Abstract
Neutrophils are implicated in the pathogenesis of tuberculosis (TB), a disease caused by Mycobacterium tuberculosis infection, but the mechanisms by which they promote disease are not fully understood. Neutrophils can express cytokines that influence TB progression, and so we compared neutrophil and T cell expression of the Th1 cytokines IFNγ and TNF, the Th2 cytokine IL-4, and regulatory cytokine IL-10 in M. tuberculosis-infected macaques to determine if neutrophil cytokine expression contributes to dysregulated immunity in TB. We found that peripheral blood neutrophils produced cytokines after stimulation by mycobacterial antigens and inactive and viable M. tuberculosis. M. tuberculosis antigen-stimulated neutrophils inhibited antigen-specific T cell IFNγ production. In lung granulomas, neutrophil cytokine expression resembled T cell cytokine expression, and although there was histologic evidence for neutrophil interaction with T cells, neutrophil cytokine expression was not correlated with T cell cytokine expression or bacteria load. There was substantial overlap in the spatial arrangement of cytokine-expressing neutrophils and T cells, but IL-10-expressing neutrophils were also abundant in bacteria-rich areas between caseum and epithelioid macrophages. These results suggest that neutrophils contribute to the cytokine milieu in granulomas and may be important immunoregulatory cells in TB granulomas.
INTRODUCTION
Neutrophils are innate immune cells that play indispensable roles in protection against microbial infection. Neutrophils are well known for their phagocytic capacity and ability to produce reactive oxygen and nitrogen intermediates, antimicrobial peptides and proteases. These antimicrobial strategies can inhibit pathogen survival and dissemination but can also damage healthy tissue and thus are highly regulated. Neutrophils are often found in the granulomas1, the multicellular lesions caused by Mycobacterium tuberculosis infection, although the contributions of neutrophils to immunity in tuberculosis (TB) are complex and controversial2. Work done in zebrafish3 and rhesus macaques4 suggest neutrophils may be protective during acute infection and studies in humans have identified an inverse correlation between peripheral blood neutrophil numbers and disease risk5. The same study demonstrated that neutrophil-expressed antimicrobial peptides can kill M. tuberculosis5 but studies on post-acute TB in mice6,7, macaques8,9, and humans10–13 suggest that neutrophilic inflammation correlates with increasingly severe disease and higher bacteria numbers. The basis for this relationship is unclear, but neutrophils may promote mycobacterial persistence by serving as a nutrient reservoir for M. tuberculosis6 or providing a short-term replicative niche14. Less is known about what neutrophils do while they are in granulomas or if they contribute to the regulatory milieu in tuberculous granulomas.
A growing body of work demonstrates that neutrophils can express pro- and anti-inflammatory cytokines including IFNγ, TNF, IL-4, and IL-10 in response to host factors and pathogen associated molecular patterns (PAMPs)15,16. IFNγ production is often associated with T cells, but multiple lines of evidence indicate that peripheral blood neutrophils can express low levels of IFNγ17 and IFNγ expression can be upregulated after IL-12, IL-15, and IL-1817,18 signaling or exposure to microbial pathogens including Paracoccidioides brasiliensis18, Salmonella enterica19, and Listeria monocytogenes20. Moreover, neutrophil IFNγ expression is enhanced by LPS and IL-12 co-stimulation17, suggesting that neutrophil activation and cytokine expression is synergized by combining cytokine and toll like receptor (TLR) signaling. Like other myeloid lineage cells, neutrophils produce TNF under a variety of settings, including in M. tuberculosis infection21,22. Neutrophil express IL-4, an M2-polarizing cytokine associated with reduced protection against M. tuberculosis23, after exposure to Leishmania major promastigotes24. IL-10 is a macrophage deactivating anti-inflammatory cytokine that promotes mycobacterial persistence25,26, but also may have protective effects in TB27 by limiting pathologic inflammatory responses. Neutrophil IL-10 expression is well characterized in mice where it occurs during sepsis28, in response to microbial products29, ligation of the pattern recognition receptor adaptor CARD930, and infection with mycobacteria31. IL-10 expression by human neutrophils is more controversial32, but occurs upon interaction with LPS-stimulated regulatory T cells or exogenous IL-1033 and in bacterial pneumonia34.
Neutrophils may represent an unappreciated layer of immunoregulation in TB but their ability to engage in cytokine-mediated communication with other cells in granulomas remains unknown. Tightly-regulated crosstalk between neutrophils, T cells, and macrophages may be required for maintaining tissue homeostasis and resolving infections35–37, but in the inflammatory environment of a TB granuloma, activated neutrophils may express cytokines that can dysregulate immune responses and contribute to pathology. To examine this area of neutrophil biology in TB, we investigated whether neutrophils from M. tuberculosis-infected macaques can express cytokines in vitro and how this impacts T cell responses. We found that M. tuberculosis bacilli induced TNF, IL-4, and IL-10 expression by neutrophils in vitro and granuloma neutrophils expressed combinations of these cytokines and could also express IFNγ. Moreover, M. tuberculosis culture filtrate protein- (CFP) stimulated neutrophils were able to inhibit PBMC IFNγ production in vitro, but did not appear to regulate T cell cytokine expression in granulomas. Likewise, neutrophil cytokine expression did correlate with changes in bacteria load per granuloma suggesting that cytokine-expressing neutrophils are not strongly influencing anti-mycobacterial immunity in vivo. Granulomas are highly organized lesions and we found that cytokine-expressing neutrophils and T cells were present in partially-overlapping regions while TNF and IL-10-expressing neutrophils were also present at the interface between epithelioid macrophages and caseum. Our results suggest that neutrophils are unexpectedly complex and have immunomodulatory properties with implications for protection and pathology after M. tuberculosis infection.
MATERIALS AND METHODS
Animal ethics statement
The samples used in this study came from cynomolgus macaques (Macaca fascicularis) that were purchased from Valley Biosystems (West Sacramento, CA), Covance (Princeton, NJ), and a rhesus macaque (M. mulatta) from Bioqual (Rockville, MD). These animals were enrolled in studies in the laboratory of JoAnne Flynn at the University of Pittsburgh (Supplemental Table 1) and the samples analyzed here were kindly given for the purpose of these study. Animals were housed and maintained by the University of Pittsburgh’s Division of Laboratory Animal Resources and all procedures were performed in accordance with regulations established by the University of Pittsburgh’s Institutional Animal Care and Use Committee (IACUC). The IACUC adheres to national guidelines established in the Animal Welfare Act (7 U.S.C. Sections 2131–2159) and the Guide for the Care and Use of Laboratory Animals (8th Edition) as mandated by the U.S. Public Health Service Policy.
Blood and tissue cell isolation
Macaques were infected with M. tuberculosis (Erdman strain) by bronchoscopic instillation as previously described38. Some of the animals received treatments prior to necropsy or were re-infected via bronchoscopic instillation after the primary infection as per their original study design (Supplemental Table 1), and while the impact of these treatments on overall host-level responses is unknown, we do not anticipate they will significantly modify the cell-level results at the time of sample acquisition and processing. Macaque blood was obtained in heparin-containing vacutainer tubes by venipuncture. For flow cytometry experiments, erythrocyte-free leukocyte preparations were obtained by lysing the whole blood in PharmLyse buffer (BD Biosciences, San Jose, CA) washing the cells twice with PBS before stimulation. For applications using purified neutrophils, neutrophils were separated from lymphocytes and monocytes by Percoll (GE Healthcare Life Sciences, Pittsburgh, PA) gradient centrifugation where they sediment with erythrocytes and neutrophils were isolated as previously described39. This approach yields a highly enriched neutrophil preparation that is not activated but remains able to respond to stimuli including M. tuberculosis antigens8. Cells from granulomas were obtained as previously indicated40 using a Medimachine (BD Bioscience, San Jose, CA) and single cell suspensions were placed on ice until being surface stained and fixed with 2% paraformaldehyde-PBS.
