Abstract
Introduction
A species of hawthorn, Crataegus mexicana (tejocote), has been marketed as a weight-loss supplement that is readily available for purchase online. While several hawthorn species have shown clinical benefit in the treatment of heart failure owing to their positive inotropic effects, little is known about hawthorn, and tejocote in particular, when consumed in excess. We describe a case of tejocote exposure from a weight-loss supplement resulting in severe cardiotoxicity.
Case Report
A healthy 16-year-old girl presented to an emergency department after ingesting eight pieces of her mother’s tejocote root weight-loss supplement. At arrival, she was drowsy, had active vomiting and diarrhea, and had a heart rate of 57 with normal respirations. Her initial blood chemistries were unremarkable, except for an elevated digoxin assay of 0.7 ng/mL (therapeutic range 0.5–2.0 ng/mL). All other drug screens were negative. She later developed severe bradycardia and multiple episodes of hypopnea that prompted a transfer to our institution, a tertiary pediatric hospital. Her ECG demonstrated a heart rate of 38 and Mobitz type 1 second-degree heart block. She was subsequently given two vials of Digoxin Immune Fab due to severe bradycardia in the setting of suspected digoxin-like cardiotoxicity after discussion with the regional poison control center. No clinical improvement was observed. Approximately 29 hours after ingestion, subsequent ECGs demonstrated a return to normal sinus rhythm, and her symptoms resolved.
Discussion
Tejocote root toxicity may cause dysrhythmias and respiratory depression. Similar to other species of hawthorn, tejocote root may cross-react with some commercial digoxin assays, resulting in a falsely elevated level.
Keywords: Crataegus, Tejocote, Hawthorn, Digoxin, Cardiovascular toxicity
Background
Hawthorn, the common name for all Crataegus plant species, is a thorny shrub long utilized for preventing and treating cardiovascular diseases [1, 2]. The berries, flowers, and leaves contain several active components including flavonoids and oligomeric proanthocyanidins. Recently, Crataegus mexicana (tejocote), a species of hawthorn native to Mexico and Guatemala, has been marketed as a weight-loss supplement in the form of dried root. However, there is little reported data about the clinical effects of Crataegus mexicana or consumption of the root specifically. We report a case of a tejocote root ingestion in a pediatric patient that resulted in cardiotoxicity and a falsely elevated digoxin level.
Case
A previously healthy 16-year-old girl presented to an outside emergency department approximately 8 hours after ingesting eight pieces of her mother’s tejocote root weight-loss supplement (Fig. 1) in an attempt to lose weight. She presented with nausea, vomiting, diarrhea, and drowsiness after she awoke due to discomfort. On presentation, she was afebrile, had normal respirations, and was normotensive with a pulse of 57. Her blood chemistries were notable for a normal potassium of 4.4 mEq/L and an elevated digoxin assay of 0.7 ng/mL (therapeutic range 0.5–2.0 ng/mL) (Vitros 4600 Chemical System, Ortho Clinical Diagnostics, Raritan, NJ, USA). The family and patient denied access to and ingestion of digoxin. During emergency department observation, she was noted to have bradycardia to 38 bpm and bradypnea of 4 breaths/min with intermittent desaturations to 70% during sleep. Her saturations and bradypnea improved after she was awoken and given 2 L/min of oxygen by nasal cannula. An ECG demonstrated sinus bradycardia with PR prolongation. She was subsequently transferred to our institution for further evaluation and monitoring.
Fig. 1.

Tejocote supplement consumed by the patient.
