Abstract
Rectal cancer accounts for one-third of newly diagnosed colorectal cancer cases. Given its anatomical location and risk for local recurrence, a multidisciplinary treatment program including surgery, radiation therapy, and chemotherapy has demonstrated improved outcomes in localized disease. Genetic analysis has become part of the standard approach for management of advanced disease and new trials are considering tailored therapies for locally advanced disease. This review describes molecular subsets of colorectal cancer; implications for clinical management, including patterns of metastatic spread and response to therapies; and emerging matched therapies. During the last decade, significant biological differences have been noted based on colorectal cancer primary location and here we focus on rectal cancers and relevant markers for this disease. As more treatment for localized rectal cancer is shifted to the neoadjuvant setting and more targeted regimens are developed for metastatic disease, radiologists will increasingly see patients defined by molecular subsets and their awareness of the genetics of rectal cancer will help further refine our understanding of this disease.
Keywords: rectal cancer, next generation sequencing, targeted therapy, genomics
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States with 50,000 cases in 2017 [1]. Rectal cancer accounts for approximately one-third of newly diagnosed cases and is considered a challenging disease given its anatomical location and risk for local recurrence. Additionally, the incidence of rectal cancer has been rising among younger patients [2]. Seminal work by Fearon and Vogelstein has elucidated the successive genetic changes that underlie transformation from an adenoma to carcinoma in CRC [3,4]. While this basic framework describes cancer development across the large bowel, recent advances in sequencing technology have identified genetic differences between rectal cancers and colon cancers. This review will focus on the genetic alterations underlying rectal cancer development and potential targets for therapy.
Molecular pathogenesis of CRC
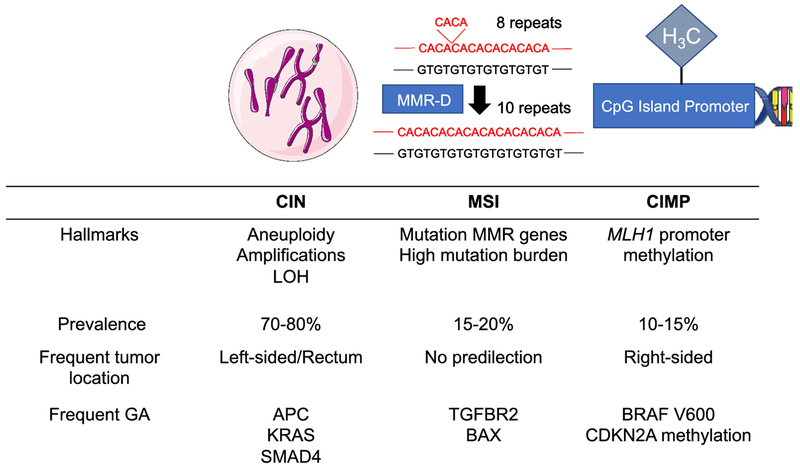
Genomic instability has long been recognized as enabling multistep tumor progression [5], and CRC is notable for a high degree of genomic instability. In CRC, this genomic instability can result from three different molecular pathways: chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP) (Figure 1). Understanding the pathway to tumorigenesis has important clinical implications in rectal cancer as it affects screening recommendations, response to chemotherapy and radiotherapy, and potential targets for matched therapies.
Figure 1. Genomic instability pathways in colorectal cancer.
Abbreviation: CIN, chromosomal instability; MSI, microsatellite instability; CIMP, CpG island methylator phenotype; LOH, loss of heterozygosity; MMR, mismatch repair; GA, genomic alteration.
Chromosomal instability pathway
CIN most frequently leads to genomic instability in CRC. CIN occurs in 70% of sporadic CRC, with increasing prevalence in the more distal aspect of the large bowel. CIN involves the gains and losses of whole chromosomes leading to aneuploidy, amplifications, and a high frequency of loss of heterozygosity [6] The CIN phenotype can result from defects in mechanisms that ensure accurate chromosome segregation including dysfunction of the mitotic checkpoint, abnormal centrosome number and function, telomere dysfunction, and failure in DNA damage response machinery [7]. Coupled with the typical karyotypic abnormalities observed in CIN tumors, the accumulation of driver genomic alterations in CRC such as APC, KRAS, and SMAD4 has been historically designated the “chromosomal instability pathway” [7]. However, these mutations are not exclusive to this pathway and can occur also in microsatellite instability high (MSI-H) or CIMP-high tumors. CIN has been associated with young onset CRC, whose incidence has been increasing steadily during the last decades, particularly in the distal colon and rectum [2,8].