Stimulation of blood cells and flow cytometry on blood and granulomas cells
Cells from peripheral blood were cultured in RPMI media (Thermo Fisher, Waltham, MA) containing 1% HEPES, AND 1% L-glutamine with 10% human AB serum (Gemini BioProducts, West Sacramento, CA) and stimulated in sterile polystyrene tubes with M. tuberculosis culture filtrate protein (CFP, 1 ug/mL; BEI Resources; Manassas, VA), or cocktails of ESAT6 and 38.1 peptides (38.1 is also known as CFP10 and indicated here as 38.1 to distinguish it from CFP; 1 ug/mL each peptide cocktail; BEI Resources, Manassas, VA), or PDBu (25 nM, Millipore-Sigma, St. Louis, MO) and ionomycin (5 μM, Millipore-Sigma) as previously described39. M. tuberculosis Erdman was grown to mid-log phase, quantified by spectrophotometry and cells were infected at a ratio of 1 bacterium per cell (MOI=1). Cells were incubated at 37°C with 5% CO2 for 30 minutes before addition of brefeldin A (BD Bioscience) followed by three more hours. Following this incubation, cells were washed, and surface stained for CD3 and CD11b, followed by fixation and intracellular staining for IFNγ (clone: B27; BD Bioscience), TNF (clone: MAB11; BD Bioscience), IL-4 (clone: 8D4–8; Thermo Fisher), and IL-10 (clone: JES2–9D7; Thermo Fisher), and calprotectin (clone MAC387; Thermo Fisher) labeled by Zenon labeling reagents (Thermo Fisher). For gating strategies for peripheral blood cells and tissue cells, see Supplemental Figure 1 and Supplemental Figure 2, respectively. Tissue cells were surface stained as for peripheral blood cells without a 3-hour incubation and intracellular cytokine staining was performed as previously indicated. Cytokine production in granulomas was identified by comparing unstimulated neutrophils and T cells from erythrocyte-free whole blood or Percoll gradient-isolated neutrophils against cells isolated from granulomas (Supplemental Figure 2). Data was acquired on an LSRII (BD Bioscience) maintained by the University of Pittsburgh’s Center for Vaccine Research or LSRFortessa (BD Bioscience) flow cytometer maintained by the Department of Infectious Diseases and Microbiology. Data was analyzed with FlowJo (BD Biosciences) version 9 for the Mac or version 10 for the PC. For comparing cytokine production by different cell types that have different autofluorescence profiles and are stained with different fluorochromes, used relative mean fluorescence intensity (MFI) where the populations’ fluorescence profiles were normalized by comparing ratios of MFIs for the cytokine-positive and cytokine-negative fractions of each population where we could not perform direct population-by-population comparisons41.
Nanostring transcriptional analysis
Neutrophil cytokine responses were measured with a macaque-specific Nanostring kit (Nanostring, Seattle, WA) targeting 770 immunology-associated genes. Highly-enriched neutrophils were aliquoted into two tubes and incubated with or without viable M. tuberculosis (MOI=1) for 3 hours before being solubilized in Trizol. RNA was isolated using RNAeasy Kits (Qiagen, Germantown, MD) after phenol chloroform isolation as per Mattila et al1. 100 ng of RNA was supplied to the University of Pittsburgh Genomics Research Core for Tapestation analysis to confirm high-quality RNA had been obtained and the Nanostring assay was performed as per manufacturer recommendations. Data was analyzed using the nSolver 4.0 software package. Briefly, raw transcript counts were normalized using negative and positive synthetic sequences provided within each codeset to account for background noise and technical variation, respectively. Normalization between samples was carried out by selecting 2 to 5 genes with the least amount of variation between samples (%CV<30%). Data are presented as normalized transcript counts.
IFNγ ELISPOT assays
IFNγ ELISPOT (MABtech, Cincinnati, OH) assays were performed as per manufacturer instructions and previously described39. Briefly, PBMCs and neutrophils were obtained from macaques as previously indicated and three cell suspensions were added to duplicate wells: neutrophils only (125,000 cells/well), PMBCs (125,000 cells per well) and neutrophils with PBMCs (1:1 ratio, total 250,000 cells per well). Cells were stimulated with CFP or a cocktail of M. tuberculosis ESAT-6 and 38.1 peptides as previously described8 and incubated overnight at 37°C with 5% CO2. T cell viability after this treatment was experimentally confirmed and not found to be impaired by overnight co-culture with CFP-stimulated neutrophils. Plates were read with a CTL SPOT reader (Immunospot, Cleveland, OH) and analyzed for the number of spots per well, spot size, and signal intensity per spot.
Flow cytometry-based TLR antagonism assay
The TLR antagonists CU CPT22 (TLR1/2 inhibitor; final concentration: 0.5 μM) and C34 (TLR4 inhibitor; final concentration: 10 μM) were purchased from Tocris (Bio-Techne, Minneapolis, MN). For TLR antagonism assays, neutrophils were isolated from Mtb-naive animals to minimize the potential that cytokines produced by Mtb-specific T cells, rather than mycobacterial antigens, would modify neutrophil responses. Neutrophils were pre-incubated with TLR antagonists for 30 minutes before addition of gamma-irradiated (dead) M. tuberculosis, and stimulation and intracellular cytokine staining was performed after three hours of incubation as previously described. Data were normalized to percent of expression relative to M. tuberculosis-stimulated control cells without TLR inhibition.
Immunohistochemistry on formalin-fixed paraffin (FFPE) tissues
Immunohistochemistry was performed on 5 μm-thick FFPE tissue sections as previously indicated1,39. Tissues were stained with combinations of primary antibodies including polyclonal rabbit anti-IFNγ (Abcam, Cambridge, MA), rabbit anti-TNF (Bioss Antibodies, Boston, MA), rabbit anti-IL-4 (Abcam), rabbit anti-IL-10 (Abcam), rabbit anti-CD3 (Agilent, Santa Clara, CA), mouse anti-calprotectin (clone: MAC378, ThermoFisher), mouse anti-CD163 (clone: 1D6, ThermoFisher), and mouse anti-human alveolar macrophage antibody (HAM56, Enzo Life Sciences, Farmingdale, NY). Tissues were subsequently stained with appropriate anti-rabbit and anti-mouse secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 1 hr at room temp, and coverslips were mounted with DAPI-containing Prolong Gold mounting medium (ThermoFisher). Cells were imaged on an Olympus confocal microscope (Olympus, Waltham, MA) running FlowView 1000 software maintained by the University of Pittsburgh’s Department of Microbiology and Molecular Genetics, or a Nikon e1000 epifluorescence microscope running Nikon Elements (Nikon Instruments, Melville, NY). Images were annotated with Photoshop CS5.1 (Adobe Systems, San Jose, CA) and projections of z-stacks were made with the FIJI build of ImageJ42. Quantitative image analysis was performed with CellProfiler43.
Statistics
A normality test was performed for all datasets, and since they were not normally distributed, nonparametric analyses were performed. Flow cytometry data was analyzed with GraphPad Prism version 7 (GraphPad Software, La Jolla, CA) using Mann-Whitney or Rank Sum tests with p<0.05 considered statistically significant. Multivariate nonparametric correlation analyses with Spearman’s ρ were performed with JMP Pro v13 (SAS Software, Cary, NC).
