Abstract
Aflibercept plus 5‐fluorouracil/levofolinate/irinotecan (FOLFIRI) is a second‐line treatment for metastatic colorectal cancer. This ancillary exploratory analysis of data in Japanese people was aimed at exploring the relationship between a set of potential prognostic biomarkers and efficacy endpoints following aflibercept plus FOLFIRI therapy. Sixty‐two patients with metastatic colorectal cancer received aflibercept (4 mg/kg) plus FOLFIRI every 2 weeks. Seventy‐eight potential protein biomarkers were chosen for analysis based on their roles in angiogenesis, tumor progression, and tumor‐stroma interaction. Plasma levels of biomarkers at baseline and at pre‐dose 3 (day 1 of treatment cycle 3) were measured in all patients by ELISA. Relationships between these levels and efficacy endpoints were assessed. Ten potential biomarkers had a ±30% change from baseline to pre‐dose 3 (adjusted P < .001), with the greatest changes occurring in placental growth factor (median: +4716%) and vascular endothelial growth factor receptor 1 (+2171%). Baseline levels of eight potential biomarkers correlated with overall survival in a univariate Cox regression analysis: extracellular newly identified receptor for advanced glycation end‐products binding protein, insulin‐like growth factor‐binding protein 1, interleukin‐8, kallikrein 5, pulmonary surfactant‐associated protein D, tissue inhibitor of metalloproteinases 1, tenascin‐C, and tumor necrosis factor receptor 2. None correlated with progression‐free survival or maximum tumor shrinkage. Pre‐dose 3 levels did not correlate with any efficacy endpoints. Preliminary data show that these eight biomarkers could be associated with overall survival. ClinicalTrials.gov identifier: NCT01882868.
Keywords: aflibercept, biomarker, colorectal cancer, FOLFIRI, metastasis
Abbreviations
- 5‐FU
5‐fluorouracil
- ANG‐2
angiopoietin‐2
- CV
coefficient of variation
- EN‐RAGE
extracellular newly identified receptor for advanced glycation end‐products binding protein
- FDR
false discovery rate
- FOLFIRI
5‐FU/levofolinate/irinotecan
- HE4
human epididymis protein 4
- IGFBP‐1
insulin‐like growth factor‐binding protein 1
- IL
interleukin
- ITAC
interferon‐inducible T‐cell alpha chemoattractant
- mCRC
metastatic colorectal cancer
- mFOLFOX6
modified folinic acid, fluorouracil, and oxaliplatin
- OS
overall survival
- PFS
progression‐free survival
- PlGF
placental growth factor
- SP‐D
surfactant protein D
- TIMP‐1
tissue inhibitor of metalloproteinases 1
- TN‐C
tenascin‐C
- TNFR2
tumor necrosis factor receptor 2
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
1. INTRODUCTION
Colorectal cancer is one of the most common malignancies in the Japanese population.1 The 5‐year survival rate for mCRC is approximately 12%.2
Aflibercept is an anti‐cancer drug that has been used in combination with FOLFIRI as a second‐line treatment for mCRC. Aflibercept acts by binding to and sequestering VEGF‐A and ‐B and PlGF, thereby preventing downstream events such as angiogenesis and metastasis. To date, the largest phase III trial on aflibercept plus FOLFIRI (EFC10262, or VELOUR)3 was an international randomized double‐blind study conducted outside of Japan and consisting of 1226 patients with mCRC who had previously been treated with oxaliplatin with or without bevacizumab. The researchers found that aflibercept plus FOLFIRI significantly improved both overall survival (OS; 13.50 vs 12.06 months, P = .0032) and progression‐free survival (PFS; 6.90 vs 4.67 months, P = .0001) compared to placebo plus FOLFIRI. Another multinational randomized double‐blind phase III study (EFC11338) consisting of 332 patients in Asia yielded similar results: aflibercept plus FOLFIRI improved both OS (14.59 vs 11.93 months) and PFS (6.93 vs 5.59 months) compared to placebo plus FOLFIRI.4
Early studies have identified biomarkers that may predict response to treatment with aflibercept plus FOLFIRI. For example, analysis of plasma samples from the phase III VELOUR study led to identification of several biomarkers potentially predictive or prognostic of OS: VEGF‐A, VEGF receptors 2 and 3, IL‐8, macrophage migration inhibitory factor (MIF), and SP‐D.5 Lambrechts et al6 found that high plasma levels of IL‐8 at baseline together with increased levels of IL‐8 during treatment were significantly associated with reduced PFS. However, there are currently no established biomarkers that predict treatment response to aflibercept plus FOLFIRI.
