Abstract
Overcoming resistance to radiation is a great challenge in cancer therapy. Here, we highlight that targeting valosin‐containing protein (VCP) improves radiation sensitivity in esophageal squamous cell carcinoma (ESCC) cell lines and show the potential of using VCP as a prognosis marker in locally advanced ESCC treated with radiation therapy. Esophageal squamous cell carcinoma cell lines with high VCP expression were treated with VCP inhibitor combined with radiotherapy. Cell proliferation, colony formation, cell death, and endoplasmic reticulum (ER) stress signaling were evaluated. Moreover, patients with newly diagnosed locally advanced ESCC who were treated with radiotherapy were analyzed. Immunohistochemistry was used to detect the expression of VCP. The correlation between overall survival and VCP was investigated. Esophageal squamous cell carcinoma cells treated with VCP inhibitor and radiotherapy showed attenuated cell proliferation and colony formation and enhanced apoptosis. Further investigation showed this combined strategy activated the ER stress signaling involved in unfolded protein response, and inhibited the ER‐associated degradation (ERAD) pathway. Clinical analysis revealed a significant survival benefit in the low VCP expression group. Targeting VCP resulted in antitumor activity and enhanced the efficacy of radiation therapy in ESCC cells in vitro. Valosin‐containing protein is a promising and novel target. In patients with locally advanced ESCC who received radiotherapy, VCP can be considered as a useful prognostic indicator of overall survival. Valosin‐containing protein inhibitors could be developed for use as effective cancer therapies, in combination with radiation therapy.
Keywords: endoplasmic reticulum stress, esophageal squamous cell carcinoma, prognosis, radiation resistance, valosin‐containing protein
1. INTRODUCTION
Esophageal cancer is a major global health concern.1 According to histological classification, squamous cell carcinoma and adenocarcinoma are the 2 main types of esophageal cancer.2 In the past few decades, adenocarcinoma has been the most common type of esophageal cancer in developed countries; however, esophageal squamous cell carcinoma (ESCC) still accounts for nearly 70% of cases of esophageal cancer around the world, especially in Asia.3, 4 Radiotherapy, in combination with chemotherapy, is widely used in the management of locally advanced ESCC.5 Despite incredible improvements in cancer treatment, radiation resistance leads to treatment failure and results in poor outcomes.6, 7 Therefore, it is crucial to select biomarkers indicative of eligible patients who could benefit from this treatment protocol.
Valosin‐containing protein (VCP), also known as p97 in mammals or cdc48 in yeast, is a hexameric ATPase of the AAA family.8 Valosin‐containing protein is described as a crucial chaperone for regulating folding or unfolding substrate proteins in the endoplasmic reticulum (ER)‐associated degradation (ERAD) pathway.9, 10 With respect to the high protein synthesis burden and possible aneuploidy in many kinds of tumor cells, the ERAD pathway has a central role in regulating cancer cell proliferation.11 Over the past few years, high expression of VCP has been described and found to correlate with disease outcomes in various cancer patients who received surgical resection.12, 13, 14, 15, 16, 17 Recently, several VCP inhibitors have been developed.18, 19, 20 Among these specific inhibitors, NMS‐873 was initially developed as an allosteric, non‐ATP‐competitive, and potent noncovalent VCP inhibitor that impairs the ERAD pathway, activates the unfolded protein response (UPR), and induces apoptosis.20 In addition, other findings have revealed VCP as a potential target and inhibiting VCP has been associated with improved antitumor activity in vitro and in vivo.21, 22 To date, the role of VCP in enhancing tumor radiation sensitivity has not been characterized in ESCC cell lines. Moreover, the significance of VCP expression in locally advanced ESCC patients who had undergone radiotherapy was of little concern.
Finding a valuable biomarker for treatment stratification and prognostic prediction is a challenge in cancer therapy. The objectives of this study were to investigate the value of VCP inhibitor in improving radiation resistance. We went further, by attempting to identify the feasibility of using VCP as a prognostic marker for patients with locally advanced ESCC who have received radiotherapy.
2. MATERIALS AND METHODS
2.1. Reagents
NMS‐873 (purity >98%) was purchased from Dalian Meilun Biotechnology (CAS: 1418013‐75‐8), the compound was dissolved in DMSO and stored at 4°C. Primary Abs used for the western blot analysis included inositol‐requiring enzyme 1α (IRE1α) (14C10; Cell Signaling Technology), X‐box binding protein 1 (XBP‐1(s)) (D2C1F; Cell Signaling Technology), phospho‐protein kinase RNA‐like ER kinase (p‐PERK) (DF7576; Affinity Biotechnology), phospho‐eukaryotic initiation factor 2α (p‐eIF2α) (119A11, Cell Signaling Technology), activating transcription factor 6 (ATF6) (D4Z8V; Santa Cruz Biotechnology), binding immunoglobulin protein (BiP) (C50B12; Cell Signaling Technology), and C/EBP homologous protein (CHOP) (D46F1; Cell Signaling Technology). Antibody to detect VCP was purchased from Abcam (ab11433), the GAPDH (KM9002T) and β‐actin (KM9001T) were obtained from Sungene Biotech. Secondary Ab included goat anti‐mouse IgG‐HRP (sc‐2005) and goat anti‐rabbit IgG‐HRP (sc‐2004) were from Santa Cruz Biotechnology.
2.2. Cell lines and cell culture
Cancer cell lines including KYSE30, KYSE70, KYSE140, KYSE150, KYSE410, KYSE450, and KYSE510 were purchased from the Type Culture Collection of the Chinese Academy of Sciences. KYSE30 cells were cultured in a 1:1 mixture of RPMI‐1640 (R8758; Sigma‐Aldrich) and Ham's F12 medium, 2% FBS (Biological Industries), and 1% streptomycin/penicillin. KYSE70, KYSE140, KYSE150, KYSE410, KYSE450, and KYSE510 cells were maintained in RPMI‐1640, 10% FBS, and 1% streptomycin/penicillin. The human immortalized normal esophageal epithelial cell line, N1217, was donated by Dr. Enmin Li from the Laboratory of Tumor Pathology (Shantou University Medical College). All cells were cultured in a humidified atmosphere at 37°C with 5% CO2. All cells were subjected to cytogenetic testing and confirmed.
2.3. Cell viability assay
In general, cancer cells were plated in a 96‐well plate format at 3000 cells per well in 0.2 mL medium and incubated overnight. Next day, the cells were treated with different amounts of NMS‐873. After incubation for 24, 48, or 72 hours, cancer cell viability was measured by the MTT assay. The optical absorbance was determined at 490 nm using a microplate reader (Thermo Fisher Scientific). Dose response curves were fitted and inhibitory concentration values were calculated for each cell line.
