Abstract
Tongue squamous cell carcinoma (TSCC) has a poor prognosis due to its early metastasis through blood and lymphatic vessels. We undertook a systematic review to investigate the prognostic significance of blood microvessel density (MVD) and lymphatic vessel density (LVD) in TSCC patients. We carried out a systematic search in Ovid Medline, Scopus, and Cochrane libraries. All studies that evaluated the prognostic significance of MVD/LVD markers in TSCC were systematically retrieved. Our results showed that MVD/LVD markers, CD31, CD34, CD105, factor VIII, lymphatic vessel endothelial hyaluronan receptor‐1, and D2‐40 were evaluated in TSCC patients until 28 June 2018. Six out of 13 studies reported markers that were associated with poor prognosis in TSCC. Two out of three studies suggested that a high number of D2‐40+ vessels predicated low overall survival (OS); the third study reported that the ratio of D2‐40+ over factor VIII + vessels is associated with low OS. Most of the other markers had controversial results for prognostication. We found higher expression of MVD/LVD markers were commonly, but not always, associated with shorter survival in TSCC patients. It is therefore not currently possible to recommend implementation of these markers as reliable prognosticators in clinical practice. More studies (especially for D2‐40) with larger patient cohorts are needed.
Keywords: biomarker, blood microvessel density, lymphatic vessel density, prognosis, tongue squamous cell carcinoma
Abbreviations
- CD
cluster of differentiation
- CI
confidence interval
- DFS
disease‐free survival
- DSS
disease‐specific survival
- FVIII
factor VIII
- HNSCC
head and neck squamous cell carcinoma
- HR
hazard ratio
- LN
lymph node
- LVD
lymphatic vessel density
- LYVE‐1
lymphatic vessel endothelial hyaluronan receptor‐1
- MAStARI
meta‐analysis of statistics assessment and review instrument tool
- MVD
microvessel density
- OS
overall survival
- PFS
progression‐free survival
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐analyses
- RDLV
relative density of lymphatic vessels
- REMARK
reporting recommendations for tumor marker prognostic studies
- RFS
recurrence‐free survival
- TSCC
tongue squamous cell carcinoma
- VC
vessel count
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Tongue squamous cell carcinoma (TSCC) is one of the most common types of head and neck squamous cell carcinoma (HNSCC) and has an increasing incidence in many European and Nordic countries.1 Cancer metastasis is the leading cause of death in TSCC patients. Unfortunately, the survival rate has not significantly improved over recent decades.2 Cancer staging is considered a vital tool in predicting the treatment and survival outcomes of TSCC patients.3 The TNM classification is currently the mainstay of clinical staging of TSCC patients.4 Despite its widespread use, this system has been criticized for not considering the biological behavior and heterogeneity of individual cancers. For example, the TNM staging scheme shows little or no prognostic value in early TSCC.5, 6 Therefore, it is important to supplement the TNM staging system with new histological features and biomarkers.7, 8 TSCC currently lacks reliable prognosticators that can predict outcome and response to therapy.
Angiogenesis (new blood vessel formation) and lymphangiogenesis (new lymph vessel formation) are vital processes for tumor development and propagation.9 These complex vasculature systems are essential not only for enriching tumor cells but also to facilitate the establishment of metastatic colonies in secondary tissues.9 Almost all types of malignant carcinomas have the potential to metastasize to regional lymph nodes and distant tissues.10 In fact, some cancers metastasize by utilizing both the blood and lymphatic vessels simultaneously, whereas others, such as TSCC, prioritize spreading through lymphatic routes to the sentinel lymph nodes.9, 11 In this context, both MVD and LVD were successfully used as parameters to study the tumor biology, prognosticators, and therapeutic targets in several cancers including HNSCC.12, 13, 14, 15 The assessment of such vascular parameters is often facilitated by the use of well‐established immunohistochemical antibodies (Abs). These Abs include a variety of blood vessel markers, such as CD34, CD31, CD105 (endoglin), and FVIII in addition to markers for lymphatic vessels, such as D2‐40 (podoplanin) and LYVE‐1.
Assessment of prognostic parameters at the time of diagnosis is essential for proper risk stratification of cancer patients.16 To the best of our knowledge, there are currently no biomarkers that reliably correlate with the prognosis and therapeutic response in TSCC patients. Several studies have investigated the potential of the tumor vasculature as a prognosticator in TSCC. Therefore, in this study we sought to systematically review the current evidence of the prognostic value of blood and lymphatic vessel markers in patients with TSCC.
2. METHODS
2.1. Protocol and registration
This review study was registered at the international prospective register of systematic reviews PROSPERO (https://www.crd.york.ac.uk/prospero/) with the registration number CRD42019115141.
