Abstract
Objective:
We sought to determine whether targeted treatment of insomnia with zolpidem-CR in suicidal adults with insomnia would provide a superior reduction in suicidal ideation as compared with placebo.
Method:
Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT) was an 8-week, 3-site, double-blind, placebo-controlled, parallel-group randomized controlled trial of zolpidem controlled release (CR) hypnotic therapy versus placebo, in conjunction with open-label selective serotonin reuptake inhibitor. Participants were medication-free 18-65 year olds, with Major Depressive Disorder, insomnia and suicidal ideation. Suicidal ideation was the main outcome, measured first by the Scale for Suicide Ideation, and second as measured by the Columbia-Suicide Severity Rating Scale.
Results:
One hundred and three participants were randomized to zolpidem-CR (N=51) or placebo (N=52), including 64 women and 39 men, mean age of 40.5 years. Zolpidem-CR had a robust anti-insomnia effect, especially in patients with the most severe insomnia symptoms. The Scale for Suicide Ideation showed no significant treatment effect with a least squares estimate of −0.56 (0.83) (95% CI: −2.19, 1.08), but the reduction in scores was positively related to improvement in insomnia after accounting for the effect of other depression symptoms (p< 0.002). The Columbia-Suicide Severity Rating Scale showed an advantage for zolpidem-CR (p=0.035), with a treatment effect of −0.26 (0.12) (95% CI: −0.50, −0.02). The advantage for zolpidem-CR in reducing suicidal ideation on the C-SSRS was greater in patients with more severe insomnia. No deaths or suicide attempts occurred.
Conclusions:
While the results do not argue for the routine prescription of hypnotics for mitigating suicidal ideation in all depressed outpatients with insomnia, they suggest that co-prescription of a hypnotic during initiation of an antidepressant may be beneficial in suicidal outpatients, especially in patients with severe insomnia.
Introduction
In the US, there have been recent reductions in mortality related to medical illness, but mortality rates from suicide have steadily increased from 12.5 to 15.0 per 100,000 annually between 2001 and 2015.1 Insomnia has been identified as a risk factor for suicidal ideation, suicidal behavior and suicide death (hereafter collectively termed ‘suicide’),2;3 but thus far there have been no randomized clinical trials examining whether targeted pharmacologic treatment of insomnia would reduce the risk of suicide. It is reasonable to hypothesize that hypnotic medication might reduce the risk of suicide, since changes in insomnia symptoms precede suicidal ideation.3;4 Patients with insomnia5 and survivors of suicide attempts show poorer performance on standardized measures of interpersonal problem solving, as compared with psychiatric patients who have not made a suicide attempt,6 and poorer performance in attention, working memory, and executive function.7–9 Thus, the impaired problem solving associated with insomnia could play a role in suicide. Hypnotic medication might reduce suicidal ideation, but the possibility of benefit from hypnotics must be balanced against the risks of suicide associated with hypnotics.10
In order to examine the impact of treatment of insomnia on suicide risk, we conducted the randomized clinical trial “Reducing Suicidal Ideation Through Insomnia Treatment – REST-IT” to examine the effects of placebo versus a bedtime dose of the hypnotic medication zolpidem controlled release (CR) on suicidal depressed outpatients with insomnia who were receiving open label selective serotonin reuptake inhibitors (SSRIs). Our hypothesis was that the addition of zolpidem-CR would result in greater reduction of suicidal ideation.
Methods
Overview
Reducing Suicidal Ideation Through Insomnia Treat was a multi-site, parallel-arm randomized clinical trial, with the Medical College of Georgia (MCG) as the coordinating/recruiting site, Duke University and University of Wisconsin as recruiting sites, and Wake Forest School of Medicine as the data management site. A data safety monitoring board provided oversight and reviewed the results of two planned interim analyses with stopping rules in effect. The results of the analyses were shared with the monitoring board, but not with the investigative team. Neither interim analysis pointed towards ‘futility’ or towards an early significant effect. As a result, the monitoring board instructed the investigative team to continue recruitment. During the 8 weeks of randomized treatment, all patients received an open label SSRI (fluoxetine=95, sertraline=3, citalopram=5) for depression, as well as zolpidem-CR versus placebo at bedtime. The initiation of the open label SSRI and the double-blinded randomization to receive either zolpidem-CR or placebo occurred simultaneously. Patients were only given enough medication to last until the next scheduled visit, plus 3 days. Follow up visits were scheduled at 1, 2, 4, 6, and 8 weeks after randomization. At the conclusion of the intervention period, arrangements for care-as-usual outpatient visits were made for each participant, and they were prescribed sufficient SSRI to last until the first care-as-usual visit. Blinded study drug (zolpidem-CR versus placebo), was stopped abruptly without tapering. Patient safety and additional clinical assessments were conducted by phone for the first two weeks after leaving randomization and while in transition to care-as-usual. Other details regarding practices to assure participant safety can be found elsewhere.11 All parties remained blind to zolpidem-CR versus placebo treatment assignment.