The objective of the present study was to explore the relationship between a set of potential prognostic biomarkers and efficacy endpoints following aflibercept plus FOLFIRI therapy.
2. MATERIALS AND METHODS
2.1. Patients
This study included Japanese patients with mCRC whose cancer had failed to respond to a prior oxaliplatin treatment regimen. Further inclusion and exclusion criteria have been described in detail elsewhere.7 All patients gave informed consent.
2.2. Study design
The study design has been described in detail elsewhere.7 A brief description follows:
This study was a prospective, multicenter, open‐label, single‐arm study. The first patient was enrolled on July 30, 2013, and the last data were collected on August 28, 2015.
The study consisted of three time periods: a baseline period, a treatment period, and a post‐treatment follow‐up period. During the baseline period, tumors were imaged and baseline data were collected. During the treatment period, the following treatment regimen was given once every 2 weeks until disease progression, unacceptable toxicity, or patient withdrawal: aflibercept (4 mg/kg) by i.v. infusion; then levofolinate (200 mg/m2) plus irinotecan (180 mg/m2), simultaneously by i.v. infusion; then a bolus of 5‐FU (400 mg/m2); and then 5‐FU (2400 mg/m2) by continuous i.v. infusion. Tumors were imaged every 6 ± 1 weeks and at the end‐of‐treatment visit 30 ± 3 days after the last study treatment. In the post‐treatment follow‐up period, tumors were imaged every 6 weeks, and survival status was determined every 2 months.
Primary endpoint (objective response rate) and secondary endpoints (PFS, OS, and pharmacokinetics) have been described previously.7 The focus of the current article is an exploratory endpoint: plasma levels of potential prognostic biomarkers.
2.3. Assessments
A total of 109 potential protein biomarkers, including cytokines and angiogenic factors (Tables S1 and S2), were chosen for analysis based on their roles in tumor progression. Plasma was collected from all 62 patients at baseline and before infusion of aflibercept in treatment cycles 1 and 3. Plasma levels of the potential biomarkers were assessed by Myriad RBM using their multiplexed immunoassays, which are based on Luminex bead technology: Assays were carried out on the bead surface and then read in a compact analyzer using multiple lasers or light‐emitting diodes and high‐speed digital‐signal processors.
Relationships between biomarker levels (at baseline and in treatment) and efficacy variables (PFS, OS, maximum shrinkage in tumor size, and best overall response) were assessed. Biomarker levels were also analyzed in patient groups stratified by whether or not the patients had previously received bevacizumab.
2.4. Statistical analysis
All analyses of plasma protein biomarker variables were defined in an ad hoc method and carried out on all patients. P values for biomarker data were adjusted by FDR methods. Welch's test was used to determine P values for biomarker levels in patients stratified by whether they had previously received bevacizumab.
Median values of biomarker data were used to examine correlations with PFS and OS. This methodology has previously been shown to be effective for use in exploratory investigations of potential relationships between biomarkers and clinical outcomes.8
2.5. Ethical considerations
As mentioned elsewhere,7 the present study was conducted in accordance with the principles of the Declaration of Helsinki, and in compliance with all international and Japanese laws, regulations, and guidelines. All aspects of the study were approved by an independent ethics committee and institutional review board.
3. RESULTS
Sixty‐two patients from 19 clinical sites in Japan were enrolled in this study. Of the 109 biomarkers measured, 31 were excluded from the analysis due to lack of informative data, as detailed in Figure 1.
Figure 1.