Microsatellite instability
In a subset of CRC patients, genetic alterations leading to the development of carcinoma result from defects in the mismatch repair (MMR) proteins, including MLH1, PMS2, MSH2, and MSH6, as well as MLH2, MSH3, PMS1, and Exol. Mutation in one of the MMR genes rendering the protein nonfunctional or hypermethylation of the MLH1 promoter leading to promoter silencing of MLH1 [9] results in abnormal DNA proofreading after replication, particularly affecting the lengths of short tandem repeats, called microsatellites, within noncoding and coding regions [10]. Asa result, microsatellites are of variable lengths in these tumors, a phenomenon known as microsatellite instability (MSI). Mutation in the MMR genes is often due to a germline alteration, as seen in hereditary non-polyposis colorectal cancer, also known as Lynch Syndrome. In these tumors, genomic instability results from the MSI pathway, and these tumors can harbor numerous mutations, particularly frameshift alterations, in microsatellites across the genome. The prevalence of MSI-H CRC decreases in advanced stages and represents around 20% of cases in stage I-II, 12% in stage III and 4% in stage IV CRC [11,12]. While the prevalence of MSI-H cancers overall is higher in right-sided tumors, those resulting from mutations in the MMR genes occur with a similar frequency of about 5% across the large bowel [13]. Thus, rectal tumors that are MSI-H are more likely due to Lynch syndrome or sporadic mutations in the MMR genes [13].
CpG island methylator phenotype
Most of the CpG sites are normally methylated in adult cells, however, CpG islands in promoter region of many genes are normally unmethylated [14]. CpG methylation in promoter regions allows regulation of gene expression as methylation leads to transcriptional silencing. As noted above, the MMR gene MLH1 promoter is often hypermethylated leading to decreased protein expression. Tumors with MLH1 methylation are microsatellite unstable but develop through a distinct pathway called the CIMP pathway. It is observed in about 15% of tumors [3], particularly in the proximal colon and is infrequent in rectal tumors [15,13].
Clinical implications of molecular subtypes
Evaluation of MMR protein status is recommended for all CRCs. As most MSI-H rectal cancers are due to genetic alterations in the MMR genes, germline assessment of these genes in patients with MMR deficient rectal tumors is important to identify patients who would benefit from genetic counseling and to guide cancer screening.
Patients with MSI-H CRC have a better prognosis in early disease but have worse outcomes for metastatic disease. Recently a series of 62 patients with MSI-H rectal cancers reported a 5-year rectal cancer-specific survival of 100% for stage I and II, 85% for stage III, and 60% for stage IV disease [16]. The pathologic complete response (pCR) rate in 29 patients who received neoadjuvant chemoradiotherapy and underwent surgery was 27.5%, which compares favorably to historical pCR rate in non-MSI-H cohorts. For locally advanced rectal cancer, MSI-H tumors have been associated with increased sensitivity to radiation treatment in preclinical studies [17–19] but large clinical series have not confirmed this increased sensitivity, and some studies have suggested relative radio-resistance [20]. These tumors appear to have more heterogeneous responses to induction chemotherapy and while they can have complete response to treatment, a substantial portion may be chemo-resistant [21].
In more advanced disease, MSI testing is important as it identifies a subset of patients with a high likelihood of response to immune checkpoint inhibitors as described below. Frameshift alterations, which are enriched in MSI-H cancers, are more immunogenic than other classes of genomic alterations [22]. In contrast, CIN, which is much more common in rectal tumors, leads to copy number alterations that are less immunogenic. The copy number alterations, however, can produce potential targets for therapy such as ERBB2 amplification, described below.
CMS subtypes
In an effort to refine the molecular classification of CRC, the CRC Subtyping Consortium has described a consensus molecular subtype (CMS) classification consisting of four subgroups based on results from six independent transcriptomic-based subtyping systems [23]. These groups are CMS1 (microsatellite instability immune), characterized by microsatellite instability and immune activation; CMS2 (canonical), characterized by marked WNT signaling activation; CMS3 (metabolic) characterized by metabolic dysregulation; and CMS4 (mesenchymal) characterized by prominent transforming growth factor activation and stromal invasion. In multivariate analysis, CMS4 was associated with worse relapse-free survival and overall survival and CMS1 was associated with poor overall survival after relapse. These molecular subtypes distribute unequally between left- and right-sided tumors. In an analysis of 1603 stage II/III CRC patients treated in the NSABP/ NRG C-07 trial, CMS2 corresponded to 53% and 21% of left- and right-sided tumors, respectively whereas CMS1 corresponded to 11% and 38% of left- and right-sided tumors, respectively (P<.001) [24]. While the CMS classification provides insight into the transcriptional program of CRC, this classification system does not yet impact clinical management.