RESULTS
Neutrophils interact with T cells and macrophages in vivo
We used IHC to confirm the localization of neutrophils in granulomas and investigate their interactions with other cells in vivo. We found that neutrophils were distributed throughout granulomas (Figure 1A, 1B) including the lymphocyte cuff and adjacent lung tissue where they often had elongated phenotypes consistent with motile cells. In the lymphocyte cuff, neutrophils could be found engaged with T cells along distinct cell-cell contacts reminiscent of immunologic synapses (Figure 1C). The frequency of neutrophil-T cell pairs was highly variable but in some 40x microscopic fields up to 19% of neutrophils in the lymphocyte cuff were engaged with T cells (n=14 images from 7 animals [2 fields/granuloma/animal], mean = 9.5%, 7.3% SD [range=0–19 pairs]). Neutrophil interactions with macrophages were difficult to identify in this region, although isolated instances of CD163+ macrophages with phagocytosed neutrophils were found in the lymphocyte cuff (Figure 1D). In necrotic granulomas, neutrophils accumulated at the interface between epithelioid macrophages and caseous necrosis, an area that can harbor substantial numbers of M. tuberculosis bacilli1, and cells in this region often had degenerative phenotypes associated with cells undergoing necrosis or apoptosis. HAM56+ epithelioid macrophages appeared to phagocytose the dying neutrophils in this region (Figure 1E). These data suggest that neutrophils interact with other cells in granulomas in previously unappreciated ways in granulomas, thus leading us to investigate whether neutrophils express cytokines that have important immunoregulatory properties in TB.
Figure 1. Neutrophils interact with other cells as they migrate through granulomas.
A. Densely packed neutrophils are present in the space surrounding acellular caseum (indicated in DAPI) in a cynomolgus macaque granuloma. Scale bar = 200 μm. B. Plotting the location of granulomas demonstrates neutrophils (blue) are present in multiple granuloma regions including the lung adjacent to granulomas, the lymphocyte cuff (CD3+ T cells; green) and are especially abundant between caseum and CD68+ epithelioid macrophages (red). C. Neutrophils (pseudocolored magenta) and T cells (green) interact along immunologic synapse-like structures (white). Scale bar = 5 μm. D. CD163+ macrophages (green) in the lymphocyte cuff phagocytose apoptotic-appearing calprotectin+ neutrophils (blue, arrow). HAM56 staining is indicated in red. Scale bar = 10 μm. E. Neutrophils accumulate at the caseum-epithelioid macrophage interface (stained with HAM56, red) where epithelioid macrophages can be seen phagocytosing neutrophils (blue, arrows). CD163+ lymphocyte cuff macrophages are indicated in green. Scale bar = 20 μm.
Spatial organization and cell-cell interactions in granulomas
M. tuberculosis antigens and bacilli induce cytokine expression in macaque peripheral blood neutrophils
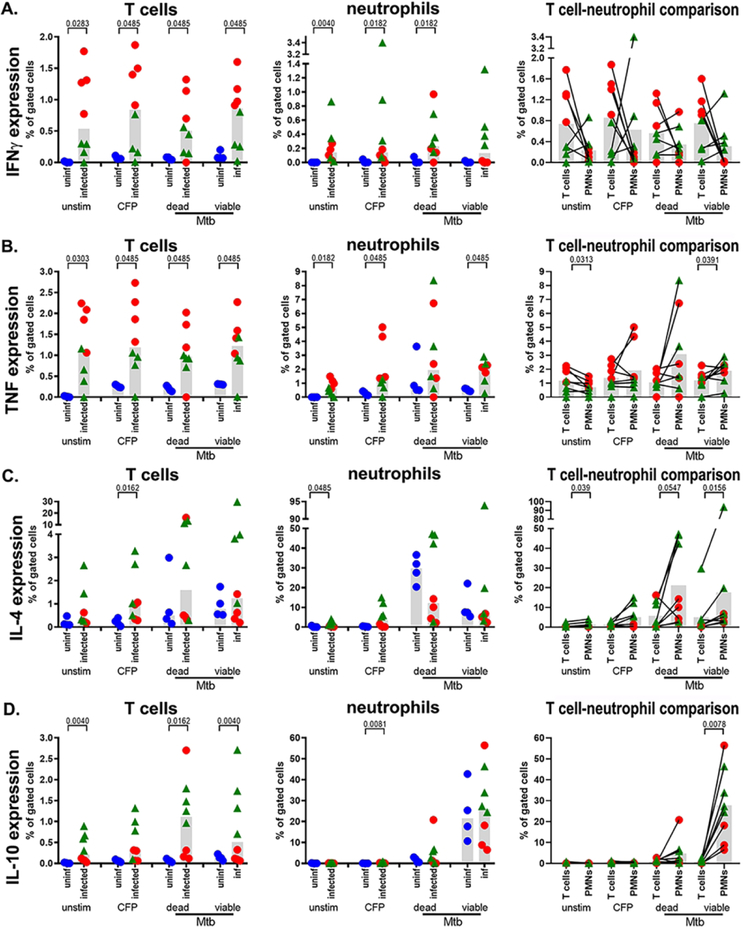
We investigated neutrophil expression of IFNγ, TNF, IL-4 and IL-10 because of the relationship these cytokines have with protection or pathology in TB, and compared expression by neutrophils and T cell to evaluate their capacity as cytokine-expressing cell type in TB. To do this, we stimulated peripheral blood neutrophils and T cells from uninfected macaques and macaques at two weeks (corresponding to initiation of adaptive immunity) or eight weeks (corresponding to robust adaptive immunity) post-infection with mycobacterial antigens or inactivated or viable M. tuberculosis bacilli. We found substantial variability in T cell and neutrophil IFNγ and TNF responses, but overall, but cells from infected animals were more responsive than uninfected animals (Figure 2A, 2B). When T cells and neutrophils from infected animals were compared, we found more TNF+ T cells in unstimulated cultures and TNF+ neutrophils in live M. tuberculosis-stimulated cultures, but similar proportions of IFNγ+ and TNF+ cells in the other conditions (Figure 2A, 2B). For IL-4 and IL-10 expression (Figure 2C, 2D), there was a trend toward more cytokine-expressing cells in infected animals, regardless of stimulation, and statistically significantly increases in IL-4+ T cells after CFP stimulation. There were increased frequencies of IL-10+ T cells in unstimulated cells and bacilli-stimulated cells from infected monkeys (Figure 2D). CFP induced a small, but statistically significant (median of 0.023% for uninfected vs median of 0.20% for infected), increase in IL-10 expression by neutrophils from infected animals. Although there were significant differences between IL-4 and IL-10 expression by unstimulated (Figure 2C) or CFP-stimulated neutrophils (Figure 2D), neutrophil IL-4 and IL-10 expression were similar between groups. When T cells and neutrophils from infected monkeys were compared, bacteria-stimulated neutrophils were more likely to be IL-4+ (Figure 2C) and more neutrophils expressed IL-10 after stimulation with viable M. tuberculosis (Figure 2D).
Figure 2. Cytokine expression by peripheral blood T cells and neutrophils.