2.4. RNA interference experiments
Specific and nontargeting siRNA were purchased from SunYa Biotechnology. KYSE70 and KYSE140 cells were seeded in 6‐well plates and grown to 40%‐50% confluency in serum‐containing medium (without penicillin/streptomycin). Tumor cells were transfected with the VCP targeting siRNAs using Lipofectamine RNAiMAX (13778150; Thermo Fisher Scientific) following the manufacturer's instructions. The knockdown efficiency was analyzed by western blot experiments.
2.5. Colony formation assays
Cells were seeded (400 cells/well for KYSE140; 200 cells/well for KYSE70) in 6‐well plates in 2 mL medium and incubated for 24 hours at 37°C in a 5% CO2 incubator. Then the culture media were replaced with media containing appropriate amounts of NMS‐873. Next day, the cells were irradiated with a TrueBeam SN1403 accelerator (Varian Medical Systems). Doses included 0, 2, 4, 6, 8, 10, and/or 12 Gy. These cells were further incubated for 7‐14 additional days until the colonies were optimal. After aspirating the media and being fixed with methanol, 0.5% crystal violet was used to stain the colonies for 10 minutes, then they were washed with water. Each well was air‐dried and colonies were visualized under a microscope; colonies containing 50 cells or more were counted using the Image‐Pro Plus software (version 6.0) program (Media Cybernetics). The classic multitarget single hit model was applied to plot the dose survival curves. Survival fraction (SF) = 1 − (1 − e−D/D0)N, where the mean lethal dose (D0) represents the dose needed to decrease the fraction of surviving cells to 37% of its previous value, N is the extrapolation number, and the quasithreshold dose (Dq) indicates repair capacity of tumor cells after radiation therapy. Radiation sensitivity parameters included survival fraction at 2 Gy (SF2), D0, Dq, and the sensitivity enhancement ratio was calculated. The sensitivity enhancement ratio was calculated by dividing D0 in the control group by D0 in the treatment group.23, 24 Three independent experiments were carried out for each cell line, with each treatment undertaken in triplicate.
2.6. Annexin V apoptosis assay
Cancer cells (8 × 105 cells for KYSE140; 4 × 105 cells for KYSE70) were seeded in 6‐well plates using appropriate culture media and incubated overnight. Cells were treated with NMS‐873 and/or irradiated with 6 Gy and allowed to incubate for 48 hours. After cells were collected and stained with annexin V‐FITC and propidium iodide (PI), apoptosis assay was carried out by flow cytometry using a BD FACSCalibur Flow Cytometer (BD Biosciences).
2.7. Western immunoblot analysis
Cells were seeded and incubated overnight. The next day, cells were treated with NMS‐873 and/or irradiated with 6 Gy and incubated for 24 hours. Cells were collected using a cell scraper and incubated on ice for 20 minutes in NP‐40 cell lysis buffer (50 mmol/L Tris [pH 8.0], 150 mmol/L NaCl, 0.5%‐1% NP‐40, protease inhibitor cocktail, dephosphorylate inhibitor tablets, and 1 mmol/L PMSF). Protein concentration was determined by using the BCA Quantification Kit (Solarbio). Protein extracts were loaded in SDS‐PAGE and transferred to PVDF membranes. After blocking with 5% nonfat dry milk in TBS‐Tween 20 (TBST) for 1 hour, electroblotted membranes were incubated with appropriate primary Abs prepared in 3% BSA in TBST at 4°C. The next day, membranes were washed with TBST 3 times and incubated with appropriate HRP‐conjugated IgG for 1 hour at room temperature. Finally, the bands were visualized using the ECL detection reagent (GE Healthcare Life Science).
2.8. Immunohistochemical staining
Medical data of newly diagnosed locally advanced ESCC patients treated with 3‐D conformal radiotherapy or intensity‐modulated radiotherapy were retrospectively analyzed. This study was approved by the institutional review board of the Affiliated Cancer Hospital of Zhengzhou University (no. 182106000062). Archival formalin‐fixed and paraffin‐embedded tissue of the eligible ESCC patients who received radiotherapy were sectioned at 4‐5 μm, and prepared for analysis. After antigen exposure, 5% goat serum was used for 1 hour and the sectioned tissues were then incubated with 1:800 mouse monoclonal antigen‐VCP Ab (Abcam) at 4°C overnight. Samples were then incubated with biotinylated secondary Ab. 3,3′‐Diaminobenzidine staining was applied to detect the targeted protein according to the manufacturer's instructions. After counterstaining with hematoxylin, samples were dehydrated with a graded series of alcohol in xylene and cover slips were placed. The staining intensity and percentage of positive cells stained were evaluated to assess the IHC staining. Scoring was undertaken by 2 pathologists, independently. Discrepancies were dealt with through discussion among senior pathologists until a final consensus was achieved. The cytoplasmic VCP staining score was calculated as follows: 0, absence of staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The percentage of VCP‐positive tumor cells was analyzed and classified as: level 0, less than 10% positive tumor cells; level 1, 10%‐25% positive tumor cells; level 2, 25%‐50% positive tumor cells; level 3, 50%‐75% positive tumor cells; and level 4, more than 75% positive tumor cells. Expression of VCP in tumor sections was scored by multiplying the intensity by the percentage of positive cells. In this analysis, the included patients were divided into 2 groups based on their final calculated scores: the low VCP expression group (scores of 0‐7) and the high VCP expression group (scores of 8‐12).
2.9. Statistical analysis
Statistical analyses were carried out using SPSS version 20.0 (IBM Software Group) and Prism 7 (GraphPad Software). Student's t test and/or one‐way or two‐way ANOVA was used for statistical analyses. The Bonferroni multiple comparisons test was applied where necessary. Overall survival (OS) was estimated using the Kaplan‐Meier methodology; the log‐rank test was used to detect potential differences amongst the various variables. Univariate and multivariate Cox proportional hazard regression models were analyzed to identify potential prognostic factors of OS. A 2‐tailed P < .05 was considered to be significant for all of the statistical analyses.
3. RESULTS
3.1. Treatment with VCP inhibitor attenuates tumor cell proliferation
To measure intracellular protein expression, whole cell lysates were generated from 7 different ESCC cells and 1 normal esophageal epithelial cell. Expression of VCP was significantly lower in normal cells compared with tumor cells. Among the 7 different ESCC cells, KYSE140 is associated with the highest expression of VCP (Figure 1A,B). Therefore, proliferation assay was carried out to determine the effect of VCP inhibitor on cancer cells (KYSE70 and KYSE140). Our results showed that NMS‐873 reduced cell proliferation in a dose‐dependent manner (Figure 1C,D). We also observed that the IC10 values are different for KYSE70 and KYSE140 (120 nmol/L and 90 nmol/L, respectively). These findings suggest that VCP inhibitor is cytotoxic to tumor cells. In addition, we tested the effect of VCP inhibitor in the remaining tumor cell lines and 1 normal esophageal epithelial cell line. The final analysis revealed that the sensitivity of VCP inhibitor was correlated with relative VCP expression (Figure 1E).