Participants
Participant recruitment came from the routine flow of self-referrals to psychiatry clinics and advertising. Participants were outpatients 18-65 years of age with a diagnosis of Major Depressive Disorder, confirmed by the Structured Clinical Interview for Diagnosis (SCID) for DSM-IV,12 and met Research Diagnostic Criteria for Insomnia.13 Participants were free of all psychotropic medications for ≥ 1 week prior to baseline assessment (except for fluoxetine, where the requirement was to be off fluoxetine for > 4 weeks), and reported a habitual sleep latency ≥ 30 minutes or wake after sleep onset ≥ 30 minutes, and sleep efficiency ≤ 85%. Baseline inclusion criteria included a 24-item Hamilton Rating Scale for Depression (HAM-D) score ≥ 20 and an Insomnia Severity Index (ISI) score > 7.14;15 Mini Mental State Examination (MMSE) scores were ≥ 24.16 Inclusion criteria also required a Scale for Suicide Ideation score ≥ 3,17 but participants were free of suicidal plans or intent as the Columbia Suicide Severity Rating Scale (C-SSRS) scores on the ideation dimension were ≤ 3.18 Patients were excluded if they had a SCID-confirmed lifetime diagnosis of bipolar disorder, schizophrenia, or alcohol or substance abuse, or a prior diagnosis of any sleep disorder other than insomnia, BMI > 50, positive urine drug screen, a positive urine pregnancy test, were non-English speaking/reading/writing, or posing imminent danger to others. Additionally, all participants underwent in-lab polysomnography or a portable home sleep test to assess for sleep apnea. Participants with an apnea-hypopnea index > 10 were excluded.19 All participants provided written, informed consent after receiving a complete description of the study, and the protocol was approved by the institutional review board of each site. Patients were paid $50 for each visit after randomization.
Randomization and Masking
Participants were stratified by site, sex, and prior history of suicide attempts and randomized within strata to receive zolpidem-CR or placebo with equal probability, using computer-generated, variably sized permuted block randomization to ensure approximately equal accrual to each treatment throughout the study. Block sizes of varying length were used to ensure that future assignments could not be inferred from past assignments. At the time of randomization, all patients received open-label fluoxetine 20 mg, sertraline 50, or citalopram 20 mg daily, but the dosage could be doubled at the end of 4 weeks if at that time HAM-D > 15. Fluoxetine was used in every case unless the participant strongly preferred sertraline or citalopram.
Recruitment began in November 2012. Study medications were over-encapsulated to look identical, masked in a central pharmacy, and sent to the investigative teams. Participants were randomized double-blind to nightly zolpidem-CR 12.5 mg or placebo until February 2013, when the FDA released guidance that zolpidem-CR should not be initially prescribed for women at a dose greater than 6.25 mg at bedtime.20 We then adjusted dosing so that participants were randomized to either zolpidem-CR 6.25 mg or matching placebo, along with open label SSRI. At the end of week 1, the zolpidem-CR/placebo dose could be increased to 12.5 mg if there was inadequate anti-insomnia effect in the absence of side effects. Participants brought pill bottles to each visit and pill counts were made to estimate adherence.
Clinical Measurements
Primary outcome variable: Suicidal Ideation
The Scale for Suicide Ideation and the C-SSRS were administered at each visit. The Scale for Suicide Ideation is a self-rated scale consisting of 19 items that evaluate suicidal desire and planning.17;21;22 Each item is rated on a 3-point scale from 0 to 2 for a maximum score of 38; a lower score indicates less severe suicidal ideation. A Scale for Suicide Ideation score ≥3 is a significant predictor for suicide death over a period of up to 20 years (Hazard Ratio = 6.6).23 The Columbia-Suicide Severity Rating Scale (C-SSRS)18 is an observer-rated scale.24 The suicidal ideation ‘intensity’ dimension of the C-SSRS is scored 1-5, with 5 representing suicidal ideation with a plan and intent. In addition to tracking the intensity of suicidal ideation, the C-SSRS was also used to assess participants’ sense of control over their suicidal thinking, the presence of deterrents that inhibited suicide, and the motivation behind their suicidal thinking (i.e., getting attention and producing a reaction in other people versus the desire to escape mental pain).18;24
Secondary Outcome variables
Sleep metrics
The Insomnia Severity Index is a self-report measure of insomnia severity, with 7 items each scored 0-4. The Insomnia Severity Index was administered at each visit, and higher scores indicate greater insomnia and scores ≤ 7 indicate no insomnia.15 Participants completed a daily, morning sleep diary during randomized treatment to record self-reported sleep latency, wake after sleep onset, number of awakenings, and total sleep time. Sleep diaries were not kept prior to randomization. The Epworth Sleepiness Scale was recorded once at baseline.25 The Dysfunctional Beliefs and Attitudes Scale was used to capture distorted beliefs about sleep.26 The Dysfunctional Beliefs and Attitudes Scale is self-administered with a set of 16 items, 26 with possible total scores ranging from 0 to 10, with higher scores indicating more dysfunctional beliefs and attitudes.
The frequency and intensity of disturbing dreams and nightmares was measured with the self-administered Disturbing Dreams and Nightmare Severity Scale, as nightmares mediate some of the risk of insomnia for suicide.27 Possible scores range from 0 to 37, with higher scores indicating greater problems with nightmares, and scores > 10 are predictive of a nightmare disorder.28
Depression severity
Depression severity was measured by the administration of the HAM-D by study staff at each visit.14
Hopelessness
Hopelessness was measured at each visit with the self-administered Beck Hopelessness Scale, which has 20-items and assesses pessimism and negative expectancies for the future with demonstrated validity across a wide age range.29;30 Among adolescents and adults, it predicts future suicidal behavior.31–33 The range of possible scores for the Beck Hopelessness Scale is 0 to 20, with higher scores indicating more hopelessness.
Health-related quality of life (HRQOL)
Two subscales of the Basis-32 were recorded at each visit, namely the daily living and role functioning subscale and relationship to self and others subscale.34 The range of possible scores for each subscale is 0 to 4, with higher scores indicating a poorer self-reported HRQOL.
Clinical Global Impression
The participants’ overall clinical status and response to treatment were assessed with the Clinical Global Impression-Severity (CGI-S), completed by a study psychiatrist at all visits. The CGI-S is scored from 1-7, with lower scores indicating less illness.35 The Clinical Global Impression- Improvement (CGI-I) was completed by a study psychiatrist at all post-randomization visits, with lower scores indicating more improvement. CGI scales were completed using all available data.