Cells were stimulated ex vivo and cytokine expression quantified by flow cytometry. Three factors including T cell cytokine expression (left column), neutrophil cytokine expression (middle column) and a comparison between T cell and neutrophil cytokine expression (right column) were assessed for (A) IFNγ, (B) TNF, (C) IL-4, and (D) IL-10 expression. Uninfected animals (n=4; uninf; blue circles), 2-week post-infection animals (n=4; red circles), and 8-week post-infection infected (n=4; green triangles) were examined. The Wilcoxon matched-pairs test was used for pairwise comparisons.
Cytokine expression by stimulated neutrophils
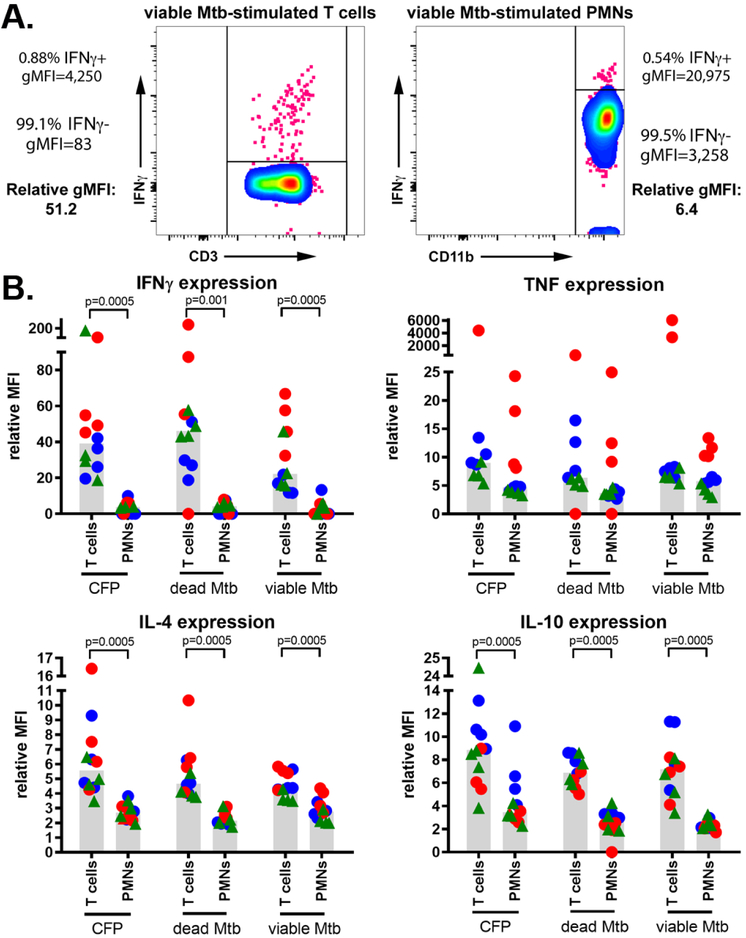
Neutrophils have fewer ribosomes than T cells44, and we predicted that neutrophils would express less cytokine per cell than T cells. We established a relative mean fluorescent intensity (MFI) metric to compare subsets with different autofluorescence profiles where ratio of geometric mean fluorescences (gMFI) for cytokine positive and cytokine negative populations in each subset were compared (Figure 3A). Consistent with our hypothesis, on a per cell basis, we found that neutrophils produced less IFNγ, IL-4, and IL-10 than comparable T cells (Figure 3B). Interestingly, there was parity between cell types for TNF expression, suggesting neutrophils vary in their ability to express cytokines in a cytokine-dependent manner. To further confirm that neutrophils were able to express cytokines, we did a Nanostring-based transcriptional analysis on highly-purified neutrophil RNA after incubation with viable M. tuberculosis and found trends that were similar to our flow cytometry-based analysis including stimulated neutrophils expressing low levels of mRNA for IFNγ, IL-4, and IL-10 but significantly elevated expression of TNF mRNA (Supplemental Figure 3). These data demonstrate that peripheral blood neutrophils can express pro- and anti-inflammatory cytokines, albeit at lower levels than T cells. Moreover, while IFNγ and TNF expression were partially dependent on host infection status, neutrophil IL-4 and IL-10 expression was independent of host infection status.
Figure 3. T cells and neutrophils have intrinsic differences in their capacities to produce cytokines.
(A) The relative gMFI of cytokine-positive cells, normalized against each cell population’s baseline fluorescence profile to generate a relative MFI and (B) cytokine expression was compared via the Wilcoxon matched-pairs test for pairwise comparisons. Markers represent samples from uninfected animals (n=4; blue circles), 2-week post-infection animals (n=4; red circles), and 8-week post infection animals (n=4; green triangles).
Neutrophil cytokine expression differs from T cell cytokine expression.
Neutrophils predominantly express a single cytokine after in vitro stimulation
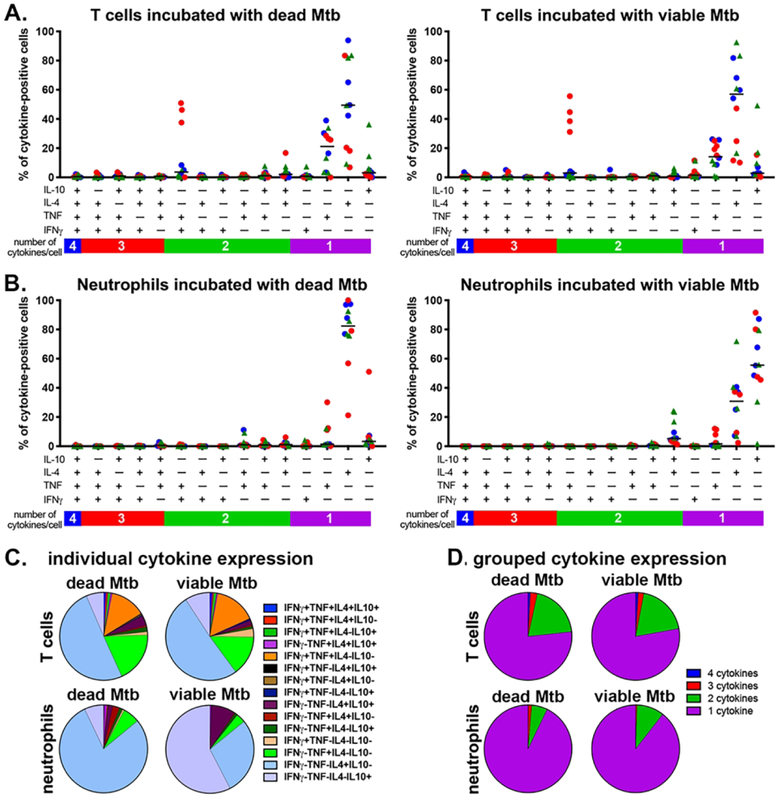
Polyfunctionality, or the ability to simultaneously produce multiple cytokines, is an important characteristic that is correlated with disease severity in TB45,46 and SIV-M. tuberculosis co-infection47. To assess whether neutrophils are polyfunctional and compare their capacities against T cells, we identified which combinations of cytokines were being expressed by cytokine-positive cells after stimulation with M. tuberculosis bacilli. Frequencies of IL-4+ or TNF+ T cells were similar for irradiated- or viable-M. tuberculosis stimulated cells (Figure 4A). There were relatively few polyfunctional T cells in the peripheral blood, although M. tuberculosis induced IFNγ+TNF+ expression in a subset of T cells from animals sampled at two weeks post-infection (Figure 4A). Cytokine-positive peripheral blood neutrophils predominantly expressed either IL-4+ or IL-10+ after stimulation with inactivated or viable M. tuberculosis, respectively (Figure 4B). When we evaluated the mean cytokine expression per group, we found T cell phenotypes were largely IL-4+, IL-10+, TNF+, or double-positive IFNγ+TNF+ after stimulation (Figure 4C) while neutrophils expressed IL-4 in response to inactivated M. tuberculosis, or IL-4+, IL-10+, or IL-4+IL-10+ in response to viable M. tuberculosis (Figure 4C). In both cell types and treatments, the dominant phenotypes were single- and two cytokine-positive cells, with very small populations of three- and four-cytokine positive cells (Figure 4D). These data indicate that peripheral blood neutrophils have limited capacities for polyfunctionality in vitro but subsets of these cells are capable of expressing more complex combinations of cytokines.