Figure 1.
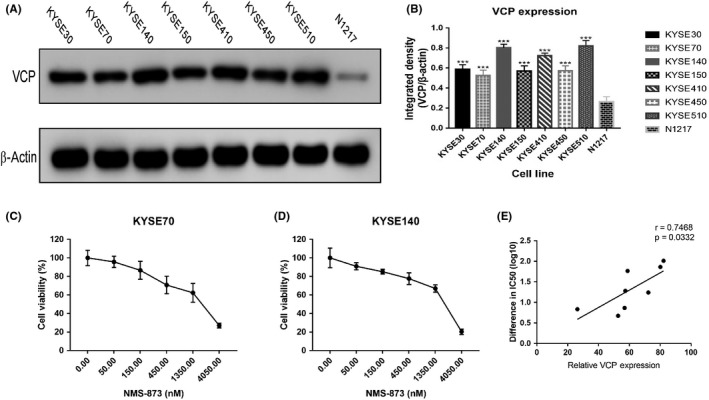
Valosin‐containing protein (VCP) protein expression in different esophageal squamous cell carcinoma (ESCC) cell lines and NMS‐873 inhibits the growth of ESCC cells. A, Expression of VCP in ESCC cells and normal esophageal epithelial cells (N1217). B, Quantification of VCP expression from (A). C, Dose‐survival curves of NMS‐873 on KYSE70 cells were estimated by MTT assay at 24 h. D, Dose‐survival curves of NMS‐873 on KYSE140 cells were estimated by MTT assay at 24 h. E, Correlation of IC 50 and VCP expression. r, Pearson correlation coefficient. Data are mean ± SD (n = 3). ***P ˂ .0001. n.s., not significant, P > .05
3.2. Inhibition of VCP enhances the efficacy of radiation
Drugs that can enhance radiation sensitivity are essential in cancer treatment. To evaluate the efficacy of VCP inhibitor in combination with radiation therapy, colony formation assays were undertaken in ESCC cells (KYSE70 and KYSE140). For subsequent investigations, both KYSE70 cells and KYSE140 cells were treated with NMS‐873 at the concentrations listed above. The results indicated that there were no visible signs of toxicity for tumor cells. Radiation therapy combined with NMS‐873 showed a significant decrease in the surviving fraction in both KYSE70 and KYSE140 cells when compared with radiation therapy alone (Figure 2A,B). These outcomes showed an increased sensitivity to radiation therapy when combined with VCP inhibitor.
Figure 2.
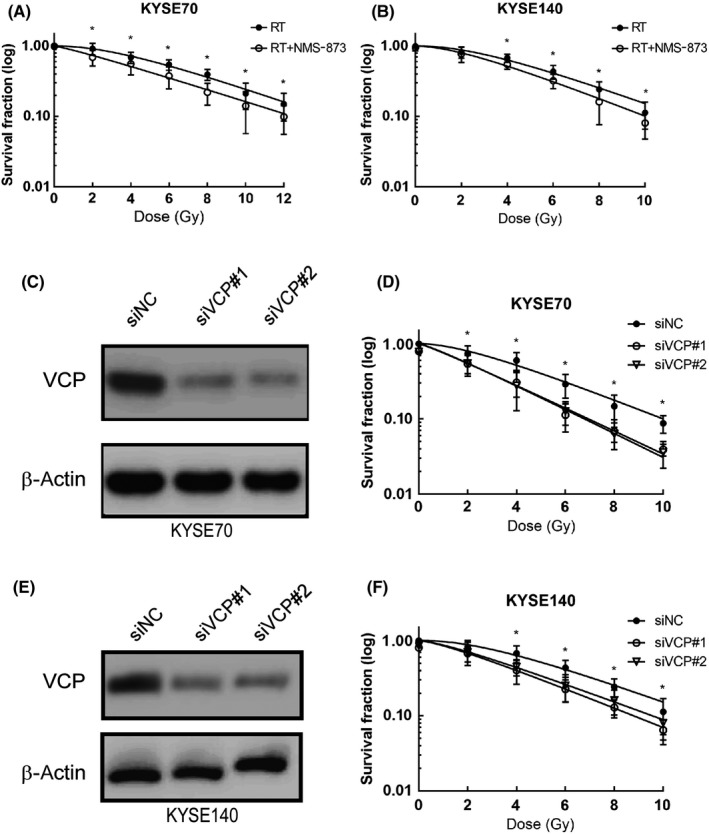
Valosin‐containing protein (VCP) inhibition enhances the sensitivity of radiation therapy (RT) in esophageal squamous cell carcinoma cells. A, Colony formation assay was undertaken in KYSE70 cells following DMSO (vehicle), NMS‐873 (120 nmol/L), RT (0, 2, 4, 6, 8, 10, and 12 Gy), or RT/NMS‐873 treatment. B, Colony formation assay was undertaken in KYSE140 cells following DMSO (vehicle), NMS‐873 (90 nmol/L), RT (0, 2, 4, 6, 8, and 10 Gy), or RT/NMS‐873 treatment. C, Western blot analysis of KYSE70 cells infected with siRNAs targeting VCP. D, Colony formation assay was undertaken in KYSE70 cells infected with siRNAs targeting VCP. E, Western blot analysis of KYSE140 cells infected with siRNAs targeting VCP. F, Colony formation assay was undertaken in KYSE140 cells infected with siRNAs targeting VCP. *P ˂ .05. n.s., not significant, P > .05; siNC, negative control siRNA
Additionally, to confirm the radiation sensitization effect of VCP inhibition, we undertook siRNA inference experiments. Knockdown of VCP resulted in increased sensitivity to radiation therapy (Figure 2C‐F). Together, these data suggested that inhibition of VCP sensitizes ESCC cells to radiation therapy.
3.3. Treatment with VCP inhibitor induces cell death through the apoptotic pathway
On the basis of the above data, radiation therapy combined with VCP inhibitor was effective in ESCC cells. To determine whether this combined strategy induces cell death by apoptosis, we undertook annexin V/PI staining assays. As shown in Figure 3A‐C, a significant increase in the percentages of cancer cells undergoing early apoptosis (annexin V+/PI−) and late apoptosis (annexin V−/PI+) was observed in both the radiation therapy group and the combined group. In addition, the combined strategy resulted in a higher apoptosis rate than radiation therapy alone. We also found that VCP inhibitor alone had limited effects on apoptosis in cancer cells.
Figure 3.