Antidepressant treatment resistance
The participants’ response or resistance to pharmacotherapy in the present episode of illness was measured at baseline by the Antidepressant Treatment History Form.36
Adverse events
Each post-randomization visit included a standardized assessment of the presence or absence of drowsiness while driving or operating dangerous machinery, any accidents or injuries, and if any injuries or accidents occurred, then the time of day and relationship to the timing of the last dose of study drug was also recorded. Additionally, the study psychiatrist elicited spontaneous reports of adverse events and serious adverse events at each treatment visit.
Actigraphy
The participants wore an actigraph on their non-dominant wrist for the duration of the randomized treatment to monitor rest/activity cycles. Actigraphy results will be described in a subsequent report.
Statistical Analyses
Recruitment targets were picked to achieve 80% power to detect a between-group difference of 2 points in the mean post-randomization Scale for Suicide Ideation score, assuming a standard deviation of 3.1, at the 5% two-sided level of significance, and additionally assuming two interim looks and a drop-out rate of 20%. A difference of 2 points on the Scale for Suicide Ideation corresponds to an increased risk of suicide death over a subsequent period of 20 years.23 These assumptions led to a target of 103 randomized participants. The primary hypothesis was tested using a mixed model analysis of covariance to assess treatment differences between the 2 study arms in mean posttreatment scores after adjusting for baseline scores and the design parameters: clinic site, sex, and prior history of suicide attempts. An autoregressive covariance structure was used to model the within-patient correlation over time. Visit was considered a class variable and a linear contrast was used to assess the treatment effect on the mean score across time. The C-SSRS was similarly examined as a second indicator of suicidal ideation. This same strategy was used to assess the effect of zolpidem-CR on other outcomes, except that the covariance structure was chosen to minimize the Bayesian Information Criterion. Separate mixed effects repeated measures analysis of covariance models including additional covariates and their interactions with zolpidem-CR were also evaluated. Insomnia (the Insomnia Severity Index) and suicidal ideation (the Scale for Suicide Ideation ) were assessed for 2 weeks following the end of treatment, and linear contrasts within a mixed effects model containing all times were used to compare week 8 against the mean of weeks 9 and 10. A Wilcoxon rank-sum test was used to assess group differences in compliance due to its skewed distribution.
Results
Baseline
The screening of participants began in November of 2012, and the last randomized participant exited the study in June of 2017 (Figure 1). One hundred and three participants were randomized. Participant characteristics are summarized in Table 1, and there were no significant differences between treatment groups at baseline. The majority of participants were women, and overall 39% of the sample represented minorities. Generalized Anxiety Disorder and Posttraumatic Stress Disorder were present in 40% and 28% of the participants, respectively, at baseline. Fifty-seven percent of the sample were naïve to antidepressant medication in this episode of Major Depressive Disorder, and 30% had a prior suicide attempt. The intensity of suicidal ideation, as measured by the Scale for Suicide Ideation, was moderate.37 At baseline, only 18% of participants described “a lot” of difficulty or an inability to control their suicidal thoughts, and 76% described deterrents that played a major role in preventing a suicidal act. Overall depression severity and insomnia severity were high.(Table 1)
Figure 1.
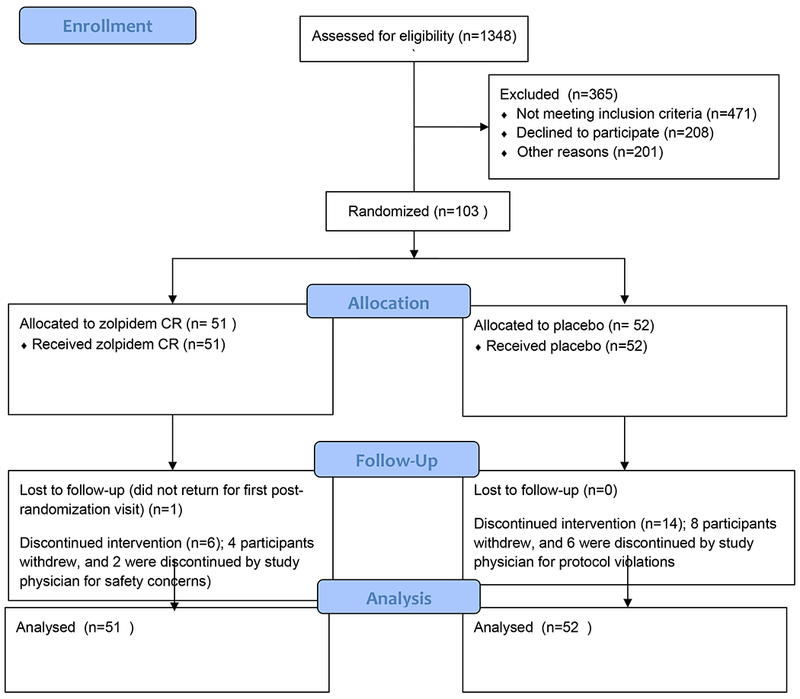
Consort Diagram
Table 1.