2.2. Search strategy
We carried out a comprehensive search in 3 electronic databases (Ovid Medline, Scopus, and Cochrane Library) combining the following search terms: (“tongue”) AND (“cancer” OR “neoplasm” OR “carcinoma” OR “squamous cell carcinoma” OR “tumor*”) AND (“angiogenesis” OR “blood vessel” OR “lymphangiogenesis” OR “lymphatic vessel” OR “lymph vessel” OR “cd31*” OR “cd34*” OR “cd45*” OR “icam‐1*” OR “cd54*” OR “lyve‐1*” OR “tie‐2*” OR “tek*” OR “vcam‐1*” OR “cd106*” OR “ve cadherin” OR “vegf‐r2” OR “vascular endothelial growth factor receptor 2” OR “FVIII‐RA” OR “FVIII” OR “factor 8” OR “von willebrand factor” OR “vwf” OR “erg” OR “vegf” OR “d2‐40” OR “podoplanin” OR “prospero‐related homeobox‐1” OR “vegf‐r3” OR “peripheral node addressin antibody”). Both the abbreviated and full name of each vessel marker were used.
The results obtained with these search terms were gathered together in RefWorks. The article search was undertaken with no time/language restrictions on 28 June 2018 and therefore articles published after that date were not considered. The PRISMA was used to illustrate the results in a flowchart of search.17 In the Ovid Medline advanced search, the set search fields were: abstract, original title, subject heading, keyword.
If the same patient cohort was involved in multiple publications, only the most recent study was included. Two authors (R.A. and M.K.) independently screened all article titles and abstracts. In the screening, duplicates were discarded and articles were verified to meet the inclusion criteria listed in Table S1. Articles not passing the inclusion criteria were excluded during the screening process.
2.3. Data extraction
The following information was extracted from each study: (i) basic article information, including first author, publication year, study period, and follow‐up duration; (ii) patient and tumor information, including the number and location of patients, mean age, gender, tumor site and size, disease stage, number of patients who underwent immunohistochemical staining and the number with positive staining results, name and source of the Ab, Ab dilution, and sample preservation (paraffin‐embedded or frozen); (iii) survival analysis, including type of survival, end‐point, Kaplan‐Meier curves and statistical results (estimated HR, 95% CI, and P values); and (iv) variables measuring vessel marker expression, including lymphatic or blood vessel density, location of the staining, and cut‐off value as a definition for positive expression.
2.4. Quality and risk of bias assessment
We assessed the reporting quality of the eligible studies according to the REMARK guidelines, a 20‐item checklist aimed at ensuring the quality and reproducibility of the reported data.18 The selected and applied REMARK guidelines of the eligible studies are listed in Table S2. For the risk of bias, two authors (R.A. and M.K.) answered 10 questions for each study using MAStARI. Answers were described as Y for “yes,” N for “no,” U for “unclear,” and NA for “not applicable”. The risk of bias was categorized as high when the study reached up to 49% of a “yes” score, moderate when the study reached a 50%‐69% of a “yes” score, and low when the study reached more than 70% of a “yes” score.
3. RESULTS
3.1. Search results
We found a total of 515 articles from 3 electronic databases (332 from Ovid Medline, 142 from Scopus, and 41 from the Cochrane Library) and 1 from a previous search. After screening titles and abstracts, 36 articles were subsequently verified for eligibility (Figure 1). Of these, only 13 articles met the inclusion criteria and were therefore included in this review. In these studies, samples from patients with TSCC were used to evaluate the following vessel markers: CD34, CD31, CD105, FVIII, D2‐40, and LYVE‐1. For MVD markers, CD34 analysis was reported in 5 studies19, 20, 21, 22, 23; Fernández et al studied CD31 and Chuang et al studied CD105.24, 25 Factor VIII was evaluated in 2 studies.26, 27 For LVD markers, D2‐40 was evaluated in 3 studies28, 29, 30 and LYVE‐1 was reported in 2 studies.21, 31 The end‐point measurement was reported as OS in 4 studies.19, 22, 29, 31 In addition, the outcome was also reported as PFS,20 DFS,21, 25, 28 DSS,24, 27, 30 RFS,26 and tumor‐specific survival.23
Figure 1.
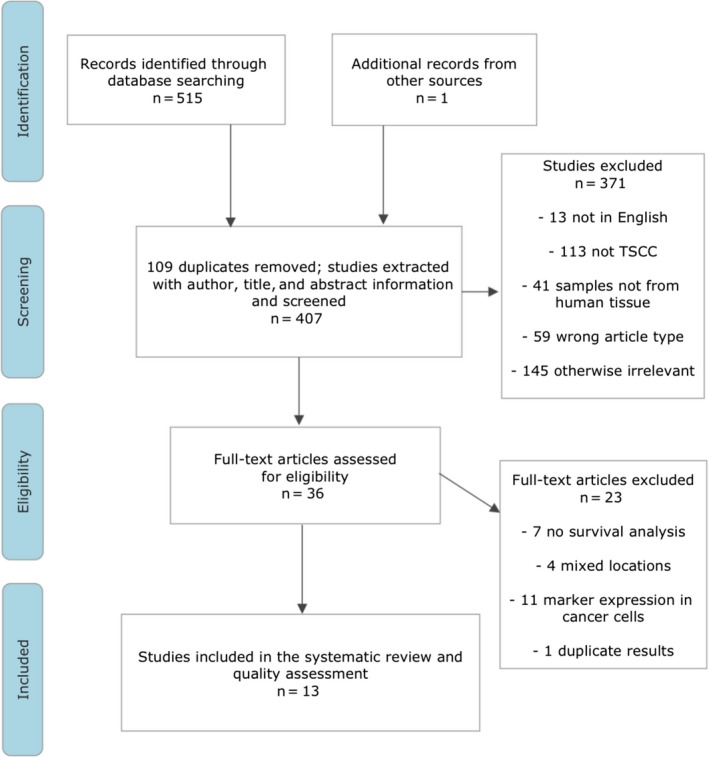
Flow chart defining the search strategy and the studies included and excluded along various steps. TSCC, tongue squamous cell carcinoma
3.2. Risk of bias results
Based on the MAStARI evaluation tool, the risk of bias in the included articles was either low (n = 9) or moderate (n = 4). The risk of bias for each study and the applied questions are shown in Table S3.