Specific genomic alterations in rectal cancer
The most recurrent genomic alterations in CRC involve the APC, TP53, and KRAS genes [25,26], and these genes are also the most commonly mutated in rectal cancers. However, there are variations in gene alterations by tumor locations – APC and TP53 mutations are more common in the rectum than in the proximal colon (78% versus 70% for APC; 81% versus 65% for TP53) and KRAS mutations are much less common in the rectum than in the proximal colon (39% versus 65%) [26]. Mutations in the V600 hotspot of BRAF rarely occur in rectal tumors. These differences in genomic alterations affect response to therapy, patterns of metastatic spread, and potential targets for matched targeted therapies.
Response to therapies
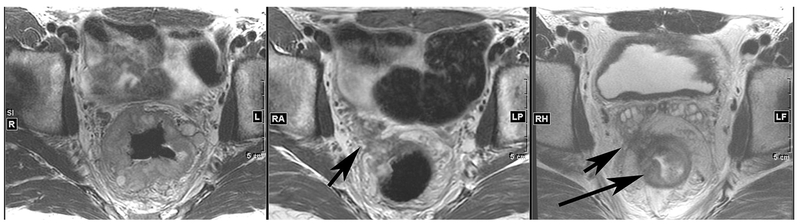
The treatment of locally advanced rectal cancer is multidisciplinary. Standard treatment often includes chemoradiotherapy, total mesorectal excision and adjuvant chemotherapy [27–29]. With this treatment sequence the incidence of local recurrence has been consistently reported below 10% and, in 15 to 27% of patients, no residual viable tumor cells are detected in the resected specimen [30,31]. A variation of this paradigm is the total neoadjuvant therapy approach, which considers chemotherapy before surgery administered either before or after neoadjuvant chemoradiotherapy [32,33]. This strategy aims for early treatment of micrometastatic disease and to increase pCR rate. In patients with clinical complete response after neoadjuvant treatment, non-operative management has also been attempted in order to avoid the impairment of quality of life associated with total mesorectal excision [34,35]. The identification of predictive biomarkers to correctly select patients for these different strategies has been challenging. As noted above, the presence of microsatellite instability may affect response to these standard therapies, potentially sensitizing to radiation treatment and leading to a larger degree of heterogeneity in response to chemotherapy. New studies are evaluating the potential of induction immunotherapy in patients with locally advanced MSI-H tumors based on the activity of this approach in advanced disease and in the neoadjuvant setting [36]. However, MSI-H tumors represent a minority of rectal cancers. A systematic review did not find any pathological factors, imaging modalities, or molecular factors consistently associated with pCR following neoadjuvant chemoradiotherapy [37]. Studies included in this review evaluated molecular biomarkers such as gene signatures by microarray, KRAS and TP53 mutations, single nucleotide polymorphisms, and protein expression profile. In a more recent multicenter study including 292 patients with stage II/III rectal cancer, the pCR rate after neoadjuvant treatment in KRAS mutant and KRAS wild type tumors was 15% and 34%, respectively [38]. KRAS mutation remained independently associated with a lower pCR rate on multivariate analysis (OR 0.34; 95% CI 0.17-0.66, P=0.01). Figure 2 shows serial images from a patient treated at our institution for locally advanced KRAS mutant rectal cancer who received induction chemotherapy followed chemoradiotherapy with modest clinical benefit. In patients with stage I tumors, KRAS mutations have also been associated with higher risk of recurrence after local excision [39].
Figure 2. Serial rectal MRIs during total neoadjuvant therapy for locally advanced KRAS G12D rectal cancer.
(Left) Baseline axial T2-weighted image shows large circumferential tumor with surrounding lymph nodes. (Center) Post-induction chemotherapy axial T2-weighted image shows moderate response with decreased tumor size but persistent tumor extending through mesorectum to contact the right seminal vesicle (arrow). (Right) Post-chemoradiotherapy axial T2-weighted image shows new edema of the rectal wall (long arrow) with little to no decrease in tumor in prior location (arrow).