Baseline Characteristics of Sample
| Characteristic | Zolpidem CR | Placebo | Overall | |||
|---|---|---|---|---|---|---|
| Randomized Participants | 51 | 52 | 103 | |||
| M | SD | M | SD | M | SD | |
| Age | 39.7 | 14.5 | 41.2 | 12.0 | 40.5 | 13.2 |
| BMI | 28.3 | 6.4 | 28.2 | 5.6 | 28.2 | 6.0 |
| N | % | N | % | N | % | |
| Female | 32 | 63 | 32 | 62 | 64 | 62 |
| Clinic | ||||||
| 1 | 23 | 45 | 27 | 52 | 50 | 49 |
| 2 | 15 | 29 | 15 | 29 | 30 | 29 |
| 3 | 13 | 25 | 10 | 19 | 23 | 22 |
| Race/Ethnicity | ||||||
| Caucasian/White | 30 | 59 | 33 | 63 | 63 | 61 |
| African American | 12 | 24 | 16 | 31 | 28 | 27 |
| Hispanic | 4 | 8 | 1 | 2 | 5 | 5 |
| Other | 5 | 10 | 2 | 4 | 7 | 7 |
| Antidepressant Trials in Current Episode | ||||||
| 0 | 29 | 57 | 30 | 58 | 59 | 57 |
| 1 | 14 | 27 | 12 | 23 | 26 | 25 |
| 2+ | 8 | 16 | 10 | 19 | 18 | 17 |
| Lifetime Suicide Attempt(s) | 15 | 29 | 16 | 31 | 31 | 30 |
| PTSD | 13 | 25 | 16 | 31 | 29 | 28 |
| Obsessive Compulsive Disorder | 2 | 4 | 3 | 6 | 5 | 5 |
| Generalized Anxiety | 20 | 39 | 21 | 41 | 41 | 40 |
| Panic Disorder with agoraphobia | 9 | 20 | 8 | 17 | 17 | 18 |
| Panic Disorder without agoraphobia | 10 | 20 | 7 | 14 | 17 | 17 |
| M | SD | M | SD | M | SD | |
| Scale for Suicide Ideation; Suicide Ideation Score | 12.2 | 5.3 | 11.8 | 5.3 | 12.0 | 5.3 |
| Scale for Suicide Ideation: Suicide Attempts | N | % | N | % | N | % |
| Never | 36 | 71 | 36 | 69 | 72 | 70 |
| Once | 8 | 16 | 8 | 15 | 16 | 16 |
| Two or more Times | 7 | 14 | 8 | 15 | 15 | 15 |
| Scale for Suicide Ideation: Death Wish | ||||||
| No suicide attempt | 36 | 71 | 36 | 69 | 72 | 70 |
| Low | 3 | 6 | 3 | 6 | 6 | 6 |
| Moderate | 6 | 12 | 4 | 8 | 10 | 10 |
| High | 6 | 12 | 9 | 17 | 15 | 15 |
| M | SD | M | SD | M | SD | |
| C-SSRS Ideation Intensity Past Week | 1.71 | 1.03 | 1.58 | 1.02 | 1.64 | 1.02 |
| N | % | N | % | N | % | |
| C-SSRS: A lot of difficulty or inability to control thoughts | 6 | 12 | 12 | 23 | 18 | 18 |
| C-SSRS: Deterrents probably or definitely stopped suicide attempt | 43 | 86 | 35 | 67 | 78 | 76 |
| CGI-S | ||||||
| Mildly | 3 | 6 | 0 | 0 | 3 | 3 |
| Moderately | 33 | 65 | 32 | 62 | 65 | 63 |
| Markedly | 13 | 25 | 18 | 35 | 31 | 30 |
| Severe | 2 | 4 | 2 | 4 | 4 | 4 |
| M | SD | M | SD | M | SD | |
| HAMD Score | 28.7 | 4.7 | 29.6 | 7.0 | 29.1 | 5.9 |
| Insomnia Severity Index | 20.7 | 4.0 | 21.0 | 4.3 | 20.9 | 4.1 |
| Beck Hopelessness Score | 13.4 | 4.8 | 12.7 | 4.6 | 13.1 | 4.7 |
| Disturbing Dreams and Nightmare Severity Index | 10.5 | 8.8 | 10.2 | 7.7 | 10.3 | 8.2 |
| Dysfunctional Beliefs and Attitudes about Sleep | 6.7 | 1.6 | 6.7 | 1.5 | 6.7 | 1.6 |
| Basis 32: Daily Living and Role Functioning | 2.4 | 0.6 | 2.2 | 0.7 | 2.3 | 0.6 |
| Basis 32: Relationship to Self and Others | 2.5 | 0.6 | 2.2 | 0.7 | 2.3 | 0.7 |
| Mini Mental State Exam Total Score | 29.6 | 0.8 | 29.3 | 1.0 | 29.4 | 0.9 |
| Epworth Sleepiness Scale Score | 7.9 | 5.0 | 8.5 | 4.7 | 8.2 | 4.9 |
Randomized Treatment
Eighty-six percent of participants assigned to zolpidem-CR completed all scheduled visits after randomization compared to 73% of placebo participants. Overall, 90% of the scheduled visits were completed. Participants took 91% of all prescribed doses of the study drug, and 94% of all prescribed doses of SSRI, with no significant differences between groups (p=0.11 and 0.29, respectively).
In the zolpidem-CR arm, 73% of patients received an increase in dose from 6.25 mg to 12.5 mg at some time, while 72% of the placebo arm received an increase in dose (p=0.92). Also, 36% of participants in the zolpidem-CR arm received a doubling of dose of SSRI, while 29% of those in the placebo arm received an increase. (p=0.44)
Outcomes
Consistent with its known hypnotic effect, the zolpidem-CR group immediately showed more improvement in Insomnia Severity Index scores compared with the placebo group, with a significant advantage over the period of randomized treatment (Figure 2). On average, the least squares mean (SE) for Insomnia Severity Index over the 8-week period was 11.2 (0.6) for the zolpidem-CR group and 13.8 (0.6) for the placebo group (p=0.004). When the sample is split by severe insomnia (Insomnia Severity Index > 21, N=50) versus mild-moderate insomnia (Insomnia Severity Index scores 8-21, N=53), the zolpidem-CR intervention provided no additional improvement in Insomnia Severity Index scores for mild-moderate insomnia (−0.14 ± 1.18; p>0.05). In contrast, for those participants with severe insomnia, the zolpidem-CR intervention produced a significant advantage in improvement in Insomnia Severity Index scores (−5.23 ± 1.43; p<0.001). Among the participants who completed at least 50% of their sleep diaries, zolpidem-CR showed numerical advantages across 8 weeks for sleep latency (−13.2 (8.0) minutes), wake after sleep onset (−20.7 (11.5) minutes) and total sleep time (12.8 (15.0) minutes).