3.3. Preoperative treatments of the studied cohorts
As the preoperative treatment could impact the expression of MVD/LVD in the studied patient samples, we screened the included reports to extract any relevant data. The samples were not subjected to any sort of preoperative treatments in a total of 7 studies.19, 20, 21, 22, 25, 29, 31 In one study from India, the patients were primarily treated by either surgery or radiotherapy.26 Some of the patients who underwent surgery were also given adjuvant radiotherapy, chemotherapy, or radiotherapy and chemotherapy.26 However, this information was either missing or not clearly stated in the other 5 studies.23, 24, 27, 28, 30
3.4. Microvessel density markers as prognosticators in TSCC
3.4.1. Prognostic value of CD34
We found 5 studies that analyzed the prognostic value of CD34 in TSCC patients. Huang et al19 did not find a statistically significant correlation between MVD (determined by CD34) and OS in a cohort of 80 TSCC patients. This was similar to the results of Toyoda et al,20 who reported no significant correlation between CD34 expression and OS or PFS in a similar sample size (n = 85). In contrast, Sasahira et al21 revealed that high CD34 expression was associated with poor prognosis and reduced DFS when they analyzed 101 TSCC patients (P = .0249). Similarly, Shao et al22 reported a significantly reduced OS in TSCC patients (n = 59) with high CD34 expression compared with those with low or moderate expression. In an older study of Forootan et al,23 the CD34‐expressing vessel count (VC) was not associated with the growth pattern or metastasis in a cohort of 51 TSCC patients. However, the Cox proportional hazards model revealed that patients with a low VC tended to have a good prognosis (P = .023). Characteristics of the studies on CD34 are summarized in Table 1.
Table 1.
Characteristics of studies of CD34 in tongue squamous cell carcinoma
| First author, year, country | Stage or tumor size | Primary Ab | Method for MVD evaluation | Cut‐off point | No. of cases in IHC | Positive cases | End‐point | Unadjusted analysis | Adjusted analysis | Results interpretation | Compliance to REMARK guidelines |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al, 2012, China | T1‐T4 | CD34 mouse mAb, Abcam, 1:200 | MVD was counted in 5 hot spots in a 200× field | >47 | 80 | 46.25% | OS | P = .427 | – | MVD had no significant effect on OS | Checklist number 1,5 and 6 not fulfilled |
| Toyoda et al, 2014, Japan | T1‐T4 | CD34 mouse mAb, Nichirei, 1:800 | MVD was counted in 4 hot spots in a 400× field (0.26 mm2 field area) | 14 (range 2‐29) | 85 | 52% | OS | P = .182 | – | MVD had no significant correlation on OS/PFS | Checklist number 5 not fulfilled |
| PFS | P = .617 | – | |||||||||
| Sasahira et al, 2010, Japan | T1‐T4 | CD34, Dako | MVD was counted in 5 hot spots in a 200× field | 54.65 ± 37.67 (mean as cut‐off value ± SD) | 101 | – | DFS | * P = .0249 | – | MVD was significantly associated with local progression, clinical stage, nodal metastasis, and DFS | Checklist numbers 1, 3, and 5 not fulfilled |
| Shao et al, 2008, China | T1‐T4 | CD34, mAb, ZYMED, 1:200 | MVD was counted in 5 hot spots in a 200× field | 78.73 ± 25.73. (mean as cut‐off value) | 59 | – | OS | HR 5.2 (95% CI, 1.78‐19.23), * P = .020 | HR 0.24 (95% CI, 0.00‐0.87), * P = .01 | MVD was significantly associated with OS and had independent prognostic effect on OS | Fulfilled all of REMARK criteria |
| Forootan et al, 1999, UK | T1‐T4 | CD34, mouse mAb, Dako | MVD was counted in the highest hot spots in a 250× field | 35 (median as cut‐off value) | 51 | 10 cases had VC < 20 | TSS | P = .178 | * P = .0234 | On univariate analysis there was no significant association between MVD and survival.Cox's regression showed that a low value of VC is a good prognostic sign | Checklist numbers 3 and 5 not fulfilled |
*P value ≤ .05.