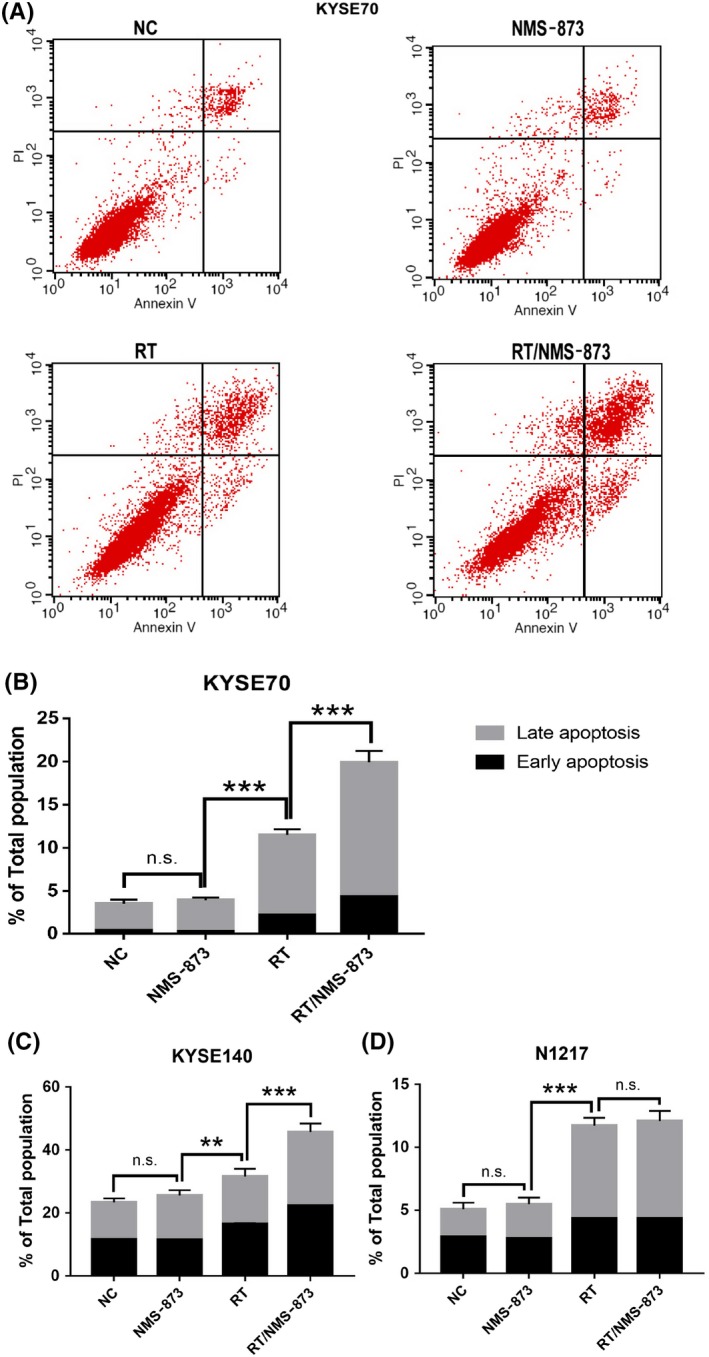
Valosin‐containing protein (VCP) inhibitor enhanced the efficacy of Radiation therapy induced apoptosis in KYSE70 and KYSE140 cells, however, this compound is ineffective in N1217 cells. A, Annexin‐V/PI staining was performed following 24 h of DMSO (vehicle), NMS873 (120 nmol/L), RT (6 Gy), or RT/NMS873 treatment in KYSE70 cells. B, Quantification of apoptosis from (A). C, Quantification of apoptosis in KYSE140 cells. D, Quantification of apoptosis in N1217 cells. NC, normal control; RT, radiation therapy; VCP, valosin‐containing protein. Data are means ± SD (n = 3), n.s., not significant, P > .05; **P ˂ .001; ***P ˂ .0001
Furthermore, we evaluated the toxicity of VCP inhibitor in normal esophageal epithelial cells. The results indicated that VCP inhibitor is slightly toxic in normal cells, and VCP inhibitor combined with radiation therapy is insignificant in inducing apoptosis (Figure 3D). Collectively, these data suggested that VCP inhibition enhanced radiation‐mediated apoptosis and this enhancement was tumor cell‐specific.
3.4. Valosin‐containing protein inhibitor and radiation therapy combined result in enhanced UPR
An accumulation of unfolded proteins and misfolded proteins resulted in ER stress response in tumor cells.25 The ER stress response is an adaptive mechanism that contributes to cell survival; however, increased ER stress is associated with CHOP overexpression, and would predispose the cells to ER stress‐mediated apoptosis.26, 27 Previous studies described radiation‐induced ER stress in cancer cells.27, 28, 29 Valosin‐containing protein inhibitors have been reported to trigger the misfolded protein accumulation and activate the UPR in various cancer cell lines.30 Therefore, we presumed that inhibiting a key component of the ER stress response might result in an elevated level of protein burden in the ER of tumor cells.
Consistent with previous analyses, we observed NMS‐873 induced amplification of ER stress markers including IRE1α, XBP‐1(s), p‐PERK, p‐eIF2α, and BiP. These findings suggest the UPR was activated in ESCC cells through both the IRE1α pathway and the PERK pathway (Figure 4A‐C). Similarly, we evaluated the effect of radiation therapy on ER stress response, and UPR pathway activation was observed. Next, we analyzed the synergistic effect of radiation therapy and NMS‐873 on ER stress response, and we detected an enhanced UPR, especially a significant amplification of the PERK‐eIF2α‐CHOP pathway. These data suggested that radiation therapy combined with VCP inhibitor was able to activate UPR, thus causing enhanced and prolonged ER stress, and resulted in CHOP‐mediated apoptosis (Figure 4D‐E). In addition, there was no difference in ATF6 expression among different types of treatment. This lack of immunoreactivity might be due to low basal expression of ATF6 in ESCC cells.
Figure 4.