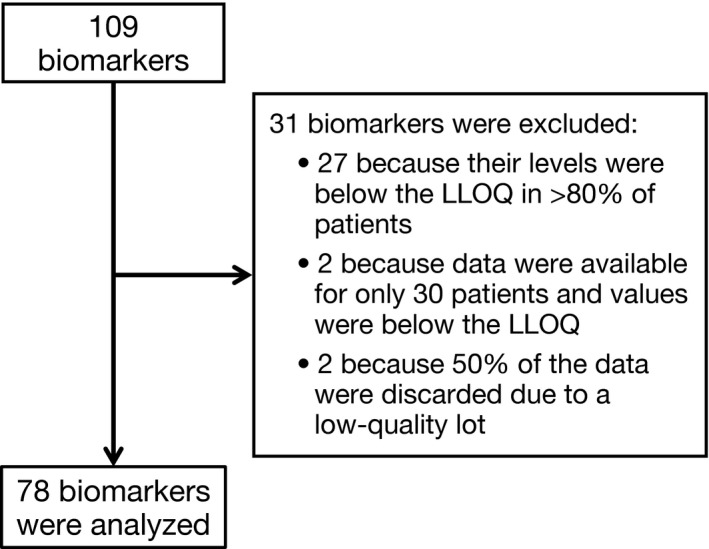
Flow chart of biomarkers measured, excluded from analysis, and analyzed. LLOQ, lower limit of quantification
Upon visual inspection of histograms of the individual biomarkers, 47 of the 78 biomarkers were found to have a non‐Gaussian distribution. In order to obtain distributions closer to Gaussian distributions, a logarithmic transformation was applied to measurements for these 47 biomarkers.
Progression‐free survival and OS were stratified by above versus below median plasma concentration for each biomarker. Baseline levels of eight biomarkers were found to correlate with OS with an adjusted P < .05 (Figures 2 and 3). The most significant differences in OS were found for TIMP‐1, IL‐8, and EN‐RAGE (all P < .001), with lower concentrations corresponding to longer OS for all three biomarkers. Spearman's rank correlation coefficient at baseline was 0.80 between TIMP‐1 and IL‐8; 0.67 between TIMP‐1 and EN‐RAGE; and 0.77 between IL‐8 and EN‐RAGE.
Figure 2.

Progression‐free survival (PFS) (A) and overall survival (OS) (B) stratified by above/below median plasma concentrations at baseline of biomarkers with adjusted P < .05 for PFS or OS. These biomarkers were extracellular newly identified receptor for advanced glycation end‐products binding protein (EN‐RAGE), insulin‐like growth factor‐binding protein 1 (IGFBP‐1), interleukin‐8 (IL‐8), kallikrein 5, pulmonary surfactant‐associated protein D (SP‐D), tenascin‐C (TN‐C), tissue inhibitor of metalloproteinases 1 (TIMP‐1), and tumor necrosis factor receptor 2 (TNFR2). ***P < .001; *P < .05; NS, not statistically significant
Figure 3.