–, not disclosed; CD, cluster of differentiation; CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; IHC, immunohistochemistry; MVD, microvascular density; OS, overall survival; PFS, progression‐free survival; REMARK, reporting recommendations for tumor marker prognostic studies; TSS, tumor‐specific survival; VC, vascular count.
3.4.2. Prognostic value of CD31 and CD105
We found only one study that used CD31 to correlate MVD and the prognosis of 43 patients with TSCC.24 In this small cohort study, Cox regression analysis did not indicate tumor vascularization as a prognostic factor of survival (P = .59). Chuang et al25 investigated the expression of CD105 in 94 TSCC patients and found that the cumulative 5‐year DFS rates of patients with low CD105 expression were significantly higher than those with high expression (P < .001). Moreover, Cox regression analysis showed that the expression of CD105 was an independent factor from other variables for survival (relative risk 8.0; 95% CI, 2.525‐25.839; P < .001). Characteristics of the studies regarding these 2 markers are summarized in Table 2.
Table 2.
Characteristics of studies regarding CD31/CD105 markers in tongue squamous cell carcinoma
| First author, year, country | Stage or tumor size | Primary Ab | Method for MVD evaluation | Cut‐off point | No. of cases in IHC | Positive cases | End‐point | Unadjusted analysis | Adjusted analysis | Results interpretation | Compliance to REMARK guidelines |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fernández et al, 2007, Spain | T1‐T4 | CD31 mAb, Dako, 1:10 | MVD was counted in 3 hot spots in a 400× field (0.369 mm2 per field) | 30.6 | 43 | 43 | 5 y DSS | P = .59 | – | There was no significant correlation between MVD and 5‐y survival | Checklist numbers 5 and 6 not fulfilled |
| Chuang et al, 2006, Taiwan | T1‐T2 | CD105 (SN6h) mouse mAb, Dako, 1:10 | MVD was counted in hot spots in a 200× field | 18.8 | 94 | 27 | 5 y DFS | * P < .001 | RR 8.077 (95% CI, 2.525‐25.839), P < .001 | CD105 was an independent prognostic factor for survival | Checklist number 5 not fulfilled |
*P value ≤ .05.
–, not disclosed; CD, cluster of differentiation; CI, confidence interval; DFS, disease‐free survival; DSS, disease‐specific survival; IHC, immunohistochemistry; MVD, microvascular density; REMARK, reporting recommendations for tumor marker prognostic studies; REMARK, reporting recommendations for tumor marker prognostic studies; RR, relative risk.
3.4.3. Prognostic value of FVIII
A series of 84 TSCC cases were enrolled in a study by Vora et al.26 The authors considered a mean of the number of microvessels from 3 vascular hot spots as representing the microvessel count for each patient. Early stage (stage I and II) cancer patients with FVIII greater than 0.0 were significantly associated with reduced OS and RFS. However, FVIII lost its prognostic significance when a general linear model was applied.26 In the other study, Kantola et al27 reported no association between FVIII and the survival rate in a cohort of 105 TSCC patients. Further information is summarized in Table 3.
Table 3.
Characteristics of studies regarding factor VIII (FVIII) markers in tongue squamous cell carcinoma
| First author, year, country | Stage or tumor size | Primary Ab | Method for MVD evaluation | Cut‐off point | No. of cases in IHC | Positive cases | End‐point | Unadjusted analysis | Adjusted analysis | Results interpretation | Compliance to REMARK guidelines |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vora et al, 2003, India | T1‐T4 | FVIII mouse mAb, clone F8/86, Dako, 1:50 | MVD was counted in 3 hot spots in a 400× field | Median 0.0 | 84 | 20% | RFS | * P = .032 | – | MVD was associated with RFS and OS | Checklist numbers 2 and 5 not fulfilled |
| OS | * P = .014 | – | |||||||||
| Kantola et al, 2000, Finland | I‐II I II‐IV | FVIII Dako | MVD was counted in 10 hot spots in a 40× field (diameter 400 μm) | ≥8.5 | 105 | – | 75% DSS survival time | P = .27 | – | No significant correlation was found between FVIII and survival | Checklist numbers 1, 3, and 5 not fulfilled |
*P value ≤ .05.
–, not disclosed; DSS, disease‐specific survival; IHC, immunohistochemistry; MVD, microvascular density; OS, overall survival; REMARK, reporting recommendations for tumor marker prognostic studies; RFS, relapse‐free survival.
3.5. Lymphatic vessel density markers as prognosticators in TSCC
3.5.1. Prognostic value of D2‐40
Al‐Shareef et al28 revealed a strong correlation between D2‐40 and LN metastasis in 80 TSCC patients. Both OS and DFS were associated with intra‐ and peritumoral LVD, as patients with a high LVD had a poor prognosis with a high possibility of recurrence. A significant reduction in OS was observed by Yan et al29 in 80 TSCC cases associated with high D2‐40, which also indicated higher nodal metastasis. In the 61 cases analyzed by Seppälä et al,30 the mean LVD did not influence patient survival. However, the relative density of lymphatic vessels (RDLV) was significantly associated with poor OS (P = .004) in TSCC patients. The authors calculated RDLV by dividing the mean number of D2‐40+ LVD by the mean number of von Willebrand factor+ LVD per microscopic field. These studies are summarized in Table 4.