Patterns of metastatic involvement
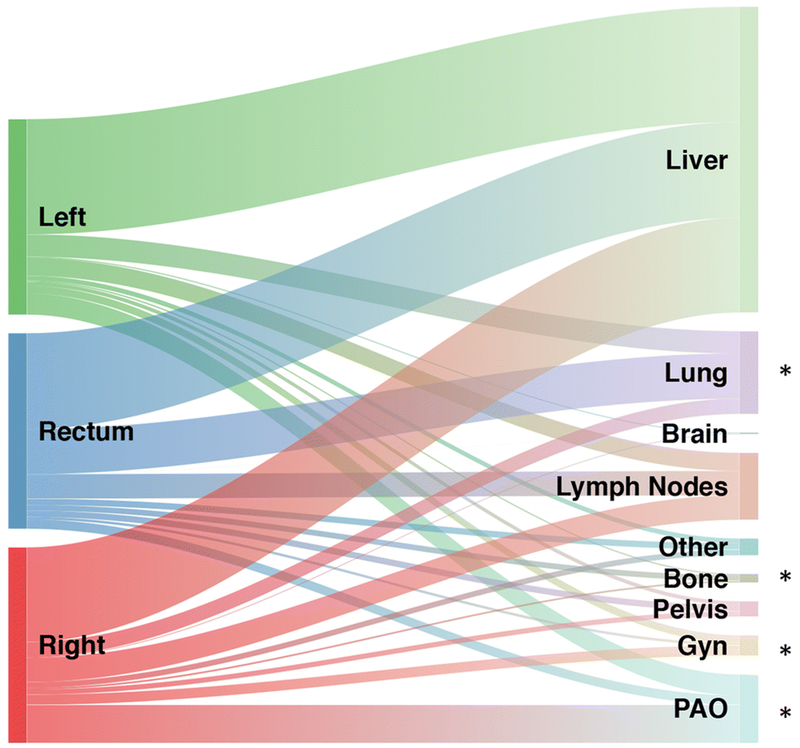
When they metastasize, rectal tumors more commonly spread to the lungs and bones and less commonly involve the peritoneum/omentum or gynecologic organs compared to right-sided and left-sided primary colon cancers. Relative flow of first site of metastasis from colorectal cancer according to different primary tumor location is described in Figure 3 [26]. The higher incidence of lung involvement has been attributed to anatomic reasons. A distal rectal tumor may metastasize initially to the lungs because the inferior rectal vein drains into the inferior vena cava, bypassing the portal venous system [40]. This is seen despite a lower frequency of KRAS mutations, which is associated with lung involvement [41,42]. Peritoneal involvement is commonly seen in MSI-H cancers that metastasize, particularly those that develop through the CIMP pathway, and in KRAS-mutant tumors, which are both more common in proximal colon cancers than in rectal cancers.
Figure 3. Sankey diagram describing relative flow of first site of metastasis from colorectal cancer according to different primary tumor location.
Abbreviation: Gyn, ovaries, fallopian tubes, uterus, cervix, and vagina; PAO, peritoneum, abdominal wall or omentum. Asterisk indicates a statistically significant difference (P<0.05).
Potential targeted therapies
The majority of rectal cancers are wild-type for the RAS genes and may therefore be targeted with the anti-EGFR antibodies cetuximab and panitumumab since RAS mutations are associated with resistance to these targeted agents [43,44]. Expression of the EGFR ligands, amphiregulin and epiregulin, is known to vary across the large bowel and predicts for response to EGFR inhibitors [45]. Anti-EGFR antibodies have also been tested in locally advanced rectal cancer, but their addition to standard therapies has not been able to improve the pCR rate [46–50].
Because of the high frequency of copy number alterations, gene amplifications are more common in distal tumors. ERBB2 (also known as HER2) amplification is emerging as a target. In a retrospective study in which 365 colorectal tumors were analyzed by in-situ hybridization, the prevalence of HER2 amplification was higher in rectal cancer (10.4%) compared to left-sided tumors (3.6%) and right-sided tumors (2.9%) (P=.013) [51]. The prognostic features of HER2 overexpression in rectal cancer was specifically studied in a retrospective cohort by Meng et al. [52]. In this cohort, HER2 overexpression by immunohistochemistry (IHC 3+ or IHC 2+ and FISH HER2-positive) was found in 115 out of 717 rectal tumors (16%). In the subgroup of HER2-positive patients the 5-year overall survival was significantly shorter than those of HER2-negative patients (63.5% vs. 73.9%, P=.013). Recent trials targeting HER2 with the combinations of trastuzumab-lapatinib and trastuzumab-pertuzumab have shown encouraging activity in metastatic HER2-amplified CRC with response rates of about 30% [53,54].
BRAF V600E mutation is rare in rectal cancer and seen in less than 1% of cases. While infrequent, testing for BRAF V600E is important to guide the use of EGFR inhibitors and of potentially matched targeted therapy. This mutation has been associated with poor prognosis and lack of response to anti-EGFR monoclonal antibodies [55,56]. Currently there are no FDA-approved targeted therapies for V600 BRAF-mutant CRC, however, combinations of a selective RAF inhibitor and EGFR antibody have shown promising preliminary activity. The National Comprehensive Cancer Network (NCCN) guidelines include the triplet of the RAF inhibitor vemurafenib, EGFR antibody cetuximab, and irinotecan for improved progression free survival compared to cetuximab and irinotecan in BRAF V600E CRC [57] and the triplet combination of the RAF inhibitor encorafenib, the MEK [the protein target of BRAF] inhibitor binimetinib, and cetuximab recently received FDA breakthrough designation for the treatment of BRAF V600E CRC after progression through standard therapy based on a response rate of about 50% in a safety lead-in cohort of 29 patients [58].