Figure 4. Cytokine expression by peripheral blood neutrophils and T cells is of limited polyfunctionality.
The phenotypes of cells incubated with gamma-irradiated (dead) M. tuberculosis (Mtb) or viable Mtb-stimulated cells (Figure 2) were assessed by Boolean gating. A. Polyfunctional profiles in T cells and (B) neutrophils after stimulation with dead and viable M. tuberculosis. Uninfected animals (uninf; blue circles), 2-week post-infection animals (red circles), and 8-week post-infection infected (red triangles) were examined. C. Mean proportion of T cell and neutrophil polyfunctional profiles per subset. D. Cytokine expression profiles grouped into 1+, 2+, 3+, or 4+ cytokine phenotypes.
Peripheral blood neutrophils have less polyfunctionality than peripheral blood T cells.
Neutrophil responses are driven by toll-like receptor (TLR) ligation
Neutrophil cytokine expression can be induced by TLR signaling31, and to understand how these responses are initiated by M. tuberculosis in vitro, we stimulated neutrophils from M. tuberculosis-naive animals with gamma-irradiated M. tuberculosis in presence of two selective TLR inhibitors, CU CPT22 for TLR1/2 and C34 for TLR4, to measure the importance of these receptors in the absence of stimulation by antigen-specific T cell-produced cytokines. We stained these cells for TNF, IL-4 and IL-10 to test this hypothesis but did not evaluate IFNγ because of its low level of expression by in peripheral blood neutrophils (Figure 2A). We found that TNF production was unaffected by TLR1/2 or TLR4 inhibition. In comparison, TLR1/2 inhibition substantially decreased IL-4 and IL-10 expression (Supplemental Figure 4) but TNF expression was not modified by TLR4 inhibition, suggesting that neutrophil IL-4 and IL-10 expression after stimulation with mycobacterial products is driven by TLR1/2 signaling.
Neutrophils can downregulate T cell responses in vitro
Neutrophils interact with T cells in granulomas (Figure 1C) and secrete immunosuppressive cytokines after stimulation (Figure 2), thus they may modulate T cell responses. We used IFNγ ELISPOT assays to determine whether neutrophils can regulate a protective T cell function. We assayed three conditions (PBMCs alone, neutrophils alone, and PBMCs co-cultured with neutrophils) where cells from M. tuberculosis-infected macaques were stimulated with a cocktail of peptides from the immunodominant M. tuberculosis antigens ESAT-6 and 38.1 (38.1 is also known as CFP-10), or CFP. We used CFP as a stimulator in this assay instead of intact bacteria because we have found it activates neutrophils8 and induces IL-10 expression (Figure 2D) but does not cause extensive cell death in overnight cultures. Previous work demonstrated that neutrophils are not strongly activated by ESAT6+38.1 peptides8 and we used this as a control for T cell responses in the absence of substantial neutrophil activation. ESAT6+38.1 stimulation did not significantly modify IFNγ secretion by PBMCs co-cultured with neutrophils, although there were significant decreases in mean spot size/cell, a metric describing the amount of IFNγ secreted per cell (Figure 5). In contrast, PBMCs co-cultured with neutrophils in the presence of CFP contained significantly fewer IFNγ secreting cells and these cells produced less IFNγ per cell than PBMCs alone (Figure 5). These data suggest that unstimulated neutrophils can have mild inhibitory effects on PBMCs while activated neutrophils can be immunosuppressive for IFNγ production.
Figure 5. Neutrophils inhibit T cell IFNγ cytokine production.
Percoll-isolated PBMCs and PBMCs co-cultured with autologous neutrophils (PMN) were cultured overnight in the presence of ESAT6 and CFP, or CFP and spots (IFNγ-producing cells), spot size, and spot intensity were quantified. Data represent the mean of two replicates per animal, with each animal represented by a marker (n=7 animals). Statistical comparisons of paired data determined by Wilcoxon Matched-Pairs Signed Rank test.
The granuloma environment enhances neutrophil cytokine expression
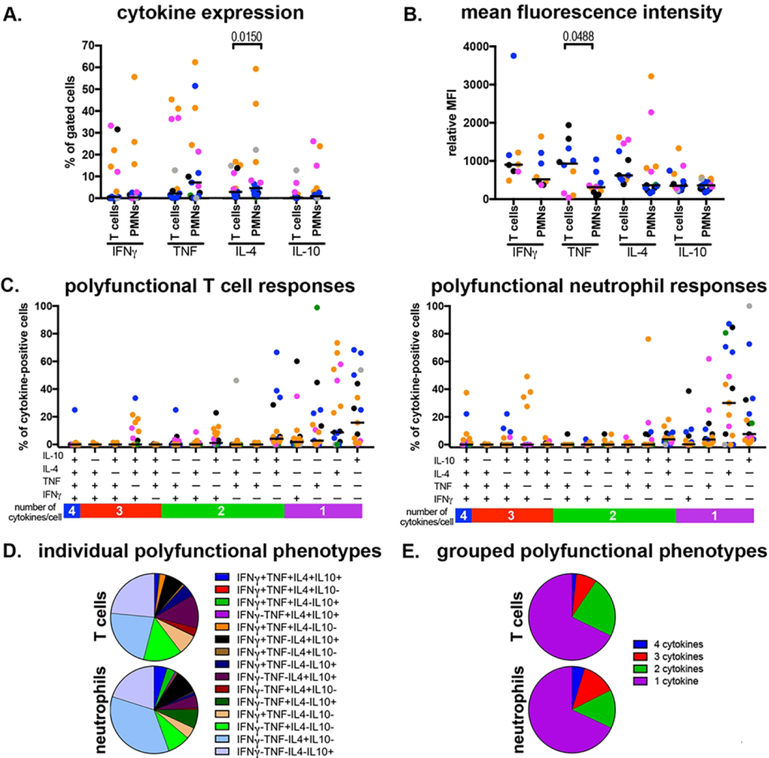
Since M. tuberculosis infection primarily occurs in the lungs rather than the blood, we compared neutrophil and T cell responses in lung granulomas. We found that T cell and neutrophil cytokine responses in lung granulomas were similar, with the only difference being significantly larger populations of IL-4+ T cells (Figure 6A). Similarly, T cells and neutrophils expressed comparable quantities of IFNγ, IL-4, and IL-10 while T cells produced significantly more TNF (Figure 6B). We examined neutrophil polyfunctionality to determine whether the combination of host factors including cytokines and damage-associated molecular patterns (DAMPs) and bacterial PAMPs in granulomas change their abilities to produce multiple cytokines and found a strong trend toward (Figure 6C) increased T cell and neutrophil polyfunctionality. For T cells, we found substantial frequencies of IL-4+IL-10+ cells (12.3%), IFNγ+IL-10+ cells (4.6%) and IFNγ+IL-4+IL-10+ cells (9.6%) (Figure 6D). For neutrophils, we found surprisingly large populations of IL-4+IL+10+ (4.8%), IFNγ+TNF+IL-10+ (3.1%) and IFNγ+TNF+IL-4+IL-10+ (4.8%) cells (Figure 6D). In both cases, however, single- (67.7% and 68.1%) and two-function (14.3% and 22.8%) T cells and neutrophils, respectively, were the dominant phenotypes observed in lung granulomas (Figure 6E).