Table 4.
Characteristics of studies regarding D2‐40 in tongue squamous cell carcinoma (TSCC)
| First author, year, country | Stage or tumor size | Primary Ab | Method for LVD evaluation | Cut‐off point | No. of cases in IHC | Positive cases | End‐point | Unadjusted analysis | Adjusted analysis | Results interpretation | Compliance to REMARK guidelines |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al‐Shareef et al, 2016, Japan | T1‐T4 | D2‐40 mouse mAb, prediluted, Nichirei Bioscience | LVD was counted in 3 hot spots using a 20× objective | Mean 6.85 vessels/μm2 | 80 | 100% | OS | * P < .001 | – | There was a significant correlation between LVD and LN metastasis in TSCC. Patients with a high LVD had a poor DFS and a high recurrence rate | Checklist numbers 1, 3, and 5 not fulfilled |
| DFS | * P < .001 | – | |||||||||
| Yan et al, 2014, China | I‐II III‐IV | D2‐40, mAb (ZM‐0465), Zhongshan Goldenbridge Biotechnology, 1:100 | LVD was counted in 3 hot spots in 200× field | Mean (21.454 ± 7.022) | 80 | 100% | OS | * P < .0001 | – | OS was significantly shorter in patients with high LVD. D2‐40 expression was higher in OTSCC than in normal tissue | Checklist number 5 not fulfilled |
| Seppälä et al, 2016, Finland | T1‐T4 | D2‐40 mAb, Dako, 1:50 | LVD was counted in 5 hot spots (0.785 mm2/field) | Mean 16.6/field | 61 | – | OS | NS | – | Mean LVD and lymph vessel diameter had no significant effect on patient OS or DSS | Checklist numbers 1 and 5 not fulfilled |
| DSS | NS | – |
*P value ≤ .05.
–, not disclosed; DFS, disease‐free survival; DSS, disease‐specific survival; IHC, immunohistochemistry; LN, lymph node; LVD, lymphatic vessel density; NS, not significant; OS, overall survival; REMARK, reporting recommendations for tumor marker prognostic studies; OTSCC, oral tongue squamous cell carcinoma.
3.5.2. Prognostic value of LYVE‐1
Ding et al31 evaluated the prognostic value of LYVE‐1 in 50 cases and revealed fewer intratumoral LYVE‐1+ vessels than peritumoral vessels. Moreover, they did not observe a significant correlation between LYVE‐1 and OS of TSCC patients. In contrast, Sasahira et al21 revealed that LVD positive for LYVE‐1 showed a poor association with DFS in 101 TSCC patients. In addition, high LVD was associated with poor prognosis (P < .0001). Both studies are summarized in Table 5.
Table 5.
Characteristics of studies regarding LYVE‐1
| Authors, year, Country | Stage or tumor size | Primary antibody | Method for evaluation LVD | Cutoff point | No. of cases in IHC | Positive cases | End‐point | Unadjusted analysis | Adjusted analysis | Results interpretations | Compliance to REMARK guidelines |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Ding et al, 2014, China | T1‐T4 | LYVE1 polyclonal, Abcam, 1:100 | LVD was counted in six hot spots in 200× field |
IT 3 (range 0‐10) PT 5 (range 0‐20) |
50 | 38% | OS |
HR: 1.604 (95% CI: 0.650‐3.960) P = .305 |
HR: 1.524 (95% CI: 0.551‐4.210) P = .417 |
There was no significant correlation between the expression of LYVE‐1 and OS of patients with OTSCC | Fulfilled all of remark criteria |
| 2. Sasahira et al, 2010, Japan | T1‐T4 | LYVE‐1, Dako | LVD was counted in five hot spots in 200× field |
104.24 ± 64.23 (Mean ± SD) |
101 | — | DFS | P < .0001* | — | LVD was significantly associated with DFS. Significant relationship was found between LVD and gender, histological differentiation, local progression, clinical stage, lymph node metastasis and local recurrence | Checklist number 1,3 and 5 not fulfilled |
Abbreviations: ‐, Not disclosed; CI, confidence interval; DFS, disease free survival; HR, hazard ratio; IT, intratumour; LVD, lymphatic vessels density; LYVE1, Lymphatic vessel endothelial hyaluronan receptor 1; OS, overall survival; PT, peritumour.
*P value ≤ .05.