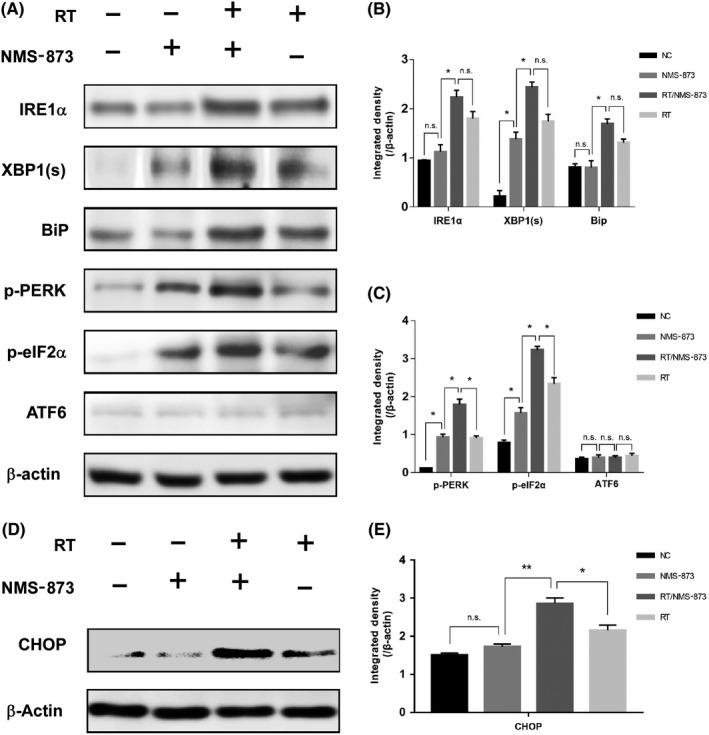
Valosin‐containing protein inhibitor combined with radiation therapy (RT) enhanced and prolonged the activation of unfolded protein response (UPR) in esophageal squamous cell carcinoma cells. A, KYSE70 cells were treated with vehicle, NMS‐873 (120 nmol/L), RT (6 Gy), or RT/NMS‐873, and the proteins in UPR pathways were analyzed by western blot. B, Quantification of inositol‐requiring enzyme 1α (IRE1α), X‐box binding protein 1 (XBP‐1(s)), and binding immunoglobulin protein (BiP) from (A). C, Quantification of phospho‐protein kinase RNA‐like endoplasmic reticulum kinase (p‐PERK), phospho‐eukaryotic initiation factor 2α (p‐eIF2α), and activating transcription factor 6 (ATF6) from (A). D, KYSE70 cells were treated with vehicle, NMS‐873 (120 nmol/L), RT (6 Gy), or RT/NMS‐873, and C/EBP homologous protein (CHOP) was detected by western blot analysis. E, Quantification of CHOP from (D). Data are mean ± SD (n = 3). *P ˂ .05; **P ˂ .001. n.s., not significant, P > .05
Taken together, VCP inhibitor alone was able to activate the UPR pathway; however, it causes only weak ER stress and has limited efficacy in inducing apoptosis. In contrast to VCP inhibition, radiation therapy alone was able to induce the UPR pathway and cause mild ER stress; nevertheless, this was insufficient to trigger apoptosis. When radiation therapy and VCP inhibitor were used in combination, both prolonged ER stress and CHOP‐mediated apoptosis were observed.
3.5. High VCP expression in ESCC is associated with poor survival
Next, we analyzed the association between VCP expression and survival in patients of locally advanced ESCC treated with radiotherapy. In general, VCP expression was significantly increased in the cytoplasm of tumor cells by immunohistochemistry (Figure 5A‐E). All the samples were evaluated carefully. High expression of VCP was detected in 46 patients, whereas low VCP expression was found in 39 patients (Table 1). A homogenous staining pattern throughout the entire specimen was observed in most of the ESCC samples. Undetectable expression of VCP is rarely seen in cancer cells. Patients with low VCP expression had better survival than those in the high VCP expression group. The median OS was 26.5 months (95% CI, 15.9‐37.2 months) in the low VCP expression group compared with 14.2 months (95% CI, 8.9‐19.5 months) in the high VCP expression group (P = .031). The 1‐ and 3‐year OS were 82.1% and 37.8%, respectively, in the low VCP expression group, and 60.9% and 19.7% in the high VCP expression group (Figure 5F).
Figure 5.

Valosin‐containing protein (VCP) expression in the nucleus and cytoplasm of esophageal squamous cell carcinoma (ESCC) cells and the association between VCP expression and survival. A,B, High expression of VCP. C,D, Low expression of VCP. E, Statistical analysis of immunohistochemical staining intensity of low expression group (n = 39) and high expression group (n = 46). F, Low VCP expression (n = 39) in ESCC patients treated with radiation therapy is associated with favorable overall survival. Data are mean ± SD. ***P ˂ .0001. n.s., not significant, P > .05
Table 1.
General characteristics of patients with esophageal squamous cell carcinoma
| Characteristics | VCP expression | Total (N = 85) | P value | |
|---|---|---|---|---|
| High (N = 46) | Low (N = 39) | |||
| Gender | ||||
| Male | 30 (35.3%) | 26 (30.6%) | 56 (65.9%) | .650 |
| Female | 16 (18.8%) | 13 (15.3%) | 29 (34.1%) | |
| Age (years) | ||||
| ≤65 | 20 (23.5%) | 19 (22.4%) | 39 (45.9%) | .557 |
| >65 | 26 (30.6%) | 20 (23.5%) | 46 (54.1%) | |
| KPS score | ||||
| ≥80 | 27 (31.7%) | 24 (28.2%) | 51 (60.0%) | .674 |
| <80 | 19 (22.4%) | 15 (17.6%) | 34 (40.0%) | |
| Smoke status | ||||
| Current | 9 (10.6%) | 8 (9.4%) | 17 (20.0%) | .987 |
| Former | 29 (34.1%) | 24 (28.2%) | 53 (62.4%) | |
| Never | 8 (9.4%) | 7 (8.2%) | 15 (17.6%) | |
| Alcohol consumption | ||||
| Yes | 29 (34.1%) | 25 (29.4%) | 54 (63.5%) | .908 |
| No | 17 (20.0%) | 14 (16.5%) | 31 (36.5%) | |
| Tumor location | ||||
| Cervical | 7 (8.2%) | 4 (4.7%) | 11 (12.9%) | .153 |
| Upper thoracic | 22 (25.9%) | 15 (17.6%) | 37 (43.5%) | |
| Middle thoracic | 11 (12.9%) | 17 (20.0%) | 28 (32.9%) | |
| Lower thoracic | 6 (7.1%) | 3 (3.5%) | 9 (10.6%) | |
| Tumor stage | ||||
| T1‐2 | 15 (17.6%) | 19 (22.4%) | 34 (40.0%) | .507 |
| T3‐4 | 31 (36.5%) | 20 (23.5%) | 51 (60.0%) | |
| LN status | ||||
| N0 | 7 (8.2%) | 6 (7.1%) | 13 (15.3%) | .391 |
| N+ | 39 (45.9%) | 33 (38.8%) | 71 (83.5%) | |
| Tumor length (cm) | ||||
| ≤5 | 13 (15.3%) | 9 (10.6%) | 22 (25.9%) | .280 |
| >5 | 33 (38.8%) | 30 (35.3%) | 63 (74.1%) | |
| Radiation dose (Gy) | ||||
| ≤50.4 | 21 (24.7%) | 17 (20.0%) | 38 (44.7%) | .708 |
| >50.4 | 25 (29.4%) | 22 (25.9%) | 47 (55.3%) | |
| Chemotherapy | ||||
| PTX + DDP | 18 (21.2%) | 14 (16.5%) | 32 (37.6%) | .728 |
| DDP + 5‐FU | 28 (32.9%) | 25 (29.4%) | 53 (62.4%) | |
| Comorbidities | ||||
| Negative | 27 (31.8%) | 24 (28.2%) | 51 (60.0%) | .628 |
| 1 | 9 (10.6%) | 5 (5.9%) | 14 (16.5%) | |
| ≥2 | 10 (11.8%) | 10 (11.8%) | 20 (23.5%) | |
| Weight loss (over 3 mo) | ||||
| ≤5% | 32 (37.6%) | 27 (31.8%) | 59 (69.4%) | .970 |
| >5% | 14 (16.5%) | 12 (14.1%) | 26 (30.6%) | |
Abbreviations: 5‐FU, 5‐fluorouracil; DDP, cisplatin; KPS, Karnofsky performance status; LN, lymph node; PTX, paclitaxel; VCP, valosin‐containing protein.