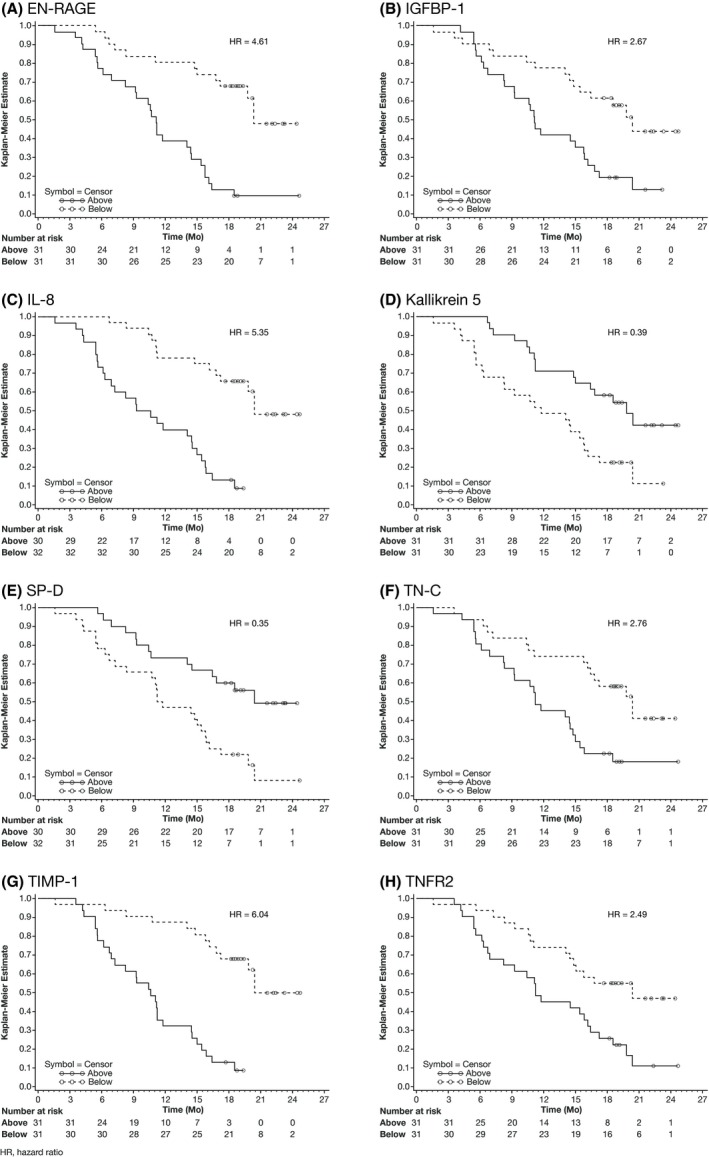
Kaplan‐Meier curves of overall survival (OS) stratified by median plasma concentration at baseline for eight biomarkers: (A) advanced glycation end‐products binding protein (EN‐RAGE), (B) insulin‐like growth factor‐binding protein 1 (IGFBP‐1), (C) interleukin‐8 (IL‐8), (D) kallikrein 5, (E) pulmonary surfactant‐associated protein D (SP‐D), (F) tenascin‐C (TN‐C), (G) tissue inhibitor of metalloproteinases 1 (TIMP‐1), and (H) tumor necrosis factor receptor 2 (TNFR2)
Median plasma concentrations used for stratification of OS were 22.50 ng/mL (CV, 295.213) for EN‐RAGE, 26.33 ng/mL (87.557) for IGFBP‐1, 6.45 pg/mL (131.301) for IL‐8, 3.30 ng/mL (33.276) for kallikrein 5, 5.43 ng/mL (67.590) for SP‐D, 430.25 ng/mL (57.457) for TN‐C, 76.00 ng/mL (43.984) for TIMP‐1, and 5.78 ng/mL (34.486) for TNFR2.
Baseline levels of none of the biomarkers were found to correlate with PFS, maximum shrinkage in tumor size, or best overall response, using an FDR correction of P value as the indicator. On‐treatment levels of none of the biomarkers were found to correlate with any of the efficacy variables.
Ten of the 78 potential biomarkers had a ±30% change in plasma concentration (P < .001) from baseline to pre‐dose 3 (Table 1). The most significant change was observed in PlGF, which had a 4716% change. In patients stratified by having received prior bevacizumab, significant differences were observed in baseline measurements of four biomarkers (Figure 4). The largest difference was observed in log‐transformed VEGF: median was 6.47 pg/mL in patients who had received prior bevacizumab versus 4.22 pg/mL in patients who had not (P = 1.4E−14). Baseline levels of PlGF and decorin were also significantly higher in patients who had received prior bevacizumab versus those who had not, whereas the baseline level of ANG‐2 was significantly lower.
Table 1.