4. DISCUSSION
Angiogenesis and lymphangiogenesis promote cancer cell growth and metastasis.14 Metastasis is estimated to be responsible for approximately 90% of cancer‐associated deaths.32 TSCC is one of the most common intraoral cancers and is characterized by an extensive and well‐developed vascular and lymphatic system and a high rate of cervical LN metastasis.33 Therefore, identification of biomarkers that associate with TSCC progression and metastasis, such as MVD and LVD, could enhance prognostic and therapeutic approaches. In the present study, several MVD and LVD markers were reviewed in 13 clinical studies that involved a total of 973 TSCC patients. Only 7 of the eligible 13 studies (53.84%) indicated a prognostic significance of one or more of the studied MVD or LVD markers. The results of almost all markers were controversial. However, studies on D2‐40 suggested some promising results. The use of these MVD and LVD markers cannot be recommended for clinical use at this time and more studies (especially on D2‐40) are needed with a larger number of TSCC cases.
Several reports indicate that CD34 can be used as a specific and sensitive biomarker in hepatocellular carcinoma and lung cancer and could therefore become an integral part of a more reliable staging system.34, 35, 36 Moreover, the use of CD34 as an angiogenic marker was superior to other markers as it yielded better results with less background and makes quantification easier.23 In TSCC, the vascular hot spots were also positively correlated with tumor size; multivariate analysis showed better prognosis in patients with low CD34 expression.23 Additionally, Sasahira et al21 showed that higher CD34 expression strongly correlated with poor survival. It was also reported by Shao et al22 that CD34 positively correlated to VEGF and to poor survival of TSCC patients. This reflects the key role of VEGF in the development of a functional vascular system in the tumor microenvironment. However, two later studies were not able to reproduce the significance of CD34 as a prognostic marker in TSCC patients.19, 20
CD31 (platelet endothelial cell adhesion molecule 1), CD105 (endoglin), FVIII, and von Willebrand factor are other blood vessel markers. Even though CD31, FVIII, and CD105 are determinants of MVD, it was concluded that CD105 expression is the best angiogenic marker and significant prognosticator of DFS in non‐small cell lung cancer patients.37 Moreover, several reports have found a positive correlation between CD105+ MVD and cancer cell metastasis, including in HNSCC patients.38, 39 Advanced oral cancer stages correlated with higher expression of CD105.40 CD31 and CD105 have thus far been studied only once in TSCC patients. Although the expression of CD105 was reported to be an independent prognostic factor for survival by multivariate analysis according to the Cox regression model,25 prognostic value was not found when CD31 was assessed. This might be due to the small sample size in their study.24 There are two studies of FVIII in TSCC that reported contradictory results.26, 27
D2‐40 (podoplanin), a mucin‐type transmembrane glycoprotein, is preferentially expressed in lymphatic endothelial cells and is considered a specific marker for the lymphatic endothelium.30, 41 The tumorigenic role of podoplanin has been suggested in several reports based on its high expression in potentially malignant lesions such as oral leukoplakia, oral carcinoma in situ, and oral squamous cell carcinoma.42, 43 The TSCC samples with high D2‐40+ LVD expression showed significant prognostic value in two studies.28, 29 Although LVD did not produce a significant correlation with patient survival in the third study, RDLV was instead significantly associated with poor OS in TSCC patients.30 However, these results should be confirmed with studies in a larger patient cohort.
Lymphatic vessel endothelial hyaluronan receptor‐1 is another specific marker for lymphatic endothelium.44 A significant relationship was found between intratumoral LVD expression and LN metastasis in HNSCC.45 In another study, Beasley et al46 revealed discrete hot spots of intratumoral LYVE‐1+ lymphatics in all HNSCC cases, which were associated with cervical LN involvement. Consistent with this, intratumoral LYVE‐1+ LVD in 97 primary HNSCC tumors increased risk for local relapse and indicated poor disease‐specific prognosis.47 In a study by Sasahira et al,21 LYVE‐1+ LVD were found at the edges of the TSCC tissues and the vessels were irregular in shape, and when accompanied with high VEGF showed shorter DFS. In a later study by Ding et al,31 there was no correlation between the expression of neither intratumoral nor peritumoral LYVE‐1 and the survival of patients with TSCC.
In conclusion, although the evidence reported in this review suggests that increased expression of MVD or LVD markers for TSCC patients could be associated with reduced survival, there is currently insufficient evidence to recommend implementation of any of these markers as part of a reliable staging system in clinical practice. This is due to several factors, such as the small patient cohorts of the studies, different assessment criteria used for MVD and LVD markers, the heterogeneity of the study samples (mixing either base of the tongue “posterior 1/3”, oral tongue “anterior 2/3,” or total tongue cancer for the analysis), and the absence of HR and CI information in the majority of the studies (11 of 13 studies). Overall, this review highlights the need for more accurate prognostic studies on TSCC.
DISCLOSURE
The authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
The authors acknowledge Tiina Heino and Katri Larmo for assisting with the systematic search. The authors acknowledge the funders of this study: the Doctoral Program in Clinical Research, University of Helsinki; the Cancer Society of Finland; the Emil Aaltonen Foundation (Emil Aaltosen Säätiö); the Finnish Dental Society (Apollonia); the Sigrid Jusélius Foundation, the Oulu University Hospital MRC grant; and the Helsinki University Central Hospital research funds.