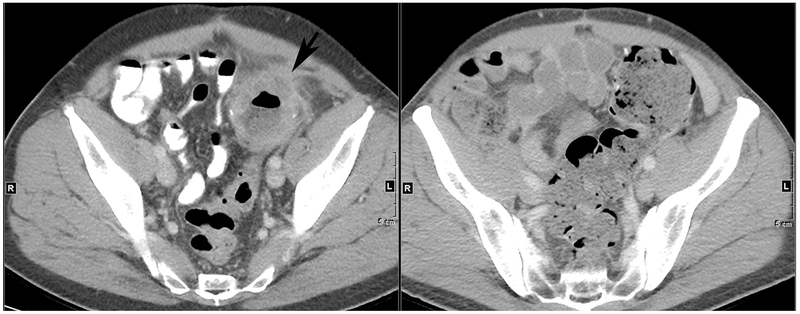
As noted above, up to 5% of rectal tumors are MSI-H, most commonly due to mutations in the MMR genes. The presence of microsatellite instability opens an important treatment option with immune checkpoint blockade. Response rates to single agent anti-PDl inhibitors range from 31-52% and combination treatment with anti-PDl and anti-CTLA4 inhibitors achieves a response rate of 55% [59–61]. These immunotherapy strategies have been associated with durable benefit in responders. Figure 4 shows serial images from a patient with MSI-H sigmoid colon cancer with local recurrence who was treated with an anti-PDl inhibitor with a clinical complete response.
Figure 4. CT imaging from a patient with resected MSI-H sigmoid colon cancer with a local recurrence.
(Left) Axial contrast enhanced CT scan at level of anastomosis revealing circumferential recurrent tumor (arrow). (Right) Axial contrast enhanced CT scan at level of anastomosis post Pembrolizumab treatment for 10 weeks showing complete normalization of anastomosis.
Conclusion
Rectal cancer is clinically distinct from colon cancer as localized disease presents a unique challenge requiring precise radiographic assessment of disease extent and careful coordination of treatment between surgical, radiation, and medical oncologists. An understanding of the genetics underlying CRC development provides a framework to further interpret the behaviors of rectal cancers. Molecular characteristics leading to clinical variations occur across the colorectum. Tumors located in the rectum share most genomic features with left-sided colon neoplasms including high rate of CIN and low frequency of adverse prognostic biomarkers such as BRAF V600 and RAS mutations. Rectal tumors exhibit a higher prevalence of copy number alterations, including clinically targetable alterations like HER2 amplification, and lower prevalence of MSI-H tumors secondary to MLH1 promoter methylation. New matched therapies have the potential to improve outcomes for metastatic disease and tailor treatment in localized disease. However, the incorporation of novel systemic therapies has not been straightforward and drugs that have demonstrated a benefit in overall survival in metastatic CRC have failed to improve outcomes in localized rectal cancer, so more work remains to be done. As radiologists are aware of the genetics of rectal cancer, their insights will help further refine our understanding of this disease.
Acknowledgements:
We acknowledge Walid Chatila and Marc Gollub for help with the figures.
Funding
Supported by the National Institutes of Health Memorial Sloan Kettering Cancer Center Core Grant (P30 CA 008748).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest
R Yaeger has served on an advisory board for GlaxoSmithKline and has received research funding from Array BioPharma, Genentech, GlaxoSmithKline, and Novartis.