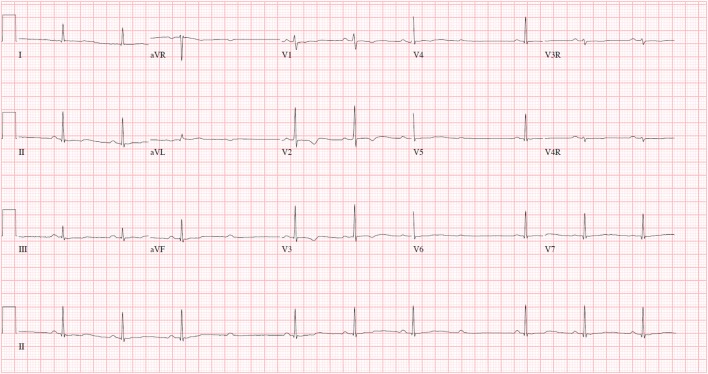
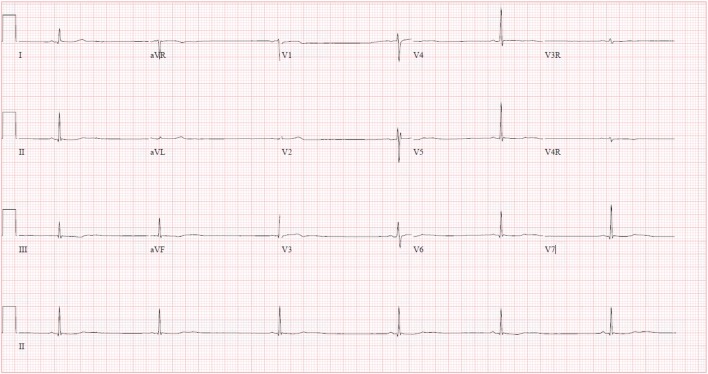
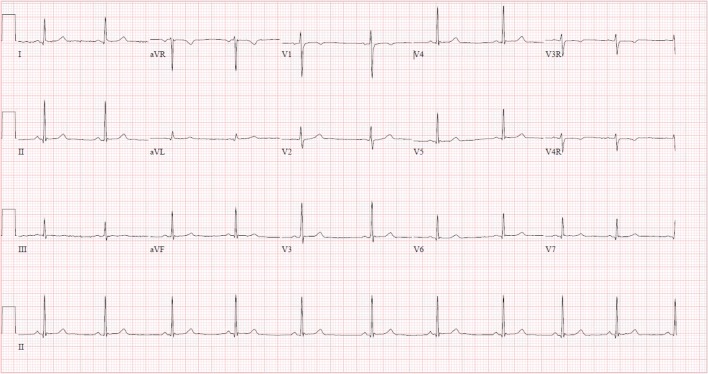
Upon arrival to our emergency department approximately 18 hours following ingestion, she reported persistent drowsiness and dizziness upon standing. Her physical exam was nonfocal. An ECG demonstrated severe bradycardia with Mobitz type 1 second-degree atrioventricular (AV) block (Fig. 2). After consultation with the regional poison control center and discussion with the patient, there was low suspicion that the patient had ingested digoxin. However, it was possible that the substance she consumed was structurally similar to digoxin as it appeared to exert a similar toxicity and cross-reacted with the digoxin immunoassay. Therefore, the patient was given two vials of Digoxin Immune Fab for her severe bradycardia. There was no improvement in her heart rate or normalization of the dysrhythmia. She was admitted to the pediatric intensive care unit for observation. Additional doses were withheld given the perceived futility and high cost of the antidote. Fortunately, her hospital course was uneventful, and her ECG demonstrated a return to normal sinus rhythm approximately 29 hours after ingestion (Fig. 3) and resolution of bradycardia approximately 3 days after ingestion (Fig. 4). The patient was subsequently discharged in a stable condition. Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Fig. 2.
ECG approximately 18 h after ingestion demonstrating Mobitz type 1 second-degree AV block. QRS interval = 70 ms. QT/QTc = 408/387 ms.
Fig. 3.
ECG approximately 29 h after ingestion demonstrating resolution of heart block, but persistent sinus bradycardia. QRS interval = 82 ms. QT/QTc interval = 460/356 ms.
Fig. 4.
ECG approximately 85 h after ingestion with a return to normal rate. QRS interval = 78 ms. QT/QTc interval = 376/382 ms.
Discussion
To our knowledge, this is the first reported case of toxicity from tejocote root (Crataegus mexicana) ingestion, and hawthorn species in general, which resulted in cardiotoxicity, respiratory depression, and altered mental status. It is also the first to demonstrate that tejocote, like other hawthorn species, cross-reacts with a commercial digoxin assay.
Hawthorn (Crataegus spp.) is well known to exert cardiovascular effects. Extract from hawthorn species is thought to exert its cardiac effects through multiple mechanisms including inhibition of the sodium-potassium ATPase and augmentation of endothelial relaxation via nitrous oxide–mediated mechanisms. Similar to Digitalis, a well-known plant source of cardiac glycosides, inhibition of the sodium-potassium ATPase leads to increased intracellular calcium and increased cardiomyocyte contractility. However, hawthorn is thought to ultimately exert a unique mechanism of action as it prolongs the action potential and refractory period for cardiac myocytes and has demonstrated antiarrhythmic properties [2].