Based on univariate analyses, high expression of VCP (P = .005, HR = 0.457 [95% CI, 0.265‐0.789]) and lymph node metastases (P = .001, HR = 0.255 [95% CI, 0.123‐0.527]) were statistically significant predictors for poor survival. Other clinicopathological features, age, sex, tumor stage, tumor length, Karnofsky performance status (KPS) score, radiation dose, chemotherapy, comorbidities, and weight loss, were insignificant prognostic factors. In order to determine the independent prognostic factors for OS, variables such as tumor stage, lymph node status, KPS score, comorbidities, and VCP expression were included in the multivariate analysis. The results showed both low VCP expression (P = .015, HR = 2.042 [95% CI, 1.151‐3.621]) and no lymph node metastasis (P = .008, HR = 0.238 [95% CI, 0.083‐0.682]) were independent prognostic factors for better survival (Table 2).
Table 2.
Cox proportional hazards regression for overall survival in patients with esophageal squamous cell carcinoma
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (>65 vs ≤65) | 1.191 (0.851‐1.668) | .309 | – | – |
| Sex (male vs female) | 0.705 (0.386‐1.287) | .255 | – | – |
| Tumor stage (T1‐2 vs T3‐4) | 0.557 (0.249‐1.248) | .155 | – | – |
| LN status (N0 vs N+) | 0.255 (0.123‐0.527) | .001 | 0.238 (0.083‐0.682) | .008 |
| Tumor length (>5 vs ≤5) | 1.576 (0.528‐4.702) | .415 | – | – |
| KPS score (≥80 vs <80) | 0.960 (0.917‐1.006) | .085 | – | – |
| Radiation dose (≤50.4 Gy vs >50.4 Gy) | 1.056 (0.381‐2.925) | .917 | – | – |
| Chemotherapy (PF vs PP) | 0.767 (0.410‐1.435) | .407 | – | – |
| Comorbidities (≥1 vs 0) | 1.634 (0.849‐3.145) | .141 | – | – |
| Weight loss, % (>5% vs ≤5%) | 0.656 (0.336‐1.283) | .218 | – | – |
| VCP expression (high vs low) | 0.457 (0.265‐0.789) | .005 | 2.042 (1.151‐3.621) | .015 |
Abbreviations: –, not included; CI, confidence interval; HR, hazard ratio; KPS, Karnofsky performance status; LN, lymph node; PF, cisplatin + 5‐fluorouracil; PP, cisplatin + paclitaxel; VCP, valosin‐containing protein.
4. DISCUSSION
The current study shows that ESCC cell lines are associated with varying levels of VCP. In line with previous reports, our analysis also showed cancer cells with high VCP expression are sensitive to VCP inhibitor. We also observed that VCP inhibitor acts as a sensitizer when combined with radiation therapy; the potential molecular mechanisms are combined strategies that result in enhanced and prolonged ER stress, which can trigger UPR, especially the PERK‐eIF2α‐CHOP pathway, thereby inducing cell death. In addition, compared with the high VCP expression group, ESCC patients with low expression of VCP treated by radiotherapy were associated with favorable survival. Further analysis suggested that VCP is an independent prognostic factor. Consequently, our results indicated that VCP is a biomarker for predicting radiation resistance and targeting VCP enhances the efficacy of radiation therapy.
Valosin‐containing protein is essential for misfolded protein disaggregation and degradation and it is also involved in genome integrity.25 It is well known that cancer cells are always exposed to various factors that alter protein homeostasis, and misfolded proteins accumulate inside the ER; therefore, invoking ER stress.31 In order to restore ER proteostasis, tumor cells evoke various kinds of adaptive mechanisms including the UPR and ERAD. With the help of VCP, one key component of the proteasome, misfolded proteins were transported from the ER to the cytosol for further degradation.25 Elevated levels of VCP appear to be cytoprotective for tumor cells, impairing rather than accentuating the killing actions of intrinsic and external factors, including nutrient starvation as well as anticancer treatment. Additionally, this cellular adaption response could enable the recurrence of cancers even with the implementation of antitumor treatments.32 Proteomic analysis of HeLa cervix carcinoma cells recovering from ER stress revealed a significant translocation of VCP from the nucleus to the cytoplasm; the change in the cellular distribution of VCP is important for the behavior and survival of cancer cells.33 In the current study, our findings suggest that VCP expression is varied in ESCC cell lines. Treatment with VCP inhibitor led to decreased cell proliferation; in particular, there is a strong correlation between VCP expression and treatment response to VCP inhibitor.
Targeting VCP is a promising strategy for antitumor therapy. NMS‐873, one of the VCP inhibitors, has been shown to cause cancer cell death by inducing ER stress.20 Our analysis also suggests a relatively mild ER stress triggered by this compound. Molecular mechanisms involved in cytotoxicity induced by NMS‐873 might both inhibit the ERAD pathway and induce the UPR pathway. Sorafenib, a multikinase inhibitor, has been proved to target VCP, thereby inducing hepatocellular cancer cell death.34 Recently, the combinatorial therapeutic strategy of targeting VCP has been explored. Valosin‐containing protein inhibitors in combination with oncolytic virus M1 was a promising treatment for hepatocellular carcinoma.35 Bastola et al described the preclinical activity of VCP inhibitors in ovarian cancer cells, and final outcomes showed that VCP inhibitors were able to induce ER stress, cause cell cycle arrest, and trigger caspase‐mediated cell death. Thus, VCP inhibitor can be used as a single agent or combined with other antitumor compounds.22
The existence of radiation resistance in cells remains one of the most critical obstacles in anticancer therapy.36 Mice xenografted glioma models treated with hypofractionated radiotherapy were analyzed using reverse‐phase protein array; the results revealed VCP levels were correlated with radiation resistance.37 Our analysis also gives evidence that VCP could act as a potential radiation resistance biomarker. In addition, we observed that radiation therapy activated the UPR signaling pathway and induced ER stress. Similar results were also reported in normal cells and tumor cells.28, 38 This is mainly because radiation therapy causes misfolded protein accumulation and activates the UPR pathway.39 Nevertheless, the role of VCP in radiation resistance was not well investigated. Detailed analyses are needed to fully understand the potential mechanisms.