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67 (1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A (2017) Colorectal cancer statistics, 2017. CA Cancer J Clin 67 (3): 177–193. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.Markowitz SD, Bertagnolli MM (2009) Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 361 (25):2449–2460. doi: 10.1056/NEJMra0804588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61 (5):759–767 [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144 (5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 6.Lengauer C, Kinzler KW, Vogelstein B (1998) Genetic instabilities in human cancers. Nature 396 (6712):643–649. doi: 10.1038/25292 [DOI] [PubMed] [Google Scholar]
- 7.Pino MS, Chung DC (2010) The chromosomal instability pathway in colon cancer. Gastroenterology 138 (6):2059–2072. doi: 10.1053/j.gastro.2009.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perea J, Rueda D, Canal A, Rodriguez Y, Alvaro E, Osorio I, Alegre C, Rivera B, Martinez J, Benitez J, Urioste M (2014) Age at onset should be a major criterion for subclassification of colorectal cancer. J Mol Diagn 16 (1):116–126. doi: 10.1016/j.jmoldx.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Fearon ER (2011) Molecular genetics of colorectal cancer. Annu Rev Pathol 6:479–507. doi: 10.1146/annurev-pathol-011110-130235 [DOI] [PubMed] [Google Scholar]
- 10.Vilar E, Tabernero J (2013) Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov 3 (5):502–511. doi: 10.1158/2159-8290.CD-12-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavin PG, Colangelo LH, Fumagalli D, Tanaka N, Remillard MY, Yothers G, Kim C, Taniyama Y, Kim SI, Choi HJ, Blackmon NL, Lipchik C, Petrelli NJ, O’Connell MJ, Wolmark N, Paik S, Pogue-Geile KL (2012) Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res 18 (23):6531–6541. doi: 10.1158/1078-0432.CCR-12-0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, Punt CJ, van Krieken JH (2009) Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 100 (2):266–273. doi: 10.1038/sj.bjc.6604867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato K, Kawazu M, Yamamoto Y, Ueno T, Kojima S, Nagae G, Abe H, Soda M, Oga T, Kohsaka S, Sai E, Yamashita Y, Iinuma H, Fukayama M, Aburatani H, Watanabe T, Mano H (2018) Fusion Kinases Identified by Genomic Analyses of Sporadic Microsatellite Instability-High Colorectal Cancers. Clin Cancer Res doi: 10.1158/1078-0432.CCR-18-1574 [DOI] [PubMed] [Google Scholar]
- 14.Issa JP (2004) CpG island methylator phenotype in cancer. Nat Rev Cancer 4 (12):988–993. doi: 10.1038/nrc1507 [DOI] [PubMed] [Google Scholar]
- 15.Sugai T, Habano W, Jiao YF, Tsukahara M, Takeda Y, Otsuka K, Nakamura S (2006) Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn 8 (2): 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, Bednarski B, Messick CA, Skibber JM, Feig BW, Lynch PM, Vilar E, You YN (2016) DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol 34 (25):3039–3046. doi: 10.1200/JCO.2016.66.6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JS, Tut TG, Yang T, Lee CS (2013) Radiotherapy response in microsatellite instability related rectal cancer. Korean J Pathol 47 (1): 1–8. doi: 10.4132/KoreanJPathol.2013.47.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchitto A, Pichierri P, Piergentili R, Crescenzi M, Bignami M, Palitti F (2003) The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 22 (14):2110–2120. doi: 10.1038/sj.onc.1206254 [DOI] [PubMed] [Google Scholar]
- 19.Barwell J, Pangon L, Hodgson S, Georgiou A, Kesterton I, Slade T, Taylor M, Payne SJ, Brinkman H, Smythe J, Sebire NJ, Solomon E, Docherty Z, Camplejohn R, Homfray T, Morris JR (2007) Biallelic mutation of MSH2 in primary human cells is associated with sensitivity to irradiation and altered RAD51 foci kinetics. J Med Genet 44 (8):516–520. doi: 10.1136/jmg.2006.048660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A (2018) Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann Surg. doi: 10.1097/SLA.0000000000003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cercek A, Fernandez GDS, Roxburgh C, Ng S, Yaeger R, Segal N, Ganesh K, Reidy D, Smith J, Nash G, Guillem J, Paty P, Shia J, Garcia-Aguilar J, Diaz L, Crane C, Goodman K, Saltz L, Weiser M, Stadler Z Mismatch repair deficient rectal cancer is resistant to induction combination chemotherapy. Paper presented at the 2018 ESMO Congress 29 (suppl_8): viii150–viii204. , [Google Scholar]
- 22.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, Chambers T, Salgado R, Savas P, Loi S, Birkbak NJ, Sansregret L, Gore M, Larkin J, Quezada SA, Swanton C (2017) Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol 18 (8): 1009–1021. doi: 10.1016/S1470-2045(17)30516-8 [DOI] [PubMed] [Google Scholar]