Potential prognostic biomarkers with a ±30% change in plasma concentration (P < .001) from baseline to pre‐dose 3
| Biomarker | Median plasma level (CV%) [min : max] | % change [min : max] | Adjusted P a | |
|---|---|---|---|---|
| Baseline | Pre‐dose 3 | |||
| PlGF (pg/mL) | 26.50 (61.941) [8.0:72.5] | 1320.00 (31.579) [72.0:1990.0] | 4716.11 [65.5:23 900.0] | 1.26E−16 |
| VEGFR‐1 (pg/mL) | 59.00 (282.331) [59.0:3899.5] | 1905.00 (45.372) [693.0:3880.0] | 2171.19 [−73.8:5628.8] | 1.78E−16 |
| VEGF (pg/mL) | 610.75 (64.106) [42.0:1470.0] | 881.00 (50.922) [497.0:3090.0] | 92.06 [−9.3:3900.0] | 2.23E−16 |
| MMP‐3 (ng/mL) | 9.35 (48.341) [4.4:27.0] | 23.00 (68.269) [5.8:92.0] | 118.33 [−72.6:613.1] | 1.15E−15 |
| VEGFR‐3 (ng/mL) | 22.00 (57.507) [5.1:71.5] | 15.00 (57.449) [3.3:45.0] | −33.64 [−75.4:27.3] | 2.15E−11 |
| FRTN (ng/mL) | 112.25 (112.920) [9.5:835.5] | 186.00 (85.930) [23.0:950.0] | 57 [−32.4:817.6] | 1.15E−09 |
| PARC (ng/mL) | 76.25 (58.546) [26.5:266.5] | 94.50 (54.861) [35.0:333.0] | 30.19 [−33.3:136.5] | 7.56E−08 |
| IGFBP‐1 (ng/mL) | 26.33 (87.557) [0.3:126.0] | 55.00 (72.197) [0.3:175.0] | 50.5 [−96.9:2763.5] | 1.99E−06 |
| cFib (μg/mL) | 36.50 (70.420) [11.0:189.0] | 25.00 (90.614) [7.5:148.0] | −36.09 [−77.6:185.4] | 5.79E−05 |
| EN‐RAGE (ng/mL) | 22.50 (295.213) [1.1:2765.0] | 13.50 (265.312) [1.1:1170.0] | −46.94 [−95.0:300.0] | 1.31E−04 |
Abbreviations: cFib, cellular fibronectin; CV, coefficient of variation; EN‐RAGE, extracellular newly identified receptor for advanced glycation end‐products binding protein; FRTN, ferritin; IGFBP‐1, insulin‐like growth factor‐binding protein 1; PARC, pulmonary and activation regulated chemokine; PlGF, placental growth factor; VEGF, vascular endothelial growth factor; and VEGFR‐1, VEGF receptor 1.
Adjusted using the false discovery rate method.
Figure 4.
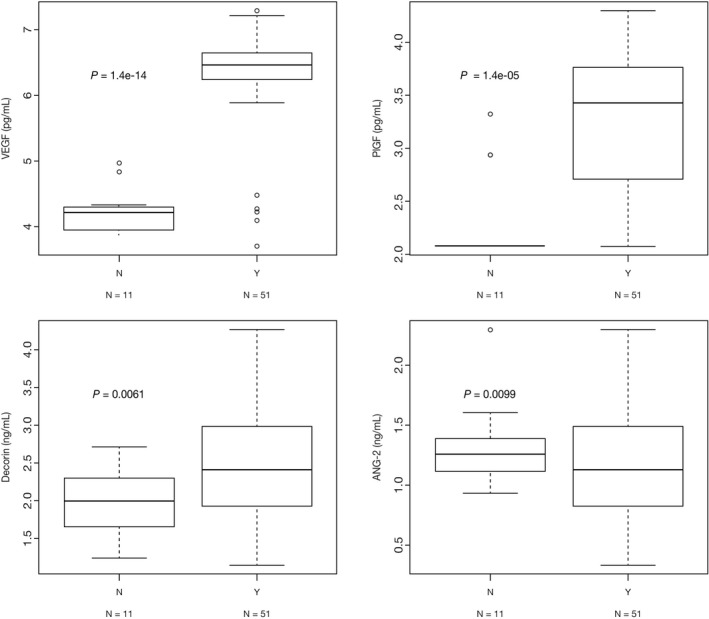
Baseline biomarker levels in patients stratified by having received prior bevacizumab. N = Patient did not receive prior bevacizumab; Y = Patient did receive prior bevacizumab. P values were determined using Welch's test. ANG‐2, angiopoietin‐2; PlGF, placental growth factor; VEGF, vascular endothelial growth factor
For 30 of the 78 biomarkers, two lots of reagent were used. Of these 30 biomarkers, nine had a batch effect with P < .001 and/or a value below the lower limit of quantification in one lot: VEGF‐C, HE4, carcinoembryonic antigen‐related cell adhesion molecule 1, cadherin‐13, endoglin, VEGFR‐1, hepatocyte growth factor receptor, human epidermal growth factor receptor 2, and ITAC. To eliminate the confounding effect, a correction was applied for all of these biomarkers except for VEGFR‐1; for VEGFR‐1, a quality problem was suspected in lot 1 prior to treatment cycle 1, which may have led to the apparent lot effect. For HE4 and ITAC, one lot was discarded, and the two biomarkers were excluded due to lack of available data.