The UPR consists of the IRE1‐XBP‐1(s) signaling pathway, the PERK‐eIF2α pathway, and the ATF6 pathway.29 Previous analyses described that both radiation therapy and VCP inhibitors were able to induce the UPR through activating the IRE1‐XBP‐1(s) signaling pathway and the PERK‐eIF2α pathway, without triggering the ATF6 pathway.35, 39 Similarly, our results also showed the expression of ATF6 showed no difference when treated with radiation therapy and/or NMS‐873. Furthermore, our analysis indicated VCP inhibitor has a synergistic effect with radiation therapy. Misfolded proteins induced by radiation therapy accumulate in cancer cells, and can be transported out of the ER through the ERAD pathway. NMS‐873 was effective in inhibiting the ERAD pathway and resulted in a large quantity of misfolded proteins accumulated in the ER. Immediately, both the activated UPR pathway and the accumulated misfolded protein caused enhanced and prolonged ER stress in tumor cells. When ER stress becomes insurmountable, cell apoptosis ensues (Figure 6).
Figure 6.
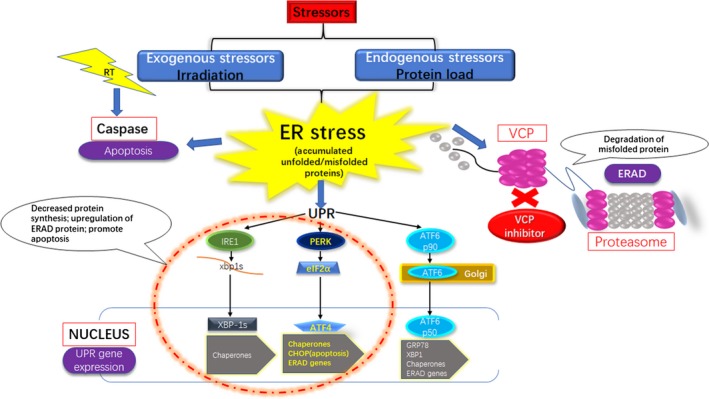
Mechanisms of targeting valosin‐containing protein (VCP) enhance the efficacy of radiation therapy (RT) in human esophageal squamous cell carcinoma cell lines. ATF, activating transcription factor; CHOP, C/EBP homologous protein; eIF2α, eukaryotic initiation factor 2α; ER, endoplasmic reticulum; ERAD, endoplasmic reticulum‐associated degradation IRE1, inositol‐requiring enzyme 1; PERK, protein kinase RNA‐like endoplasmic reticulum kinase; UPR, unfolded protein response; XBP‐1s, X‐box binding protein 1
Valosin‐containing protein has been investigated for a long time. In previous studies, the prognostic potential of VCP expression was evaluated in different types of solid cancers and hematological tumors. Yamamoto et al15, 16 reported that high expression of VCP was associated with increased incidence of cancer progression in gastric carcinoma and esophageal cancer patients who received surgical resection. In human papillomavirus (HPV)‐negative oropharyngeal squamous cell carcinoma patients treated with surgery, VCP expression evaluated by immunohistochemistry indicated that this protein could be used as a prognostic marker, and VCP intensity was apparently uncorrelated with HPV status.12 Zhu et al recently investigated the correlation between VCP expression and primary orbital mucosa‐associated lymphoid tissue in lymphoma patients treated by curative resection; the results showed that high expression of VCP was significantly correlated with recurrence, enlarged tumor size, and reduced survival.17 The novelty of our analysis is that elevated expression of VCP was indicative of unfavorable survival in ESCC patients who received radiotherapy. We also showed that VCP can serve as an independent prognostic factor for this patient cohort. Additionally, positive lymph node was associated with tumor invasion and poor survival in esophageal cancer.40, 41 The present study also found negative lymph node was an independent favorable prognostic marker in locally advanced ESCC patients treated with radiotherapy.
There were several limitations in the present study. Our results indicated ESCC cell lines have higher VCP expression compared with normal cells, thus implying that VCP is an attractive target for antitumor therapy. However, potential causes of elevated VCP in ESCC cells should be further investigated. Given the anticancer activity of VCP inhibitor in ESCC cells, additional preclinical and clinical studies are necessary. In addition, the evaluation of VCP expression was based on intensity of immunohistochemistry, and potential bias could exist. Valosin‐containing protein as a radiation resistance biomarker was not well characterized from a clinical perspective. Therefore, our analysis should be interpreted with caution and additional studies are needed to support the results.
In conclusion, the findings of this study suggest that VCP plays a vital role in regulating the cell viability of ESCC cells. Targeting VCP offers a new strategy in enhancing radiation sensitivity. In addition, an elevated expression of VCP is associated with unfavorable survival in locally advanced ESCC patients treated with radiotherapy. Valosin‐containing protein expression has the potential to be used as an independent prognostic factor.
DISCLOSURE
The authors have declared no conflicts of interest to this work.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81372436, 81773230) and the Open Cooperation of Science and Technology of Henan (182106000062). We appreciate the support by Jia X, Wang L, Marco P, Nie W, Zhao D, Wang M, Jiang M, Wang S, and Liu K from the China‐US Hormel Cancer Institute.