Figure 6. Neutrophils and T cells can express a similar range of cytokines in lung granulomas.
Lung granulomas (n=16) were disaggregated into single-cell suspensions for analysis by flow cytometry. A. Proportion of cytokine-expressing T cells and neutrophils (PMNs) in lung granulomas. B. Comparison of relative cytokine expression (relative MFI) for cytokine-positive T cells and neutrophils in lung granulomas. To be included, a granuloma must have cytokine-positive neutrophils and T cells. C. Polyfunctionality in lung granuloma T cells (left) and neutrophils (right). D. Mean frequencies of different polyfunctional phenotypes in granuloma T cells and neutrophils. E. Grouped polyfunctional phenotypes in granuloma T cells and neutrophils. Each marker indicates one granuloma, and each color represents a different monkey. Statistical comparisons of paired data determined by Wilcoxon Matched-Pairs Signed Rank test.
Neutrophil and T cell cytokine expression in lung granulomas.
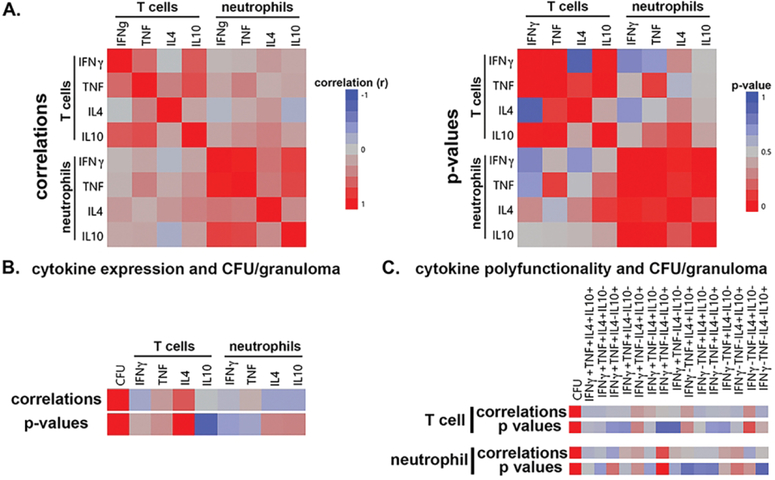
The observation that neutrophils and T cells interact in granulomas (Figure 1C), and neutrophils can influence T cell IFNγ expression ex vivo (Figure 5), suggests that neutrophils may be able to modulate T cell responses in vivo. To examine this question, we performed correlation analyses of cytokine expression between cell subsets, polyfunctionality, and then examined how neutrophil or T cell cytokine expression relates to bacterial burden. We found significant correlations within a cell type for overall cytokine expression (p<0.05), but little evidence for a relationship between cell types (Figure 7A), suggesting that each cell type is activated by a common set of stimuli in lung granulomas and, within the limits of this analysis, the cells are not directly regulating each other. We also investigated whether neutrophil cytokine expression correlated with bacteria load in the lesions where bacteria loads were quantified at necropsy (n=16 lung granulomas). These analyses did not identify strong associations between bacteria load and single-cytokine expression (Figure 3B) or polyfunctionality (Figure 3C) in either cell subset, suggesting that, in the granulomas we analyzed, bacteria numbers were independent of the cytokine expression measured here. Taken together, these results suggest that T cells and neutrophils do not regulate each other’s cytokine expression in granulomas and that cytokine expression in granulomas is more complex than can be explained by the number of bacteria per granuloma.
Figure 7. Correlations of T cell and neutrophil cytokine expression and bacteria load per granuloma.
A. Correlating T cell and neutrophil cytokine expression in lung granulomas. Correlations (left) and p value significance (right) are presented. B. Correlation of T cell and neutrophil cytokine expression and bacteria per granuloma (CFU). Correlation and significance are denoted by colors indicated in (A). C. Correlation of polyfunctional T cell and neutrophil cytokine expression with CFU per granuloma. Correlation and significance are denoted by colors indicated in A.
Correlations of T cell and neutrophil cytokine expression and bacteria load per granuloma.
Cytokine expressing neutrophils localize to different granuloma regions
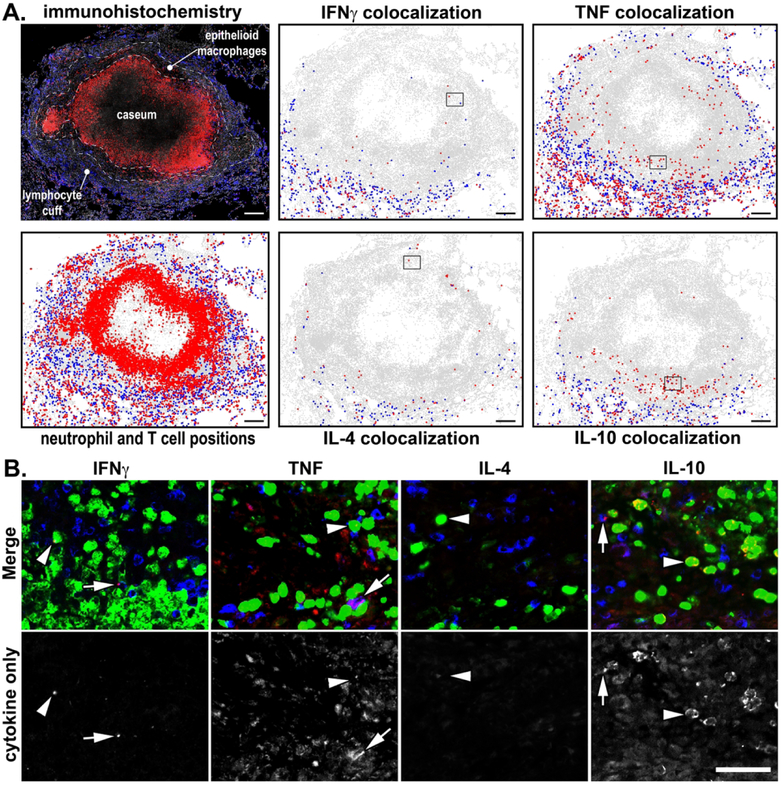
Granulomas have functionally unique microenvironments defined by different cell populations, antigen abundance, and oxygen tension1,48. To assess whether cytokine expression differs by microenvironment, we used IHC to identify the location of cytokine-positive neutrophils and T cells in different granuloma regions including the lymphocyte cuff, epithelioid macrophage region, and caseum (Figure 8A, 8B). Overall, the frequency of cytokine positive cells was consistent with our flow cytometry-based results with 2.2%, 10.2%, 2.0%, and 3.8% of neutrophils colocalizing with IFNγ, TNF, IL-4, and IL-10, respectively, while 21.9%, 53.8%, 12.0%, and 13.5% of T cells colocalizing with IFNγ, TNF, IL-4, and IL-10, respectively. We found most cytokine-positive cells were located in the granuloma’s lymphocyte cuff and at the interface between adjacent lung tissue (Figure 8B). We also noted that TNF+ and IL-10+ neutrophils were also present in the region adjacent to caseum, an area commonly associated with few T cells and large bacterial burdens1. Taken together, these data suggest that neutrophils express cytokines in multiple granuloma microenvironments and suggest that both host cell factors and mycobacterial antigens may drive neutrophil activation and cytokine expression.