4. DISCUSSION
The objective of the present study was to explore the relationship between a set of potential prognostic biomarkers and efficacy endpoints following second‐line treatment of mCRC with aflibercept plus FOLFIRI. We found eight biomarkers that are potentially prognostic for response to second‐line aflibercept plus FOLFIRI. It is important to note that because this was a single‐arm study, it could not be determined whether these eight biomarkers are actually prognostic, only that they are potentially prognostic for treatment response.
Among the eight potentially prognostic biomarkers found were TIMP‐1 and IL‐8: a lower baseline concentration of each of these biomarkers correlated with a longer OS. Other researchers have also implicated IL‐8 as a potentially prognostic biomarker. Lambrechts et al6 found that high plasma levels of IL‐8 at baseline together with increased levels of IL‐8 during first‐line treatment with 4 mg/mL aflibercept plus mFOLFOX6 (85 mg/m2 oxaliplatin and 350 mg/m2 leucovorin given simultaneously by i.v. infusion over 2 hours, followed by 400 mg/m2 5‐FU as a bolus and then 2400 mg/m2 5‐FU by i.v. infusion over 46 hours), given every 2 weeks,9 were significantly associated with reduced PFS compared to mFOLFOX6 alone. A retrospective analysis of data from the VELOUR study also implicated IL‐8 as a potential prognostic biomarker, as well as SP‐D,5 similar to the current study.
Interleukin‐8 has been found to be potentially prognostic for response not only to aflibercept but also to bevacizumab, which, like aflibercept, is an angiogenesis inhibitor. In one study, relative change in IL‐8 was associated with response to bevacizumab in patients with metastatic breast cancer.10 In other studies, IL‐8 levels or changes in IL‐8 level were not found to be prognostic for objective response rate, PFS, or OS in patients with colorectal cancer,11, 12 although in one of those studies, tissue samples rather than serum were studied.12
In the current study, 10 biomarkers, including four involved in the VEGF pathway, had a ±30% change in plasma concentration from baseline to pre‐dose 3. The highest increase observed at pre‐dose 3 was for VEGF and PlGF which was likely due to stabilization of these two proteins by aflibercept. Aflibercept plasma trough concentrations showed the existence of a certain concentration of bound aflibercept at pre‐dose 3. Mean plasma trough concentrations of free and adjusted bound aflibercept at pre‐dose 3 were 7.1 μg/mL (CV, 53.00%) and 4.4 μg/mL (CV, 15.93%), respectively. Despite these large changes, most of these biomarkers did not correlate with PFS or OS. The only ones that did were IGFBP‐1 and EN‐RAGE, which had percent changes of 50.5 (adjusted P = 1.99E−06) and −46.94 (adjusted P = 1.31E−04), respectively.
