Abstract
Apolipoprotein E (ApoE) ε4 allele and cerebral blood flow (CBF) changes are related to the increased risk of cognitive impairment independently. However, whether there are interactions between ApoE ε4 and CBF on memory performance in older adults with normal cognition remains unknown. This study determined whether the association between CBF and memory performance could be moderated by ApoE ε4 within a sample of cognitively normal older adults from the ADNI. 62 participants, including 23 with ApoE ε4 (ApoE ε4+) and 39 without ApoE ε4 (ApoE ε4−), underwent resting CBF measurement and memory testing. CBF was measured by arterial spin labeling MRI and memory performance was evaluated by the Rey Auditory Verbal Learning Test. By using linear regression models, CBF was negatively associated with memory function in ApoE ε4+ group, whereas positively in ApoE ε4− group by contrast. This study suggests that different CBF-memory relationships can be detected in cognitively normal ApoE ε4 carriers compared to ApoE ε4 non-carriers. Associations between hyperperfusion and worse memory performance in ApoE ε4 carriers may reflect vascular and/or cellular dysfunction.
Keywords: Arterial spin labeling (ASL), Cerebral blood flow (CBF), ApoE ε4, Memory function
Introduction
Cerebral blood flow (CBF) refers to the rate at which arterial blood delivers to the tissue capillary bed. It is a measure of neural function and brain metabolism. The precise measurement of CBF can be obtained by ASL, a novel MRI technique. CBF reduction (hypoperfusion) is related to cognitive impairment, indicating that cerebrovascular mechanisms are of great importance in the maintenance of cognitive performance (Knopman and Roberts 2010; Montagne et al. 2015b; Wierenga et al. 2014).
The conclusions of previous studies determining the correlation between cognitive performance and CBF in cognitively normal older adults were inconsistent. Some reported positive correlation using arterial flow measurements of carotid and basilar (Rabbitt et al. 2006), while some showed negative association using ASL MRI (Bertsch et al. 2009). For instance, Heo et al. (2010) found that CBF of hippocampal was positively correlated with spatial memory function by using flow-enhanced signal intensity MRI. By contrast, another study by Steffener et al. (2013) found that memory performance was negatively associated with CBF in the posterior central gyrus, part of the temporal cortex and hippocampus, but positively associated with CBF in the orbital frontal lobe. Those results showed that associations between CBF and cognitive function may have clinical significance for the prevention of AD.
Whether the associations between CBF and cognitive function could be regulated by AD risks is still understudied. It is well established that cerebrovascular dysfunction is related to mild cognitive impairment (MCI) and AD (Kelleher and Soiza 2013). ApoE ε4 is crucial to the integrity of cerebral vascular structure (Bell et al. 2012; Zlokovic 2011). The risk for AD is two to three folds higher in individuals carrying one ApoE ε4 allele (Corder et al. 1993) and 12-folds higher in those carrying two ApoE ε4 alleles (Corder et al. 1993; Roses 1996; Saunders et al. 1993). However, previous studies determining the association between ApoE ε4 and CBF showed mixed results. For example, compared to ApoE ε4− individuals, ApoE ε4+ individuals represented increased CBF in the medial temporal lobe, left lingual gyrus, precuneus and the right insular gyrus according to positron emission tomography (PET) studies (Bangen et al. 2012; Thambisetty et al. 2010; Wierenga et al. 2013). On the contrast, an ASL study showed that decreased regions of CBF in ApoE ε4+ group compared to ApoE ε4− group include right caudate, left middle temporal gyrus, right inferior parietal lobe, and right insula (Kim et al. 2013). Those studies seemed in contradiction with each other, which may be due to the different measurement methods of CBF (i.e., PET versus ASL MRI), but they all revealed the correlation between ApoE ε4 and CBF. However, those studies mainly concentrated on individuals with MCI or AD, and research on whether ApoE ε4 could modulate the association between CBF and memory function in cognitively normal older adults has been scanty.
This study investigated whether there was an association between regional ASL CBF and memory performance, and whether this association was modulated by ApoE ε4 in cognitively normal older adults.
Materials and methods
Participants
Data used in this study came from the ADNI database (adni.loni.usc.edu). The ADNI was launched by Principal Investigator Michael W. Weiner, MD in 2003. The primary goal of ADNI is to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessments can be combined to measure the progression of MCI and early AD. Following the establishment of the ADNI, ADNI-GO, ADNI-2 and ADNI-3 have been launched one after another. Participants included in this manuscript were cognitively normal older adults from the ADNI-2.
Inclusion criteria
Age between 55 and 90 years old;
Years of education > 6;
Mini-mental state examination (MMSE) score ≥ 24;
Clinical Dementia Rating (CDR) score = 0;
Participants who have completed RAVLT.