Luo H, Song H, Mao R, et al. Targeting valosin‐containing protein enhances the efficacy of radiation therapy in esophageal squamous cell carcinoma. Cancer Sci. 2019;110:3464‐3475. 10.1111/cas.14184
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38‐44. [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 5. Chen F, Luo H, Xing L, Liang N, Xie J, Zhang J. Feasibility and efficiency of concurrent chemoradiotherapy with capecitabine and cisplatin versus radiotherapy alone for elderly patients with locally advanced esophageal squamous cell carcinoma: experience of two centers. Thorac Cancer. 2018;9:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo H, Cui YY, Zhang JG, et al. Meta‐analysis of survival benefit with postoperative chemoradiotherapy in patients of lymph node positive esophageal carcinoma. Clin Transl Oncol. 2018;20:889‐898. [DOI] [PubMed] [Google Scholar]
- 7. Bernard V, Bishop AJ, Allen PK, et al. Heterogeneity in treatment response of spine metastases to spine stereotactic radiosurgery within “radiosensitive” subtypes. Int J Radiat Oncol Biol Phys. 2017;99:1207‐1215. [DOI] [PubMed] [Google Scholar]
- 8. van den Boom J, Meyer H. VCP/p97‐mediated unfolding as a principle in protein homeostasis and signaling. Mol Cell. 2018;69:182‐194. [DOI] [PubMed] [Google Scholar]
- 9. Abisambra JF, Jinwal UK, Blair LJ, et al. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum‐associated degradation. J Neurosci. 2013;33:9498‐9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA‐ATPase in the ubiquitin system. Nat Cell Biol. 2012;14:117‐123. [DOI] [PubMed] [Google Scholar]
- 11. Deshaies RJ. Proteotoxic crisis, the ubiquitin‐proteasome system, and cancer therapy. BMC Biol. 2014;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer MF, Seuthe IM, Drebber U, et al. Valosin‐containing protein (VCP/p97)‐expression correlates with prognosis of HPV‐ negative oropharyngeal squamous cell carcinoma (OSCC). PLoS ONE. 2014;9:e114170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsujimoto Y, Tomita Y, Hoshida Y, et al. Elevated expression of valosin‐containing protein (p97) is associated with poor prognosis of prostate cancer. Clin Cancer Res. 2004;10:3007‐3012. [DOI] [PubMed] [Google Scholar]
- 14. Valle CW, Min T, Bodas M, et al. Critical role of VCP/p97 in the pathogenesis and progression of non‐small cell lung carcinoma. PLoS ONE. 2011;6:e29073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamamoto S, Tomita Y, Hoshida Y, et al. Expression level of valosin‐containing protein (p97) is associated with prognosis of esophageal carcinoma. Clin Cancer Res. 2004;10:5558‐5565. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto S, Tomita Y, Hoshida Y, et al. Expression level of valosin‐containing protein is strongly associated with progression and prognosis of gastric carcinoma. J Clin Oncol. 2003;21:2537‐2544. [DOI] [PubMed] [Google Scholar]
- 17. Zhu WW, Kang L, Gao YP, et al. Expression level of valosin containing protein is associated with prognosis of primary orbital MALT lymphoma. Asian Pac J Cancer Prev. 2013;14:6439‐6443. [DOI] [PubMed] [Google Scholar]
- 18. Anderson DJ, Ronan LM, Stevan D, et al. Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell. 2015;28:653‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fang CJ, Gui L, Zhang X, et al. Evaluating p97 inhibitor analogues for their domain selectivity and potency against the p97‐p47 complex. ChemMedChem. 2015;10:52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magnaghi P, D'Alessio R, Valsasina B, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol. 2013;9:548‐556. [DOI] [PubMed] [Google Scholar]
- 21. Xh L, Zh Z, Zl L, Sh H, Qf L. Inhibiting valosin‐containing protein suppresses osteosarcoma cell metastasis via AKT/nuclear factor of kappa B signaling pathway in vitro. Indian J Pathol Microbiol. 2013;56:190‐195. [DOI] [PubMed] [Google Scholar]
- 22. Bastola P, Neums L, Schoenen FJ, Chien J. VCP inhibitors induce endoplasmic reticulum stress, cause cell cycle arrest, trigger caspase‐mediated cell death and synergistically kill ovarian cancer cells in combination with Salubrinal. Mol Oncol. 2016;10:1559‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315‐2319. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, Han N, Jiang Y, et al. EGFR confers radioresistance in human oropharyngeal carcinoma by activating endoplasmic reticulum stress signaling PERK‐eIF2alpha‐GRP94 and IRE1alpha‐XBP1‐GRP78. Cancer Med. 2018;7:6234‐6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer. 2016;2:252‐262. [DOI] [PubMed] [Google Scholar]
- 26. Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress‐induced gene expression in mammalian cells. Mol Cell. 2000;6:1099‐1108. [DOI] [PubMed] [Google Scholar]
- 27. Koizumi M, Tanjung NG, Chen A, et al. Administration of salubrinal enhances radiation‐induced cell death of SW1353 chondrosarcoma cells. Anticancer Res. 2012;32:3667‐3673. [PubMed] [Google Scholar]
- 28. Moretti L, Cha YI, Niermann KJ, Lu B. Switch between apoptosis and autophagy: radiation‐induced endoplasmic reticulum stress? Cell Cycle. 2007;6:793‐798. [DOI] [PubMed] [Google Scholar]
- 29. Riha R, Gupta‐Saraf P, Bhanja P, Badkul S, Saha S. Stressed out ‐ therapeutic implications of ER stress related cancer research. Oncomedicine. 2017;2:156‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sano R, Reed JC. ER stress‐induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460‐3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol. 2013;5:a012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 33. Magagnin MG, Sergeant K, van den Beucken T, et al. Proteomic analysis of gene expression following hypoxia and reoxygenation reveals proteins involved in the recovery from endoplasmic reticulum and oxidative stress. Radiother Oncol. 2007;83:340‐345. [DOI] [PubMed] [Google Scholar]
- 34. Yi P, Higa A, Taouji S, et al. Sorafenib‐mediated targeting of the AAA(+) ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol Cancer Ther. 2012;11:2610‐2620. [DOI] [PubMed] [Google Scholar]
- 35. Zhang H, Li K, Lin Y, et al. Targeting VCP enhances anticancer activity of oncolytic virus M1 in hepatocellular carcinoma. Sci Transl Med. 2017;9:eaam7996. [DOI] [PubMed] [Google Scholar]
- 36. Kuwahara Y, Tomita K, Urushihara Y, Sato T, Kurimasa A, Fukumoto M. Association between radiation‐induced cell death and clinically relevant radioresistance. Histochem Cell Biol. 2018;150:649‐659. [DOI] [PubMed] [Google Scholar]
- 37. Biau J, Chautard E, De Koning L, et al. Predictive biomarkers of resistance to hypofractionated radiotherapy in high grade glioma. Radiat Oncol. 2017;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim EJ, Lee YJ, Kang S, Lim YB. Ionizing radiation activates PERK/eIF2alpha/ATF4 signaling via ER stress‐independent pathway in human vascular endothelial cells. Int J Radiat Biol. 2014;90:306‐312. [DOI] [PubMed] [Google Scholar]
- 39. Zhang B, Wang Y, Pang X, Su Y, Ai G, Wang T. ER stress induced by ionising radiation in IEC‐6 cells. Int J Radiat Biol. 2010;86:429‐435. [DOI] [PubMed] [Google Scholar]
- 40. Rice TW, Ishwaran H, Hofstetter WL, et al. Esophageal cancer: associations with (pN+) lymph node metastases. Ann Surg. 2017;265:122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo H, Cui YY, Zhang JG, et al. Meta‐analysis of survival benefit with postoperative chemoradiotherapy in patients of lymph node positive esophageal carcinoma. Clin Transl Oncol. 2018;20:889‐898. [DOI] [PubMed] [Google Scholar]