Figure 2.
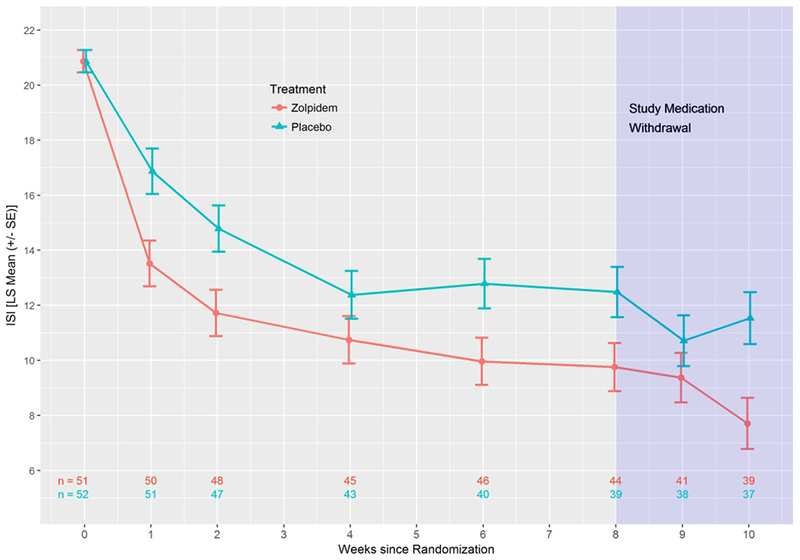
Least Squares Means (± SE) for the Insomnia Severity Index (ISI) for all participants
There was no significant effect of treatment on suicidal ideation as quantified by the Scale for Suicide Ideation (p=0.50). The least squares estimate of the Scale for Suicide Ideation treatment effect was −0.56 (0.83) (95% CI: −2.19, 1.08; Cohen’s d = −0.11), with no effect of site (Figure 3). However, we found a significant longitudinal linear association between Insomnia Severity Index and Scale for Suicide Ideation scores across treatment that remained after adding a covariate for depression scores with the sleep and suicide items removed (b=0.12, se = 0.04, p=0.002). There was a significant decrease in Scale for Suicide Ideation scores over time in both groups. At the end of randomized treatment, 61% of the zolpidem-CR patients and 57% of the placebo patients had achieved a Scale for Suicide Ideation score of “0” (59% overall). Prior treatment with an antidepressant medication in the index episode of Major Depressive Disorder was not a significant moderator of the effect of zolpidem-CR on Scale for Suicide Ideation scores.
Figure 3.
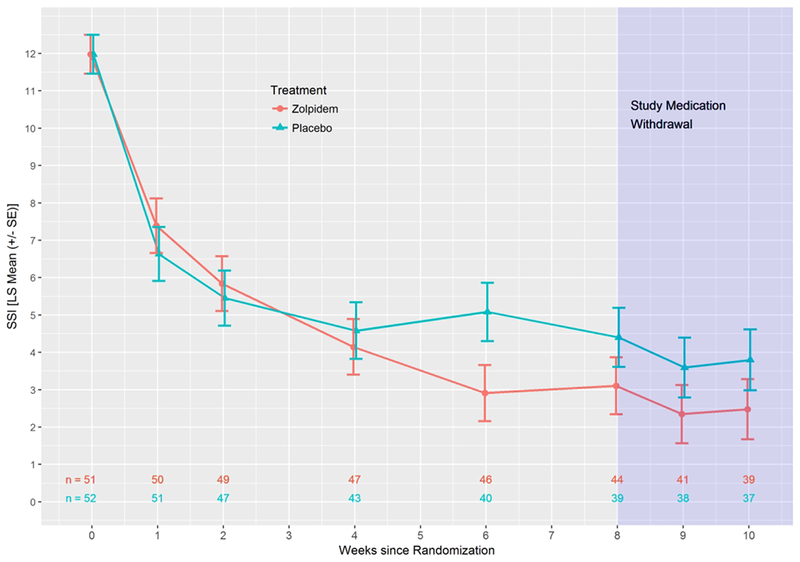
Least Squares Means (± SE) for Scale for Suicidal Ideation (SSI) for all participants
As a second indicator of suicidal ideation, the suicide ideation score of the C-SSRS showed an advantage for zolpidem-CR (p=0.035). The least squares estimate of the treatment effect was −0.26 (0.12) (95% CI: −0.50, −0.02) (Cohen’s d = −0.26) (Figure 4). The zolpidem-CR intervention was associated with a numerically greater reduction in the C-SSRS suicide ideation scores in those participants with severe baseline insomnia (−0.41 ± 0.21), as compared with those with mild-moderate baseline insomnia (−0.08 ± 0.15).
Figure 4.