Figure 8. Spatial localization of T cell and neutrophil cytokine expression in lung granulomas.
A. Immunohistochemistry was performed on a necrotic granuloma, and the location of cytokine-positive T cells (blue) and neutrophils (red) was identified and plotted. The lymphocyte cuff, epithelioid macrophage region, and caseous center are included for context. Scale bars = 200 μm. B. Boxes in the plotted positions (A) are included to show the staining characteristics for cytokine-positive T cells (blue) and neutrophils (green), arrowheads indicate cytokine+ neutrophils and arrows indicate cytokine+ T cells. Cytokine expression within each panel is indicated in red. Scale bar, lower right = 50 μm.
Spatial localization of T cell and neutrophil cytokine expression in lung granulomas.
DISCUSSION
Neutrophils are implicated in the pathogenesis of TB, but important aspects of their biology, including how they interact with other cells in granulomas to promote disease, remain unknown. Immunomodulatory properties may exacerbate disease by dysregulating the balance of pro- and anti-inflammatory responses in granulomas. To explore this hypothesis, we investigated neutrophils cytokine expression in response to mycobacterial antigens and in vivo to identify potential contributions to the regulatory milieu in cynomolgus and rhesus macaques, nonhuman primates that mimic nearly all aspects of human TB. We found that macaque neutrophils expressed a surprisingly broad repertoire of cytokines that overlapped with T cell-expressed cytokines, but expression was independent of adaptive immunity and could be induced by M. tuberculosis bacilli. Interestingly, our data suggest that neutrophils are differentially affected by host factors and bacterial antigens, and neutrophils stimulated with intact bacilli in vitro expressed TNF, IL-4, or IL-10, but little IFNγ, while neutrophils in granulomas also expressed IFNγ. Moreover, exogenously-stimulated peripheral blood neutrophils exhibited little polyfunctionality while neutrophils in granulomas were considerably more polyfunctional, oftentimes more polyfunctional than T cells from the same lesion. This raises important questions about the overall impact of neutrophil cytokine expression in granulomas relative to T cells, but in most cases, T cell are more numerous than neutrophils in granulomas and express more cytokine per cell than neutrophils, thus T cells are likely to have a greater contribution to a granuloma’s cytokine milieu than neutrophils. That said, neutrophils are present in more of a granuloma’s microenvironments than T cells, and are activated by both host and microbial factors, and this may differentially impact the effect of their cytokine expression.
Granuloma bactericidal activity and homeostasis requires a microenvironment-appropriate equilibrium between pro-inflammatory and anti-inflammatory cytokines to maximize macrophage activation and minimize immune response-mediated damage to uninvolved tissue. In nonhuman primates, this concept is best defined for T cells, and granulomas with balanced T cell IL-17 and IL-10 expression are more likely to develop sterilizing immunity than granulomas with different cytokine profiles27. Our data did not support a similar relationship for neutrophil cytokine expression and bacteria per granuloma in vivo, but this may be attributable to the small subset of granulomas we examined or basic aspects of neutrophil biology not investigated here. Likewise, although we identified interactions between neutrophils and T cells in granulomas, we could not resolve how this impacts cytokine expression or activation. Unlike tightly-regulated T cell cytokine expression, neutrophils can be activated by PAMPs via TLR ligation and host factors including cytokines, and this complex web of interactions may better define the relationship between neutrophils and bacteria load39. Under this framework, elevated neutrophil cytokine expression may reflect stimuli-rich environments and neutrophil responses may have increasingly negative effects on granuloma homeostasis as cytokine expression, bacteria numbers, and necrosis increase in severe disease. The target of neutrophil-produced cytokines is unclear but granulomas have characteristic patterns of STAT phosphorylation associated with macrophage polarity including IFNγ-stimulated phospho-STAT1+ epithelioid macrophages and IL-10-stimulated phopho-STAT3+ lymphocyte cuff cells49 and neutrophilic inflammation does not appear to change this overall organization (data not shown). This suggests that neutrophil-expressed anti-inflammatory cytokines are not regulating the M. tuberculosis-infected epithelioid macrophages but are likely to have a greater influence on lymphocyte cuff macrophages.
Recent work suggests that neutrophils may have at least two polarization states, N1 and N2, reminiscent of the polarization states in macrophages and T cells50,51. We found that pro- and anti-inflammatory neutrophils are abundant in granulomas, but instead of falling into discrete N1 or N2 categories based on cytokine expression we found a range of overlapping pro- or anti-inflammatory cytokine expression rather than a binary set of phenotypes. Moreover, the lifespan of a neutrophil once it enters a granulomas is unknown and could range from hours to days52 so rather than remaining in a single polarization state, neutrophils could move through the N1-N2 spectrum as they experience stimuli specific to different microenvironments. In addition to putatively N2-polarized neutrophils, the anti-inflammatory neutrophils we identified could resemble granulocytic myeloid-derived suppressor cells (MDSCs) that can downregulate anti-mycobacterial CD4+ T cell responses53. We found that activated peripheral blood neutrophils suppressed PBMC IFNγ production ex vivo whereas the neutrophils in granulomas had more complex phenotypes that included co-expression of pro- and anti-inflammatory cytokines in a fashion that is not associated with traditional MDSCs.
Neutrophils are critical components of vertebrate immunity and our data support a growing body of evidence suggesting neutrophils actively shape their immune environment by modifying neighboring cells’ behaviors. Our findings demonstrate that activated neutrophils are unappreciated sources of cytokine expression in TB that rival T cells for the diversity of cytokines they can express, the quantity of cytokine per cell, and the capacity to simultaneously express multiple cytokines. Moreover, neutrophils express cytokines in more granuloma microenvironments than T cells, potentially giving them the ability to influence different cell subsets. Consequently, neutrophil cytokine expression may represent another layer of regulation or pathogenesis in granulomas with implications for treatment of TB.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge JoAnne Flynn and Philana Ling Lin for supplying macaque blood and tissues for this project and the technical assistance provided by Mark Rodgers, Carolyn Bigbee, Cassaundra Ameel, and the Flynn Lab veterinary staff. Partial support for HPG was provided by a Biomedical Research Grant from the American Lung Association. Partial support from JTM was provided by funding from NIH AI134183, NIH AI118195, and startup funds from the University of Pittsburgh Graduate School of Public Health’s Department of Infectious Diseases and Microbiology.
Footnotes
Conflict of Interest Disclosure: The authors declare no conflicts of interest in this work.
