbook of Colon and Rectal Surgery. Cham: Springer International Publishing; 2016. [Google Scholar]
- 29.Tortora GJ, Derrickson BH. Introduction to the Human Body. John Wiley & Sons; 2011. [Google Scholar]
- 30.Agarwal S Anatomy of the Pelvic Floor and Anal Sphincters. JIMSA. 2012;25:1–3. [Google Scholar]
- 31.Durot C, Dohan A, Boudiaf M, Servois V, Soyer P, Hoeffel C. Cancer of the Anal Canal: Diagnosis, Staging and Follow-Up with MRI. Korean J Radiol. 2017;18:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J-M, Kim NK. Essential Anatomy of the Anorectum for Colorectal Surgeons Focused on the Gross Anatomy and Histologic Findings. Ann Coloproctol. 2018;34:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Kinugasa Y, Hwang SE, Murakami G, Rodríguez-Vázquez JF, Cho BH. Denonvilliers’ fascia revisited. Surg Radiol Anat. Springer Paris. 2015;37:187–197. [DOI] [PubMed] [Google Scholar]
- 34.Lowry AC, Simmang CL, Boulos P, et al. Consensus statement of definitions for anorectal physiology and rectal cancer. Colorectal Dis. 2001;3:272–275. [DOI] [PubMed] [Google Scholar]
- 35.Hosseini-Nik H, Hosseinzadeh K, Bhayana R, Jhaveri KS. MR imaging of the retrorectalpresacral tumors: an algorithmic approach. Abdom Imaging. Springer US. 2015;40:2630–2644. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Ding Z-H, Li G-X, Yu J, Wang Y-N, Hu Y-F. Perirectal fascia and spaces: annular distribution pattern around the mesorectum. Dis Colon Rectum. 2010;53:1315–1322. [DOI] [PubMed] [Google Scholar]
- 37.Massalou D, Moszkowicz D, Mariage D, Baqué P, Camuzard O, Bronsard N. Is it possible to give a single definition of the rectosigmoid junction? Surg Radiol Anat. Springer Paris. 2018;40:431–438. [DOI] [PubMed] [Google Scholar]
- 38.Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdominal Radiology. Springer US. 2018;92:148–10. [DOI] [PubMed] [Google Scholar]
- 39.Huang A, Zhao H, Ling T, Quan Y, Zheng M, Feng B. Oncological superiority of extralevator abdominoperineal resection over conventional abdominoperineal resection: a meta-analysis. Int J Colorectal Dis. Springer Berlin Heidelberg. 2014;29:321–327. [DOI] [PubMed] [Google Scholar]
- 40.Denost Q, Rullier E. Intersphincteric Resection Pushing the Envelope for Sphincter Preservation. Clin Colon Rectal Surg. Thieme Medical Publishers. 2017;30:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirouzu K, Murakami N, Akagi Y. Intersphincteric resection for very low rectal cancer: A review of the updated literature. Ann Gastroenterol Surg. John Wiley & Sons, Ltd. 2017;1:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakhman Y, Nougaret S, Miccò M, et al. Role of MR Imaging and FDG PET/CT in Selection and Follow-up of Patients Treated with Pelvic Exenteration for Gynecologic Malignancies. Radiographics. 2015;35:1295–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Althumairi AA, Gearhart SL. Local excision for early rectal cancer: transanal endoscopic microsurgery and beyond. J Gastrointest Oncol. 2015;6:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]