- 23.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, B Perez-Villamil, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S (2015) The consensus molecular subtypes of colorectal cancer. Nat Med 21 (11):1350–1356. doi: 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SR, Song N, Yothers G, Gavin PG, Allegra CJ, Paik S, Pogue-Geile KL (2018) Tumour sidedness and intrinsic subtypes in patients with stage II/III colon cancer: analysis of NSABP C-07 (NRG Oncology). Br J Cancer 118 (5):629–633. doi: 10.1038/bjc.2017.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Network (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 (7407):330–337. doi: 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, Jayakumaran G, Middha S, Zehir A, Donoghue MTA, You D, Viale A, Kemeny N, Segal NH, Stadler ZK, Varghese AM, Kundra R, Gao J, Syed A, Hyman DM, Vakiani E, Rosen N, Taylor BS, Ladanyi M, Berger MF, Solit DB, Shia J, Saltz L, Schultz N (2018) Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 33 (1):125–136.e123. doi: 10.1016/j.ccell.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 69 (10):613–616 [DOI] [PubMed] [Google Scholar]
- 28.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R, German Rectal Cancer Study G (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351 (17): 1731–1740. doi: 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 29.Petersen SH, Harling H, Kirkeby LT, Wille-Jorgensen P, Mocellin S (2012) Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 3:CD004078. doi: 10.1002/14651858.CD004078.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27 (31):5124–5130. doi: 10.1200/JC0.2009.22.0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R, Rich TA, Skibber J (1999) Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 44 (5):1027–1038 [DOI] [PubMed] [Google Scholar]
- 32.Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR (2018) Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol 4 (6):e180071. doi: 10.1001/jamaoncol.2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K, Consortium ToRCRtC (2015) Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 16 (8):957–966. doi: 10.1016/S1470-2045(15)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr., Silva e Sousa AH Jr., Campos FG, Kiss DR, Gama-Rodrigues J (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240 (4):711–717; discussion 717-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewe KW, Buijsen J, Beets GL (2011) Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 29 (35):4633–4640. doi: 10.1200/JCO.2011.37.7176 [DOI] [PubMed] [Google Scholar]
- 36.Chalabi M, Fanchi L, Berg JVd, Beets G, Lopez-Yurda M, Aalbers A, Grootscholten C, Snaebjornsson P, Maas M, Mertz M, Nuijten E, Kuiper M, Kok M, Leerdam MV, Schumacher T, Voest E, Haanen J Neoadjuvant ipilimumab plus nivolumab in early stage colon cancer. Paper presented at the 2018 ESMO Congress Abstract LBA37_PR., [Google Scholar]
- 37.Ryan JE, Warrier SK, Lynch AC, Ramsay RG, Phillips WA, Heriot AG (2016) Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis 18 (3):234–246. doi: 10.1111/codi.13207 [DOI] [PubMed] [Google Scholar]
- 38.Chow OS, Kuk D, Keskin M, Smith JJ, Camacho N, Pelossof R, Chen CT, Chen Z, Avila K, Weiser MR, Berger MF, Patil S, Bergsland E, Garcia-Aguilar J (2016) KRAS and Combined KRAS/TP53 Mutations in Locally Advanced Rectal Cancer are Independently Associated with Decreased Response to Neoadjuvant Therapy. Ann Surg Oncol 23 (8):2548–2555. doi: 10.1245/sl0434-016-5205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sideris M, Moorhead J, Diaz-Cano S, Bjamason I, Haji A, Papagrigoriadis S (2016) KRAS Mutant Status, pl6 and beta-catenin Expression May Predict Local Recurrence in Patients Who Underwent Transanal Endoscopic Microsurgery (TEMS) for Stage I Rectal Cancer. Anticancer Res 36 (10):5315–5324. doi: 10.21873/anticanres.11104 [DOI] [PubMed] [Google Scholar]
- 40.Chiang JM, Hsieh PS, Chen JS, Tang R, You JF, Yeh CY (2014) Rectal cancer level significantly affects rates and patterns of distant metastases among rectal cancer patients post curative-intent surgery without neoadjuvant therapy. World J Surg Oncol 12:197. doi: 10.1186/1477-7819-12-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaeger R, Cowell E, Chou JF, Gewirtz AN, Borsu L, Vakiani E, Solit DB, Rosen N, Capanu M, Ladanyi M, Kemeny N (2015) RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer 121 (8): 1195–1203. doi: 10.1002/cncr.29196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, Jamagin WR, Fong YC, DeMatteo RP, Allen PJ, Shia J, Ang C, Vakiani E, D’Angelica MI (2014) KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 120 (24):3965–3971. doi: 10.1002/cncr.28954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douillard JY, Oliner KS, Siena S, Tabemero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasihska-Morawiec M, Smakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369 (11): 1023–1034. doi: 10.1056/NEJMoal305275 [DOI] [PubMed] [Google Scholar]