Literature cited
- 1.Mattila JT et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. Journal of immunology (Baltimore, Md.: 1950) 191, 773–784, doi: 10.4049/jimmunol.1300113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A & Martineau AR Neutrophils in tuberculosis: friend or foe? Trends Immunol 33, 14–25, doi: 10.1016/j.it.2011.10.003 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Sugawara I, Udagawa T & Yamada H Rat neutrophils prevent the development of tuberculosis. InfectImmun 72, 1804–1806 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen SG et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nature medicine 24, 130–143, doi: 10.1038/nm.4473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martineau AR et al. Neutrophil-mediated innate immune resistance to mycobacteria. The Journal of clinical investigation 117, 1988–1994, doi: 10.1172/jci31097 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra BB et al. Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat Microbiol 2, 17072, doi: 10.1038/nmicrobiol.2017.72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nandi B & Behar SM Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med 208, 2251–2262, doi: 10.1084/jem.20110919 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattila JT, Maiello P, Sun T, Via LE & Flynn JL Granzyme B-expressing neutrophils correlate with bacterial load in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Cellular microbiology, doi: 10.1111/cmi.12428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopal R et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med 188, 1137–1146, doi: 10.1164/rccm.201304-0803OC (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry MP et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977, doi: 10.1038/nature09247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmbhatt S et al. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin Exp Immunol 146, 243–252, doi: 10.1111/j.1365-2249.2006.03211.x (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki T et al. Differential cell analysis in bronchoalveolar lavage fluid from pulmonary lesions of patients with tuberculosis. Chest 102, 54–59 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Lowe DM et al. Neutrophilia independently predicts death in tuberculosis. Eur Respir J 42, 1752–1757, doi: 10.1183/09031936.00140913 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eum SY et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137,122–128, doi: 10.1378/chest.09-0903 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyadova IV Neutrophils in Tuberculosis: Heterogeneity Shapes the Way? Mediators Inflamm 2017, 8619307, doi: 10.1155/2017/8619307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tecchio C & Cassatella MA Neutrophil-derived chemokines on the road to immunity. Semin Immunol 28, 119–128, doi: 10.1016/j.smim.2016.04.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ethuin F et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Laboratory investigation; a journal of technical methods and pathology 84, 1363–1371, doi: 10.1038/labinvest.3700148 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues DR et al. Interferon-gamma production by human neutrophils upon stimulation by IL-12, IL-15 and IL-18 and challenge with Paracoccidioides brasiliensis. Cytokine 69, 102–109, doi: 10.1016/j.cyto.2014.05.009 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Spees AM et al. Neutrophils are a source of gamma interferon during acute Salmonella enterica serovar Typhimurium colitis. Infect Immun 82, 1692–1697, doi: 10.1128/iai.01508-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J & Ferguson TA Identification of an IFN-gamma-producing neutrophil early in the response to Listeria monocytogenes. Journal of immunology (Baltimore, Md. : 1950) 182, 7069–7073, doi: 10.4049/jimmunol.0802410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawant KV, Cho H, Lyons M, Ly LH & McMurray DN Guinea pig neutrophil- macrophage interactions during infection with Mycobacterium tuberculosis. Microbes Infect 12, 828–837, doi: 10.1016/j.micinf.2010.05.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokkali S, Rajavelu P, Sudhakar R & Das SD Phenotypic modulation in Mycobacterium tuberculosis infected neutrophil during tuberculosis. Indian J Med Res 130, 185–192 (2009). [PubMed] [Google Scholar]
- 23.Potian JA et al. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. 208, 1863–1874, doi: 10.1084/jem.20091473 %J The Journal of Experimental Medicine (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keyhani A, Riazi-Rad F, Pakzad SR & Ajdary S Human polymorphonuclear leukocytes produce cytokines in response to Leishmania major promastigotes. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 122, 891–897, doi: 10.1111/apm.12252 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Redford PS, Murray PJ & O’Garra A The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol 4, 261–270, doi: 10.1038/mi.2011.7 (2011). [DOI] [PubMed] [Google Scholar]
- 26.O’Leary S, O’Sullivan MP & Keane J IL-10 blocks phagosome maturation in mycobacterium tuberculosis-infected human macrophages. American journal of respiratory cell and molecular biology 45, 172–180, doi: 10.1165/rcmb.2010-03190C (2011). [DOI] [PubMed] [Google Scholar]
- 27.Gideon HP et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog 11, e1004603, doi: 10.1371/journal.ppat.1004603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasten KR, Muenzer JT & Caldwell CC Neutrophils are significant producers of IL-10 during sepsis. Biochem Biophys Res Commun 393, 28–31, doi: 10.1016/j.bbrc.2010.01.066 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deguine J, Wei J, Barbalat R, Gronert K & Barton GM Local TNFR1 Signaling Licenses Murine Neutrophils for Increased TLR-Dependent Cytokine and Eicosanoid Production. Journal of immunology (Baltimore, Md. : 1950) 198, 2865–2875, doi: 10.4049/jimmunol.1601465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorhoi A et al. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med 207, 777–792, doi: 10.1084/jem.20090067 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Majlessi L, Deriaud E, Leclerc C & Lo-Man R Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31, 761–771, doi: 10.1016/j.immuni.2009.09.016 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Tamassia N et al. Cutting edge: An inactive chromatin configuration at the IL-10 locus in human neutrophils. Journal of immunology (Baltimore, Md. : 1950) 190, 1921–1925, doi: 10.4049/jimmunol.1203022 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Lewkowicz N et al. Induction of human IL-10-producing neutrophils by LPS- stimulated Treg cells and IL-10. Mucosal Immunol 9, 364–378, doi: 10.1038/mi.2015.66 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Spuck S et al. G-CSF application in patients with severe bacterial pneumonia increases IL-10 expression in neutrophils. Respir Med 97, 51–58 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Cassatella MA, Costantini C & Jaillon S Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11, 519–531, doi: 10.1038/nri3024 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Nicolas-Avila JA, Adrover JM & Hidalgo A Neutrophils in Homeostasis, Immunity, and Cancer. Immunity 46, 15–28, doi: 10.1016/j.immuni.2016.12.012 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Greenlee-Wacker MC Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev 273, 357–370, doi: 10.1111/imr.12453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin PL et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 77, 4631–4642, doi: 10.1128/iai.00592-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattila JT, Maiello P, Sun T, Via LE & Flynn JL Granzyme B-expressing neutrophils correlate with bacterial load in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Cellular microbiology 17, 1085–1097, doi: 10.1111/cmi.12428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin PL et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun 74, 3790–3803, doi: 10.1128/IAI.00064-06 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warsinske HC, Pienaar E, Linderman JJ, Mattila JT & Kirschner DE Deletion of TGF-beta1 Increases Bacterial Clearance by Cytotoxic T Cells in a Tuberculosis Granuloma Model. Front Immunol 8, 1843, doi: 10.3389/fimmu.2017.01843 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindelin J et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682, doi: 10.1038/nmeth.2019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter AE et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7, R100, doi: 10.1186/gb-2006-7-10-r100 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scapini P, Calzetti F & Cassatella MA On the detection of neutrophil-derived vascular endothelial growth factor (VEGF). J Immunol Methods 232, 121–129 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA & Ota MO Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. European journal of immunology 39, 723–729, doi: 10.1002/eji.200838693 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Caccamo N et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. European journal of immunology 40, 2211–2220, doi: 10.1002/eji.201040455 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Mattila JT, Diedrich CR, Lin PL, Phuah J & Flynn JL Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. Journal of immunology (Baltimore, Md.: 1950) 186, 3527–3537, doi: 10.4049/jimmunol.1003773 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Via LE et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun 76, 2333–2340, doi: 10.1128/IAI.01515-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marino S et al. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect Immun 83, 324–338, doi: 10.1128/iai.02494-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fridlender ZG et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta:”N1” versus “N2” TAN. Cancer cell 16, 183–194, doi: 10.1016/j.ccr.2009.06.017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deniset JF & Kubes P Recent advances in understanding neutrophils. F1000Research 5, doi: 10.12688/f1000research.9691.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tak T, Tesselaar K, Pillay J, Borghans JA & Koenderman L What’s your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol 94, 595–601, doi: 10.1189/jlb.1112571 (2013). [DOI] [PubMed] [Google Scholar]
- 53.du Plessis N et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med 188, 724–732, doi: 10.1164/rccm.201302-02490C (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.