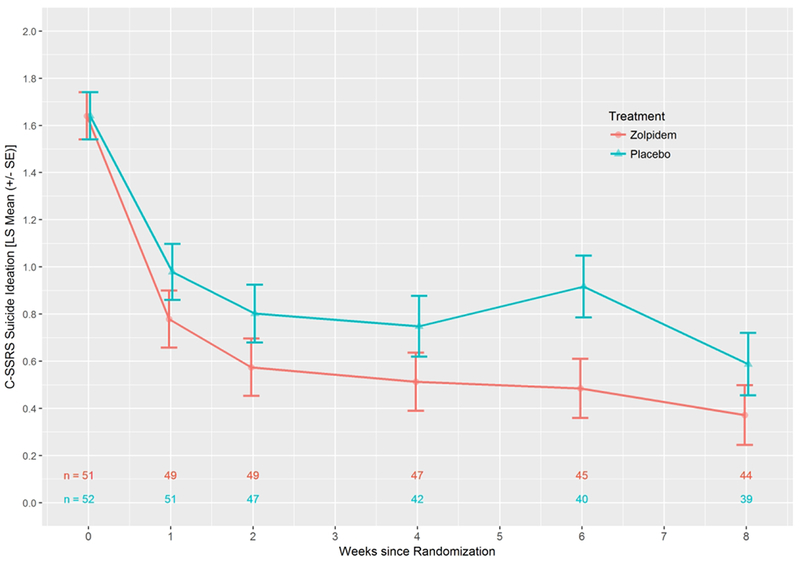
Least Squares Means (± SE) for Columbia Suicide Severity Rating Scale (C-SSRS) Suicide Ideation for all participants
There were significant improvements over time in depression scores (p<0.001), but with no differences between treatment. CGI-S showed significant improvement in both groups over time (p<0.001), with a trend advantage for the zolpidem-CR group (−0.25 (−0.53, 0.02), p=0.07). CGI-I also showed improvement for both groups across time (p<0.001), but with a significant advantage for the zolpidem-CR group (−0.28 (−0.52, −0.04), p = 0.022) that remained after controlling for baseline CGI-S (−0.25 (−0.49, −0.01), p<0.05). There were significant improvements over time in hopelessness scores (p<0.001), quality of life subscales (both p<0.001), dysfunctional beliefs and attitudes about sleep (p<0.001), and nightmares (p<0.001) overall. However, there were no differences between treatment groups for any of these secondary outcomes.
Follow up post-randomization
Weekly telephonic follow-up for the two weeks after the conclusion of randomized treatment showed no loss of effect in Insomnia Severity Index or Scale for Suicide Ideation scores in either group (Figures 2 and 3).
Adverse Events
There were no deaths and no suicide attempts during any phase of this study. Pregnancy and subsequent miscarriage in one participant in the zolpidem-CR group was the sole serious adverse event observed, and it was judged not to be related to study participation. Regarding non-serious adverse events, 61% of the zolpidem-CR group and 71% of the placebo group experienced an adverse event, with the most common categories being “cold symptoms and flu”, “nausea and diarrhea”, and “headache” in descending rank order.
Discussion
This investigation found that the addition of zolpidem-CR to an SSRI was superior to placebo plus an SSRI in reducing insomnia symptoms in suicidal Major Depressive Disorder patients, similar to what we have described before in non-suicidal samples of Major Depressive Disorder patients with insomnia.38 The advantage for zolpidem-CR was principally in those patients with severe insomnia at baseline. We did not find that adding zolpidem-CR to SSRI provided an advantage in decreasing suicidal ideation scores on the Scale for Suicide Ideation, but we did find an advantage for zolpidem-CR in the suicide ideation score on the C-SSRS. As with the effect in insomnia scores, the impact of zolpidem-CR on C-SSRS suicide ideation scores was numerically greater in those patients with severe baseline insomnia. The clinical significance of the advantage for the zolpidem-CR group in C-SSRS suicide ideation scores was modest (−0.41 ± 0.21) even in the severe insomnia group, consistent with an advantage of a 50% decrease (half-point drop) drop between the item anchors “Have you actually had any thoughts of killing yourself?” versus “Have you wished you were dead or wished you could go to sleep and not wake up?”18;24
It is noteworthy that one measure of suicidal ideation (the Scale for Suicide Ideation) failed to show a treatment effect of zolpidem-CR, while another measure of suicidal ideation (the C-SSRS) did show an effect. However, there is no published literature on the relative performance of the Scale for Suicide Ideation versus the C-SSRS in randomized clinical trials, leaving no guidance on which measure should be preferred in randomized clinical trials of suicidality. We found significant correlation between the two measures of intensity of suicidal ideation, (Supplemental Table), but the structure of the scoring is sufficiently different that the scales may be measuring different constructs of ‘intensity’. The improvements in suicidality and insomnia that were seen during the randomized phase persisted after the discontinuation of bedtime medication into the 2-week telephonic follow-up. This finding is similar to what we reported with the addition of eszopiclone to SSRIs in depressed patients with insomnia.39
Still, significant improvements in insomnia intensity and suicidal ideation were seen also in the fluoxetine-plus-placebo group. While SSRIs are known to have adverse effects on objective sleep continuity, 40;41 patients may nevertheless report perceived improvements in insomnia, even as the objective measures worsen.42 This may related to a more positive appraisal of sleep as the depression lifts, or related to placebo effects.43
In our view, the safety findings of the study are as important as the efficacy findings. First, we found that it is possible to selectively recruit and safely retain suicidal Major Depressive Disorder outpatients in a pharmacotherapy randomized clinical trial and that they were highly adherent to the randomized clinical trial’s schedule of events.44 As a result, this study establishes methods that may be generalized to carry out double-blind, randomized trials of treatments for suicidality in outpatients. Second, we detected no worsening of suicidal ideation or the emergence of suicidal behavior under the controlled circmnstance of a randomized clinical trial, leading us to conclude that there are no reasons to systematically exclude suicidal outpatients from psychotropic randomized clinical trials.45