- 44.Cercek A, Braghiroli MI, Chou JF, Hechtman JF, Kemeny N, Saltz L, Capanu M, Yaeger R (2017) Clinical Features and Outcomes of Patients with Colorectal Cancers Harboring NRAS Mutations. Clin Cancer Res 23 (16):4753–4760. doi: 10.1158/1078-0432.CCR-17-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ (2007) Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25 (22):3230–3237. doi: 10.1200/JCO.2006.10.5437 [DOI] [PubMed] [Google Scholar]
- 46.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, Chua YJ, Wong R, Barbachano Y, Oates J, Chau I (2012) Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 30 (14):1620–1627. doi: 10.1200/JC0.2011.39.6036 [DOI] [PubMed] [Google Scholar]
- 47.Merx K, Martens UM, Kripp M, Hoehler T, Geissler M, Gaiser T, Mai S, Kienle P, Belle S, Ploger C, Hieber U, Wenz F, Post S, Hofheinz RD (2017) Panitumumab in Combination With Preoperative Radiation Therapy in Patients With Locally Advanced RAS Wild-type Rectal Cancer: Results of the Multicenter Explorative Single-Arm Phase 2 Study NEORIT. Int J Radiat Oncol Biol Phys 99 (4):867–875. doi: 10.1016/j.ijrobp.2017.06.2460 [DOI] [PubMed] [Google Scholar]
- 48.Eisterer W, De Vries A, Ofner D, Rabl H, Koplmuller R, Greil R, Tschmelitsch J, Schmid R, Kapp K, Lukas P, Sedlmayer F, Hofler G, Gnant M, Thaler J, Austrian B, Colorectal Cancer Study G (2014) Preoperative treatment with capecitabine, cetuximab and radiotherapy for primary locally advanced rectal cancer--a phase II clinical trial. Anticancer Res 34 (11):6767–6773 [PubMed] [Google Scholar]
- 49.Kim SY, Shim EK, Yeo HY, Baek JY, Hong YS, Kim DY, Kim TW, Kim JH, Im SA, Jung KH, Chang HJ (2013) KRAS mutation status and clinical outcome of preoperative chemoradiation with cetuximab in locally advanced rectal cancer: a pooled analysis of 2 phase II trials. Int J Radiat Oncol Biol Phys 85 (1):201–207. doi: 10.1016/j.ijrobp.2012.03.048 [DOI] [PubMed] [Google Scholar]
- 50.Horisberger K, Treschl A, Mai S, Barreto-Miranda M, Kienle P, Strobel P, Erben P, Woernle C, Dinter D, Kahler G, Hochhaus A, Post S, Willeke F, Wenz F, Hofheinz RD, Margit (2009) Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. Int J Radiat Oncol Biol Phys 74 (5): 1487–1493. doi: 10.1016/j.ijrobp.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 51.Seo AN, Kwak Y, Kim DW, Kang SB, Choe G, Kim WH, Lee HS (2014) HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One 9 (5):e98528. doi: 10.1371/journal.pone.0098528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng X, Huang Z, Di J, Mu D, Wang Y, Zhao X, Zhao H, Zhu W, Li X, Kong L, Xing L (2015) Expression of Human Epidermal Growth Factor Receptor-2 in Resected Rectal Cancer. Medicine (Baltimore) 94 (47):e2106. doi: 10.1097/MD.0000000000002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S (2016) Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 17 (6):738–746. doi: 10.1016/S1470-2045(16)00150-9 [DOI] [PubMed] [Google Scholar]
- 54.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, Burris H, Bose R, Yoo B, Stein A, Beattie M, Kurzrock R (2018) Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol 36 (6):536–542. doi: 10.1200/JCO.2017.75.3780 [DOI] [PubMed] [Google Scholar]
- 55.Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29 (15):2011–2019. doi: 10.1200/JCO.2010.33.5091 [DOI] [PubMed] [Google Scholar]
- 56.van Brummelen EMJ, de Boer A, Beijnen JH, Schellens JHM (2017) BRAF Mutations as Predictive Biomarker for Response to Anti-EGFR Monoclonal Antibodies. Oncologist 22 (7): 864–872. doi: 10.1634/theoncologist.2017-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopetz S, McDonough SL, Morris VK, Lenz H-J, Magliocco AM, Atreya CE, Diaz LA, Allegra CJ, Wang SE, Lieu CH, Eckhardt SG, Semrad TJ, Kaberle K, Guthrie KA, Hochster HS (2017) Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). Journal of Clinical Oncology 35 (4_suppl):520–520. doi: 10.1200/JCO.2017.35.4_suppl.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cutsem EV, Cuyle P-J, Huijberts S, Yaeger R, Schellens JHM, Elez E, Tabernero J, Fakih M, Montagut C, Peeters M, Desai J, Yoshino T, Ciardiello F, Wasan HS, Kopetz S, Maharry K, Christy-Bittel J, Gollerkeri A, Grothey A (2018) BEACON CRC study safety lead-in (SLI) in patients with BRAFV600E metastatic colorectal cancer (mCRC): Efficacy and tumor markers. Journal of Clinical Oncology 36 (4_suppl):627–627. doi: 10.1200/JCO.2018.36.4_suppl.62729283749 [DOI] [Google Scholar]
- 59.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372 (26):2509–2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, Andre T (2018) Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36 (8):773–779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 61.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, Andre T (2017) Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18 (9): 1182–1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]