Our results are consistent with those for bevacizumab in which bevacizumab was also found to increase circulating PlGF in colorectal cancer13 and in ovarian cancer.14 Its effects on VEGF have been variable.13, 14
Baseline levels of four biomarkers were significantly different in patients who had received prior bevacizumab versus those who had not: VEGF, PlGF, and decorin were significantly higher in patients who had received prior bevacizumab, whereas ANG‐2 was significantly lower. Similarly, in an exploratory analysis of the VELOUR study, VEGF‐A and PlGF were found to correlate with prior bevacizumab therapy15 in patients who received aflibercept and FOLFIRI and in those who received placebo and FOLFIRI.16 None of the four biomarkers were found to be potentially prognostic for treatment response.
Thirty‐one of the original 109 biomarkers chosen based on their roles in tumor progression were removed from analysis because of insufficient data. It is possible that some of these 31 biomarkers are actually prognostic for response to treatment with aflibercept plus FOLFIRI. This remains to be studied.
One limitation of the present study is that there were a relatively small number of study participants. Another limitation is that it consisted of only a single arm, so comparisons in biomarker level between a study treatment arm and a control arm could not be made. In addition, although the methodology used herein to explore potential correlations between biomarkers and survival times, based on median values, has been validated previously,8 future studies may choose to incorporate alternative analysis methods using absolute rather than relative cut‐off values. It must also be noted that this study analysis did not identify predictive biomarkers but merely identified potential biomarkers whose levels correlated with overall survival. To determine whether the eight candidate biomarkers found in this study actually predict prognosis, it would be important to do a double‐arm prospective study with a larger number of patients. Furthermore, the possibility that the selected biomarkers are false positives cannot be ruled out. Nevertheless, these data would be valuable for biomarker selection in a meta‐analysis of data from multiple studies.
In conclusion, eight biomarkers potentially prognostic for OS were identified: TIMP‐1, IL‐8, EN‐RAGE, SP‐D, TN‐C, IGFBP‐1, kallikrein 5, and TNFR2. Further studies are warranted.
DISCLOSURE
T.H. received honoraria from Taiho, Chugai, Takeda, Yakult, and Merck Serono; a consulting fee from NanoCarrier; and research funding from MSD, Ono, Sanofi, Daiichi‐Sankyo, Sumitomo Dainippon Pharma, Taiho, Teijin, and NanoCarrier. T.K. received research finding from Yakult. N.S. received honoraria from Chugai and Eli Lilly, and research funding from Chugai, Eli Lilly, Dainippon Sumitomo, Taiho, MSD, Ono, Daiichi‐Sankyo, and Sanofi. T.U. received honoraria from Merck Serono, Taiho, Chugai, and Takeda, and research funding from Sanofi. K.Y. received honoraria from Chugai, Takeda, Yakult, Daiichi‐Sankyo, Merck Serono, Bristol, Bayer, Eli Lilly, and Taiho, and research funding from BMS and Sanofi. H.F. received research funding from Sanofi. S.T. received honoraria from Asahikasei and research funding from Merck Serono, Ono, and Sanofi. Y.K. received honoraria from BMS and Sanofi, and research funding from Eli Lilly, BMS, and Sanofi. T.E. received honoraria from Eli Lilly, and research funding from Boehringer, Daiichi‐Sankyo, Dainippon Sumitomo, Eli Lilly, Merck Serono, MSD, Novartis, Ono, and Taiho, and scholarship from Ono. S.T. received honoraria from Asahikasei, and research funding from Merck Serono, Ono and Sanofi. E.O. received honoraria from Bayer, Chugai, Eli Lilly, Merck Serono, Taiho, Takeda, and Yakult. T.Y. received honoraria from Chugai, Eli Lilly, Merck Serono, Sanofi, and Taiho, and research funding from Boehringer Ingelheim, Chugai, Dainippon Sumitomo, GlaxoSmithKline, MSD, Novartis, and Sanofi. The affiliated medical institutions of physician authors received study funding from Sanofi. T.S. and M.C. are employees of Sanofi. Funding for this research was provided by Sanofi.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank all patient participants. This study was sponsored by Sanofi writing assistance by Michelle L. Jones, PhD, ELS, and funded by Sanofi. Yoshinori Sunaga, Sanofi K. K., Tokyo, Japan analyzed the data and contributed to interpretation of the study.