Reducing Suicidal Ideation Through Insomnia Treatment has strengths and limitations. Strengths include the innovation of a sample entirely comprised by patients with ongoing suicidal ideation (albeit without plan or intent), a relatively high proportion of minority participants, successful safety planning, and good retention. Limitations include the exclusion of suicidal patients with an active plan and imminent intent for suicide, as those patients would not be suitable for an outpatient study. Further, patients with bipolar disorder, primary psychotic disorders, and substance abuse were excluded, as were patients older than 65. The study did not include a specific measure of anxiety severity. The period of randomized treatment and follow up were short, and polysomnography was not an outcome measure. Also, as the C-SSRS was our second indicator of suicidality, if a Bonferroni correction were applied with α=0.025, then the C-SSRS finding would be judged of marginal significance. The study did not compare a hypnotic against cognitive behavior therapy for insomnia (CBT-I), and CBT-I has proven helpful for insomnia in depressed patients.46 Indeed, an open-label study of CBT-I was associated with reductions of suicidal ideation in depressed patients with insomnia.47 Clinical guidelines for chronic insomnia endorse CBT-I as a first line treatment.48;49 However, the onset of treatment effect of CBT-I is likely to be slower than that of a hypnotic, and time-to-onset-of-effect may be an important consideration in the beginning phases of treatment with suicidal patients. Additionally, the relationship between insomnia and suicidality must be understood in light of the possibility that underlying circadian dysfunction explain the results.50;51
Conclusion
Insomnia symptoms are drivers of suicidal ideation.3 Reducing Suicidal Ideation Through Insomnia Treatment shows that hypnotics are effective for insomnia in suicidal Major Depressive Disorder outpatients, and that the resolution of suicidal ideation positively co-varies with resolution of insomnia symptoms. Correspondingly, the addition of zolpidem-CR produced greater reductions in suicidality as compared with placebo, as measured by the C-SSRS. Still, SSRI-monotherapy was associated with some improvement in both insomnia and suicidality, and hence routine prescription of hypnotics as a means of mitigating suicidal ideation may not be necessary in all Major Depressive Disorder outpatients. However, they do suggest that with proper safety procedures it is possible to provide short-term relief of insomnia and more rapid reduction in suicidal ideation by time-limited prescribing of small quantities of hypnotics, without incurring major risk of emergent suicidal ideation, especially in those suicidal Major Depressive Disorder outpatients with the most severe insomnia.11
Supplementary Material
Acknowledgement:
The REST-IT team thanks Drs. John Mann, Anna Iltis, Dusan Hadzi-Pavlovic, John Winkelman, Maurizio Fava and Mr. Andy Hagler for their thoughtful service on our Data Safety Monitoring Board.
Funding: Supported by NIMH MH095776 (MCG), MH095780 (Duke), and MH095778 (WI)
Disclosures
Dr. McCall receives honoraria from Wolters Kluwer Publishing, CME Outfitters, and Anthem Inc., and research support from Merck and MECTA Corp, and is a scientific advisor for Sage Therapeutics and Jazz. Dr Benca is a consultant for Eisai, Jazz and Merck & Co., and receives grant support from Merck. Dr Rumble receives research support from Merck & Co. Dr. Szabo receives grant support from the NIH, Janssen, and Otsuka Pharmaceuticals and is a consultant to Neurocrine Biosciences, Teva Pharmaceuticals, Otsuka Pharmaceuticals, and Jazz Pharmaceuticals. Dr Krystal receives grant support from NIH, Janssen, Jazz, Axsome and Reveal Biomarkers, and is a consultant to Adare, Eisai, Ferring, Galderma, Idorsia, Jazz, Janssen, Takeda, Merck, Neurocrine, Pernix, Physician’s Seal. The other authors note no disclosures.
Footnotes
Trial registration number: ClinicalTrials.gov Identifier:
Reference List
- 1.Ivey-Stephenson AZ, Crosby AE, Jack SPD, et al. Suicide trends among and within urbanization levels by sex, race/ethnicity, age group, and mechanism of death - United States, 2001-2015. MMWR Surveill Summ 2017;66: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Current Psychiatry Reports 2013;15: 389-doi: 10.1007/s11920-013-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuromski KL, Cero I, Witte TK. Insomnia symptoms drive changes in suicidal ideation: a latent difference score model of community adults over a brief interval. Journal of Abnormal Psychology 2017;126: 739–749 [DOI] [PubMed] [Google Scholar]
- 4.McCall WV, Blocker JN, D′Agostino R Jr, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Medicine 2010;11: 822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, et al. Insomnia and daytime cognitive performance: a meta-analysis . Sleep Rev Med 2012;16: 83–94 [DOI] [PubMed] [Google Scholar]
- 6.Pollock L, Williams J. Problem-solving in suicide attempters. Psychol Med 2004;34: 163–167 [DOI] [PubMed] [Google Scholar]
- 7.Keilp J, Gorlyn M, Russell M, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt . Psychol Med 2013;43: 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keilp J, Gorlyn M, Oquendo M, et al. Attention deficit in depressed suicide attempters . Psychiatry Res 2008;159: 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keilp J, Sackeim H, Brodsky B, et al. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry 2001,158: 735–741 [DOI] [PubMed] [Google Scholar]
- 10.McCall W, Benca R, Rosenquist P, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry 2017;174: 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCall W, Benca R, Rosenquist P, et al. A multi-site randomized clinical trial to reduce suicidal ideation in suicidal adult outpatients with major depressive disorder: development of a methodology to enhance safety. Clinical Trials 2015;12: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 13.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep 2004; 27: 1567–1596 [DOI] [PubMed] [Google Scholar]
- 14.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2001;2: 297–307 [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198 [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 1979;47: 343–352 [DOI] [PubMed] [Google Scholar]
- 18.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments In: First MB, ed. Standardized Evaluation in Clinical Practice. Washington, DC: APPI Press; 2003:103–130 [Google Scholar]