Hawthorn is considered safe in therapeutic doses, and serious adverse effects are rare in the literature. In one systematic review of 5577 patients taking standard doses of hawthorn extract, only 166 patients reported transient, non-life threatening adverse effects such as dizziness, GI complaints, headache, and palpitations [3]. This is consistent with multiple postmarketing surveillance studies in Europe [4]. In the SPICE trial, a randomized control trial with 2681 patients comparing hawthorn with placebo in the treatment of NYHA class II or III congestive heart failure, no significant difference in adverse events was found between the two groups [5].
There are no published reports of hawthorn root toxicity that have been published to our knowledge.
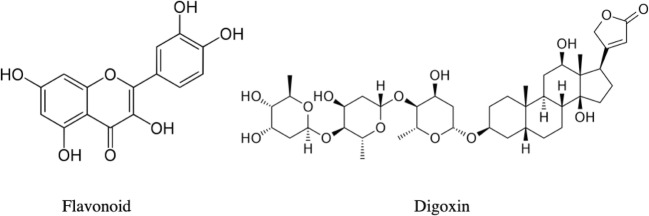
Notably, we also report that the patient’s digoxin immunoassay level was elevated, but the patient did not clinically respond to two vials of Digoxin Immune Fab. Dasgupta et al. previously demonstrated cross-reactivity of hawthorn extract with certain digoxin assays in a non-clinical context, a finding supported by documented structural similarities between hawthorn and Digitalis species [6]. It is not clear which active components of hawthorn are structurally similar to digoxin, but flavonoids appear to share some structural similarity (Fig. 5). Therefore, we hypothesize that the findings of hawthorn toxicity and digoxin toxicity may overlap. Both Crataegus and Digitalis inhibit the sodium-potassium ATPase, and one study has suggested that they bind at the same site [6]. The authors also found that the compound in the hawthorn extract appeared to be more potent than that of digoxin, which may have implications for patients who take both substances. Similar to the present case, heart block has been previously associated with pediatric Digitalis ingestion [7, 8]. Specifically, Mobitz type 1 second-degree AV block has been associated with severe Digitalis toxicity [7].
Fig. 5.
Example of a flavonoid, the class of hydroxylated polyphenolic compound thought to be one of the active ingredients in Crataegus spp. and digoxin for structural comparison [9, 10].
Conclusions
Tejocote (Crataegus mexicana) root ingestion may result in cardiotoxicity when consumed in excess. Components of hawthorn spp. may cross-react with some commercial digoxin assays and may produce similar toxicities to Digitalis spp. and cardiac glycosides in general. Providers should be aware of the potential toxicity and side effects of these and other readily available tejocote root products.
Compliance with Ethical Standards
Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Conflict of Interest
None.
Sources of Funding
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang Q, Zuo Z, Harrison F, Chow M. Hawthorn. J Clin Pharmacol. 2002;42:605–612. doi: 10.1177/00970002042006003. [DOI] [PubMed] [Google Scholar]
- 2.Holubarsch C, Colucci WS, Eha J. Benefit-risk assessment of Crataegus extract WS 1442: an evidence-based review. Am J Cardiovasc Drugs. 2018;18:25–36. doi: 10.1007/s40256-017-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniele C, Mazzanti G, Pittler MH, Ernst E. Adverse-event profile of Crataegus spp.: a systematic review. Drug-Safety. 2006;29:523–535. doi: 10.2165/00002018-200629060-00005. [DOI] [PubMed] [Google Scholar]
- 4.Guo R, Pittler MH, Ernst E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst Rev. 2008. 10.1002/14651858.CD005312.pub2. [DOI] [PubMed]
- 5.Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur J Heart Fail. 2008;10:1255–1263. doi: 10.1016/j.ejheart.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta A, Kidd L, Poindexter BJ, Bick RJ. Interference of hawthorn on serum digoxin measurements by immunoassays and pharmacodynamic interaction with digoxin. Arch Pathol Lab Med. 2010;134:1188–1192. doi: 10.5858/2009-0404-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Gittelman MA, Stephan M, Perry H. Acute pediatric digoxin ingestion. Pediatr Emerg Care. 1999;15:359–362. doi: 10.1097/00006565-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Thacker D, Sharma J. Digoxin toxicity. Clin Pediatr. 2007;46:276–279. doi: 10.1177/0009922806294805. [DOI] [PubMed] [Google Scholar]
- 9.Flavonoid: David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev. 2016;10(20):84. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deguigne M, Brunet M, Abbara C, Turcant A, Le Roux G, Lelièvre B. Enzalutamide and analytical interferences in digoxin assays. Clin Toxicol. 2018;56(11):1150–1154. doi: 10.1080/15563650.2018.1469758. [DOI] [PubMed] [Google Scholar]