Hamaguchi T, Denda T, Kudo T, et al. Exploration of potential prognostic biomarkers in aflibercept plus FOLFIRI in Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019;110:3565–3572. 10.1111/cas.14198
REFERENCES
- 1. Toukei GT. Cancer Registration Statistics. National Cancer Center Japan. http://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed January 18, 2019.
- 2. American Cancer Society, Inc . Survival rates for colorectal cancer, by stage. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html. Accessed November 17, 2018.
- 3. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J Clin Oncol. 2012;30(28):3499‐3506. [DOI] [PubMed] [Google Scholar]
- 4. Sanofi . A study of aflibercept versus placebo with FOLFIRI in patients with metastatic colorectal cancer previously treated with an oxaliplatin chemotherapy (AFLAME); 2015. https://clinicaltrials.gov/ct2/show/results/NCT01661270?sect=X80156. Accessed November 18, 2018.
- 5. Sims TN, Gao B, Phillips R, Lowy I. Potential predictive and prognostic biomarkers identified in baseline plasma samples from the VELOUR trial. J Clin Oncol. 2015;33(Suppl 3):638. [Google Scholar]
- 6. Lambrechts D, Thienpont B, Thuillier V, et al. Evaluation of efficacy and safety markers in a phase II study of metastatic colorectal cancer treated with aflibercept in the first‐line setting. Br J Cancer. 2015;113(7):1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denda T, Sakai D, Hamaguchi T, et al. Phase II trial of aflibercept plus FOLFIRI as a second‐line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019;110(3):1032‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keyvanjah K, DePrimo SE, Harmon CS, et al. Soluble KIT correlates with clinical outcome in patients with metastatic breast cancer treated with sunitinib. J Transl Med. 2012;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Folprecht G, Pericay C, Saunders MP, et al. Oxaliplatin and 5‐FU/folinic acid (modified FOLFOX6) with or without aflibercept in first‐line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol. 2016;27(7):1273‐1279. [DOI] [PubMed] [Google Scholar]
- 10. Lam SW, Nota NM, Jager A, et al. Angiogenesis‐ and hypoxia‐associated proteins as early indicators of the outcome in patients with metastatic breast cancer given first‐line bevacizumab‐based therapy. Clin Cancer Res. 2016;22(7):1611‐1620. [DOI] [PubMed] [Google Scholar]
- 11. Kargi A, Yalcin AD, Erin N, Savas B, Polat HH, Gorczynski RM. Erratum to “IL8 and serum soluble TRAIL levels following anti‐VEGF monoclonal antibody treatment in patients with metastatic colon cancer” [Clin Lab. 2012;58:501‐505]. Clin Lab. 2012;10:1103‐1107. [PubMed] [Google Scholar]
- 12. Bruhn MA, Townsend AR, Khoon Lee C, et al. Proangiogenic tumor proteins as potential predictive or prognostic biomarkers for bevacizumab therapy in metastatic colorectal cancer. Int J Cancer. 2014;135(3):731‐741. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi H, Arao T, Matsumoto K, et al. Biomarkers of reactive resistance and early disease progression during chemotherapy plus bevacizumab treatment for colorectal carcinoma. Oncotarget. 2014;5(9):2588‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horowitz NS, Penson RT, Duda DG, et al. Safety, efficacy, and biomarker exploration in a phase II study of bevacizumab, oxaliplatin, and gemcitabine in recurrent Müllerian carcinoma. Clin Ovarian Cancer Other Gynecol Malig. 2011;4(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tabernero J, Paccard C, Chiron M, Dochy E, Van Cutsem E. Placental growth factor and the angiogenic environment based on analysis of baseline plasma biomarkers from the VELOUR trial. J Clin Oncol. 2017;35(Suppl 4):592. [Google Scholar]
- 16. Van Cutsem E, Dochy E, Paccard C, Chiron M, Tabernero J. Impact of prior bevacizumab treatment on VEGFA and PlGF levels and patient outcomes: A retrospective analysis of baseline plasma samples from the VELOUR trial. Ann Oncol. 2017;28(Suppl 3):141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