- 19.McCall W, Benca R, Rumble M, et al. Prevalance of obstructive sleep apnea in suicidal patients with major depressive disorder. Journal of Psychiatric Research 2019;116: 147–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA, http:, www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm334798.htm. FDA requiring lower recommended dose for certain sleep drugs containing zolpidem. US Department of Health and Human Services; 2013: [Google Scholar]
- 21.Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther 1997;35: 1039–1046 [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Brown GK, Steer RA, et al. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide Life Threat Behav 1999;29: 1–9 [PubMed] [Google Scholar]
- 23.Brown GK, Beck AT, Steer RA, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. Journal of Consulting and Clinical Psychology 2000;68: 371–377 [PubMed] [Google Scholar]
- 24.Posner K, Oquendo M, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry 2007;164: 1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14: 540–545 [DOI] [PubMed] [Google Scholar]
- 26.Morin CM, Gramling SE. Sleep patterns and aging: comparison of older adults with and without insomnia complaints. Psychol Aging 1989;4: 290–294 [DOI] [PubMed] [Google Scholar]
- 27.McCall W, Batson N, Webster M, et al. Nightmares and dysfunctional beliefs about sleep mediate the effect of insomnia symptoms on suicidal ideation. Journal of Clinical Sleep Medicine 2013;9: 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krakow B, Melendrez DC, Johnston LG, et al. Sleep dynamic therapy for Cerro Grande fire evacuees with posttraumatic stress symptoms: a preliminary report. Journal of Clinical Psychiatry 2002;63: 673–684 [DOI] [PubMed] [Google Scholar]
- 29.Beck A, Steer R. Beck Hopeless Scale Manual. New York: Psychological Corporation; 1988 [Google Scholar]
- 30.Steer R, Beck A. Use of the Beck Depression Inventory, Hopelessness Scale, Scale for Suicidal Ideation, and Suicidal Intent Scale with Adolescents. Advances in Adolescent Mental Health 1988;3: 219–231 [Google Scholar]
- 31.Beck A, Brown G, Berchick R, et al. Relationship between hopelessness and ultimate suicide: A replication with psychiatric outpatients. American Journal of Psychiatry 1990;147: 190–195 [DOI] [PubMed] [Google Scholar]
- 32.Goldston D, Daniel S, Reboussin B, et al. Cognitive Risk Factors and Suicide Attempts Among Formerly Hospitalized Adolescents: A Prospective Naturalistic Study. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40: 91–99 [DOI] [PubMed] [Google Scholar]
- 33.Scott J, House R, Yates M, et al. Individual Risk Factors for Early Repetition of Deliberate Self-Harm. British Journal of Medical Psychology 1997;70: 387–393 [DOI] [PubMed] [Google Scholar]
- 34.Eisen SV, Dill DL, Grob MC. Reliability and validity of a brief patient-report instrument for psychiatric outcome evaluation. Hosp Community Psychiatry 1994;45: 242–247 [DOI] [PubMed] [Google Scholar]
- 35.Guy W ECDEU Assessment Manual for Psychopharmacology Rockville, MD: US Department of health, Education, and Welfare Public health Service: Alcohol, Drug, and Mental Health Administration; 1976: [Google Scholar]
- 36.Prudic J, Sackeim HA, Devanand DP. Medication Resistance and Clinical Response to Electroconvulsive Therapy. Psychiatry Research 1990;31: 287–296 [DOI] [PubMed] [Google Scholar]
- 37.McCall WV, Batson N, Webster M, et al. A psychometric cut-point to separate emergently suicidal depressed patients from stable outpatients. Indian Journal of Psychiatry 2013;55: 283–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry 1999;60: 668–676 [DOI] [PubMed] [Google Scholar]
- 39.Krystal A, Fava M, Rubens R, et al. Evaluation of Eszopiclone Discontinuation After Cotherapy with Fluoxetine for Insomnia with Coexisting Depression. Journal of Clinical Sleep Medicine 2007;3: 48–55 [PubMed] [Google Scholar]
- 40.Oswald I, Adam K. Effects of paroxetine on human sleep. Br J Clin Pharmacol 1986;22: 97–99 [PMC free article] [PubMed] [Google Scholar]
- 41.Dorsey C, Lukas S, Cunningham S. Fluoxetine-induced sleep disturbance in depressed patients. Neuropsychopharmacology 1996;14: 437–442 [DOI] [PubMed] [Google Scholar]
- 42.Gillin JC, Rapaport M, Erman MK, et al. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry 1997;58: 185–192 [DOI] [PubMed] [Google Scholar]
- 43.Perlis M, McCall V, Jungquist C, et al. Placebo effects in primary insomnia. Sleep Medicine Reviews 2005;9: 381–389 [DOI] [PubMed] [Google Scholar]
- 44.Smith P, Poindexter E, Cukrowicz K. The effect of participating in suicide research: does participating in a research protocol on suicide and psychiatric symptoms increase suicide ideation and attempts? Suicide Life Threatening Behavior 2010;40: 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sisti DA, Joffe S. Implications of zero suicide for suicide prevention research. JAMA 2018;doi: 10.1001/jama.2018.13083: [DOI] [PubMed] [Google Scholar]
- 46.Manber R, Buysse D, Edinger J, et al. Efficacy of Cognitive Behavior Therapy for Insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: a randomized clinical trial. Journal of Clinical Psychiatry 2016;77: e1317–e1323 [DOI] [PubMed] [Google Scholar]
- 47.Trockel M, Karlin B, Taylor C, et al. Effects of Cognitive Behavioral Therapy for Insomnia on Suicidal Ideation in Veterans. Sleep 2015;38: 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Int Medicine 2017;165: 125–133 [DOI] [PubMed] [Google Scholar]
- 49.Sateia MM, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine 2017;13: 307–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rumble M, Dickson D, McCall W, et al. The relationship of person-specific eveningness chronotype, greater seasonality, and less rhythmicity to suicidal behavior: a literature review. Journal of Affective Disorders 2018;227: 721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCall W A rest-activity biomarker to predict response to SSRIs in major depressive disorder. J Psychiatric Research 2015;64: 19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