Almahmoudi R, Kasanen M, Sieviläinen M, et al. Prognostic value of blood and lymphatic vessel markers in tongue cancer: A systematic review. Cancer Sci. 2019;110:3424‐3433. 10.1111/cas.14189
Salo and Al‐Samadi supervised the work equally.
REFERENCES
- 1. Annertz K, Anderson H, Palmér K, Wennerberg J. The increase in incidence of cancer of the tongue in the Nordic countries continues into the twenty‐first century. Acta Otolaryngol. 2012;132(5):552‐557. [DOI] [PubMed] [Google Scholar]
- 2. Sim YC, Hwang JH, Ahn KM. Overall and disease‐specific survival outcomes following primary surgery for oral squamous cell carcinoma: analysis of consecutive 67 patients. J Korean Assoc Oral Maxillofac Surg. 2019;45(2):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I). Oral Oncol. 2010;46(9):630‐635. [DOI] [PubMed] [Google Scholar]
- 4. Huang SH, O'Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18(7):40. [DOI] [PubMed] [Google Scholar]
- 5. Po Wing Yuen A, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma‐A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez‐Gimeno score, and pathologic features. Head Neck. 2002;24(6):513‐520. [DOI] [PubMed] [Google Scholar]
- 6. Yanamoto S, Yamada S, Takahashi H, et al. Predictors of locoregional recurrence in T1‐2N0 tongue cancer patients. Pathol Oncol Res. 2013;19(4):795‐803. [DOI] [PubMed] [Google Scholar]
- 7. Almangush A, Heikkinen I, Mäkitie AA, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta‐analysis. Br J Cancer. 2017;117(6):856‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elseragy A, Salo T, Coletta RD, et al. A proposal to revise the histopathologic grading system of early oral tongue cancer incorporating tumor budding. Am J Surg Pathol. 2019;43(5):703‐709. [DOI] [PubMed] [Google Scholar]
- 9. Bielenberg DR, Zetter BR. The contribution of angiogenesis to the process of metastasis. Cancer J. 2015;21(4):267‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zwaans BM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvasc Res. 2007;74(2–3):145‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sagheb K, Sagheb K, Rahimi‐Nedjat R, Taylor K, Al‐Nawas B, Walter C. Sentinel lymph node biopsy in T1/T2 squamous cell carcinomas of the tongue: a prospective study. Oncol Lett. 2016;11(1):600‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta‐analysis. Cancer Res. 2004;64(9):2941‐2955. [DOI] [PubMed] [Google Scholar]
- 13. Audet N, Beasley NJ, MacMillan C, Jackson DG, Gullane PJ, Kamel‐Reid S. Lymphatic vessel density, nodal metastases, and prognosis in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131(12):1065‐1070. [DOI] [PubMed] [Google Scholar]
- 14. Carla C, Daris F, Cecilia B, Francesca B, Francesca C, Paolo F. Angiogenesis in head and neck cancer: a review of the literature. J Oncol. 2012;2012:358472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundov Z, Tomic S, Alfirevic S, et al. Prognostic value of MVD, LVD and vascular invasion in lymph node‐negative colon cancer. Hepatogastroenterology. 2013;60(123):432‐438. [DOI] [PubMed] [Google Scholar]
- 16. Halabi S, Owzar K. The importance of identifying and validating prognostic factors in oncology. Semin Oncol. 2010;37(2):e9‐e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. Open Med. 2009;3(3):e123‐e130. [PMC free article] [PubMed] [Google Scholar]
- 18. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCI‐EORTC Working Group on Cancer Diagnostics . Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang C, Sun Z, Sun Y, et al. Association of increased ligand cyclophilin A and receptor CD147 with hypoxia, angiogenesis, metastasis and prognosis of tongue squamous cell carcinoma. Histopathology. 2012;60(5):793‐803. [DOI] [PubMed] [Google Scholar]
- 20. Toyoda M, Kaira K, Ohshima Y, et al. Prognostic significance of amino‐acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer. 2014;110(10):2506‐2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sasahira T, Kirita T, Kurihara M, et al. MIA‐dependent angiogenesis and lymphangiogenesis are closely associated with progression, nodal metastasis and poor prognosis in tongue squamous cell carcinoma. Eur J Cancer. 2010;46(12):2285‐2294. [DOI] [PubMed] [Google Scholar]
- 22. Shao Z, Zhang WF, Chen XM, Shang ZJ. Expression of EphA2 and VEGF in squamous cell carcinoma of the tongue: correlation with the angiogenesis and clinical outcome. Oral Oncol. 2008;44(12):1110‐1117. [DOI] [PubMed] [Google Scholar]
- 23. Forootan SS, Jones AS, Helliwell TR. Neoangiogenesis and squamous cell carcinoma of the tongue. Cancer Detect Prev. 1999;23(2):137‐146. [DOI] [PubMed] [Google Scholar]
- 24. Fernández MM, García‐Rozado A, Parente PL. Is microvascular density an independent prognostic factor in squamous cell carcinoma of the tongue? Acta Otorrinolaringol Esp. 2007;58:341‐346. [PubMed] [Google Scholar]
- 25. Chuang HC, Su CY, Huang HY, Chien CY, Chen CM, Huang CC. High expression of CD105 as a prognostic predictor of early tongue cancer. Laryngoscope. 2006;116(7):1175‐1179. [DOI] [PubMed] [Google Scholar]
- 26. Vora HH, Shah NG, Patel DD, Trivedi TI, Chikhlikar PR. Prognostic significance of biomarkers in squamous cell carcinoma of the tongue: multivariate analysis. J Surg Oncol. 2003;82(1):34‐50. [DOI] [PubMed] [Google Scholar]
- 27. Kantola S, Parikka M, Jokinen K, et al. Prognostic factors in tongue cancer ‐ relative importance of demographic, clinical and histopathological factors. Br J Cancer. 2000;83(5):614‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Shareef H, Hiraoka SI, Tanaka N, et al. Use of NRP1, a novel biomarker, along with VEGF‐C, VEGFR‐3, CCR28 and SEMA3E, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncol Rep. 2016;36(5):2444‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan J, Jiang Y, Ye M, Liu W, Feng L. The clinical value of lymphatic vessel density, intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 expression in patients with oral tongue squamous cell carcinoma. J Cancer Res Ther. 2014;10(suppl):C125‐C130. [DOI] [PubMed] [Google Scholar]
- 30. Seppälä M, Pohjola K, Laranne J, et al. High relative density of lymphatic vessels predicts poor survival in tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273(12):4515‐4524. [DOI] [PubMed] [Google Scholar]
- 31. Ding L, Zhang Z, Shang D, et al. α‐Smooth muscle actin‐positive myofibroblasts, in association with epithelial‐mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43(5):335‐343. [DOI] [PubMed] [Google Scholar]
- 32. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559‐1564. [DOI] [PubMed] [Google Scholar]
- 33. Noguti J, De Moura CF, De Jesus GP, et al. Metastasis from oral cancer: an overview. Cancer Genomics Proteomics. 2012;9(5):329‐335. [PubMed] [Google Scholar]
- 34. Salizzoni M, Romagnoli R, Lupo F, et al. Microscopic vascular invasion detected by anti‐CD34 immunohistochemistry as a predictor of recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2003;76(5):844‐848. [DOI] [PubMed] [Google Scholar]
- 35. Saad RS, Luckasevic TM, Noga CM, Johnson DR, Silverman JF, Liu YL. Diagnostic value of HepPar1, pCEA, CD10, and CD34 expression in separating hepatocellular carcinoma from metastatic carcinoma in fine‐needle aspiration cytology. Diagn Cytopathol. 2004;30(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 36. Mineo TC, Ambrogi V, Baldi A, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB‐IIA non‐small cell lung cancer. J Clin Pathol. 2004;57(6):591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka F, Otake Y, Yanagihara K, et al. Evaluation of angiogenesis in non‐small cell lung cancer: comparison between anti‐CD34 antibody and anti‐CD105 antibody. Clin Cancer Res. 2001;7(11):3410‐3415. [PubMed] [Google Scholar]
- 38. Kumar S, Ghellal A, Li C, et al. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59(4):856‐861. [PubMed] [Google Scholar]
- 39. Martone T, Rosso P, Albera R, et al. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncol. 2005;41(2):147‐155. [DOI] [PubMed] [Google Scholar]
- 40. Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. 2002;24(2):151‐156. [DOI] [PubMed] [Google Scholar]
- 41. Agarwal D, Pardhe N, Bajpai M, et al. Characterization, localization and patterning of lymphatics and blood vessels in oral squamous cell carcinoma: a comparative study using D2‐40 and CD‐34 IHC marker. J Clin Diagn Res. 2014;8(10):ZC86‐ZC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Vicente JC, Rodrigo JP, Rodriguez‐Santamarta T, Lequerica‐Fernández P, Allonca E, García‐Pedrero JM. Podoplanin expression in oral leukoplakia: tumorigenic role. Oral Oncol. 2013;49(6):598‐603. [DOI] [PubMed] [Google Scholar]
- 43. Funayama A, Cheng J, Maruyama S, et al. Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology. 2011;78(3):171‐180. [DOI] [PubMed] [Google Scholar]
- 44. Koukourakis MI, Giatromanolaki A, Sivridis E, et al. LYVE‐1 immunohistochemical assessment of lymphangiogenesis in endometrial and lung cancer. J Clin Pathol. 2005;58(2):202‐206.er. 2000 Sep;83(5):614‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frech S, Hörmann K, Riedel F, Götte K. Lymphatic vessel density in correlation to lymph node metastasis in head and neck squamous cell carcinoma. Anticancer Res. 2009;29(5):1675‐1679. [PubMed] [Google Scholar]
- 46. Beasley NJ, Prevo R, Banerji S, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62(5):1315‐1320. [PubMed] [Google Scholar]
- 47. Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63(8):1920‐1926. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