Exclusion criteria
A history of cerebral infarction or brain injury;
Existence of other neurological diseases that may cause cognitive impairment such as severe depression, brain tumor, Parkinson’s Disease, brain trauma and normal pressure hydrocephalus, etc.;
Existence of other systemic diseases that may lead to cognitive impairment, such as impairments of liver or kidney function, thyroid dysfunction, folate and/or vitamin B12 deficiency, specific infections (e.g. syphilis and HIV), etc.;
Consumption of drugs that may affect cognitive function, including sedatives, anxiolytics, nootropics or cholinergic drugs;
Systemic disease with significant symptoms, such as heart failure, tumor, drug dependence, drug addiction, etc.;
A total of 70 participants were selected in accordance with the above criteria, of whom 25 carried with ApoE ε4 and 45 without ApoE ε4. We further excluded participants who were diagnosed as cognitively normal but met criteria for MCI, with scores of neuropsychological measures one standard deviation lower than normative expectations within a cognitive domain (Bondi et al. 2014; Edmonds et al. 2015; Jak et al. 2009). A final sample of 62 participants were included.
Neuropsychological assessment
Memory function was evaluated by the RAVLT. The detailed procedures were as follows: first, the participants were required to learn 15 words (list A) for 5 times and recall freely (trials 1–5). Then, the participants were asked to learn 15 interfered words (list B) and recall list B freely, followed by recalling list A immediately (trial 6) and 30 min later (trial 7). We calculated total scores of the trials 1–5 as memory performance.
MRI data acquisition
MRI was conducted on a Siemens MAGNETOM Verio 3.0 Tesla scanner. Structural images and ASL images were downloaded. The structural MRI data were acquired utilizing a three-dimensional (3D) magnetization-prepared rapid acquisition with gradient echo (MPRAGE) T1-weighted sequence. Pulsed ASL (Wong et al. 1997) data were acquired by QUIPS II with thin-slice TI1 periodic saturation sequence (Luh et al. 1999). The acquisition parameters of 3D_T1 were: repetition time (TR) = 2300 ms, echo time (TE) = 2.98 ms, inversion time (TI) = 900 ms, field of view (FOV) = 256 mm × 240 mm, slice number: 176 (sagittal) and flip angle (FA) = 9°. The acquisition parameters of pulsed ASL were: TR = 3400 ms, TE = 12 ms, TI1 = 700 ms, TI2 = 1900 ms, FOV = 256 mm × 256 mm, slice number: 24 (axial), thickness = 4 mm and image matrix = 64 × 64.
MRI data processing
ASL data processing was conducted by SPM8. To minimize the effect of head motion, ASL images were aligned to the intermediate time point. Perfusion weighted images were calculated from the difference between control and labeled images. ASL images were normalized to the Montreal Neurological Institute (MNI) space. Then, each participant’s brain was spatial smoothed with a Gaussian kernel at full-width-at-half-maximum (FWHM) of 8 mm × 8 mm × 8 mm. Finally, to correct CBF in the gray matter, partial volume effect (PVE) correction was performed.
The following cerebral regions which are closely related to aging and AD were selected as regions of interest (ROI) in this study: medial temporal lobe (hippocampus, parahippocampal gyrus and uncus), parietal lobe (supramarginal gyrus, angular gyrus, precuneus and posterior cingulate), and frontal lobe (anterior cingulate gyrus, middle and medial frontal gyrus). Average CBF was extracted for each ROI.
Statistical analysis
In order to compare intergroup differences in demographic data and cognitive function scores, the continuous variables in this study were analyzed by the independent sample t test and categorical variables were analyzed by the Chi-squared test. A hierarchical linear regression model was employed to investigate whether there are interactions between ApoE genotype and CBF on memory function. CBF of ROIs, ApoE genotype and the interaction term were chosen as independent variables and memory performance was the dependent variable. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 19.
Results
There were no significant differences between the two groups with respect to age, sex, education and cognitive performers (Table 1). Significant interactions of CBF and ApoE genotype on memory performance were found in three ROIs by regression analyses. Spearman’s correlation showed that memory scores were negatively associated with CBF of the medial temporal lobe (r = − 0.45, p = 0.03), parietal lobe (r = − 0.56, p = 0.007), and frontal lobe (r = − 0.51, p = 0.02) in the ApoE ε4+ group. Contrarily, memory scores were positively associated with CBF of the medial temporal lobe (r = 0.48, p = 0.002), parietal lobe (r = 0.37, p = 0.02), and frontal lobe (r = 0.38, p = 0.03) in the ApoE ε4− group (Fig. 1).
Table 1.
Demographic statistics and cognitive characteristics of the participants
| ApoE ε4− (n = 39) | ApoE ε4+ (n = 23) | F/X2 | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 71.08 | 6.87 | 73.27 | 5.94 | F = 2.8 | 0.24 |
| Sex (female/%) | 30/76% | – | 16/70% | – | X2 = 0.7 | 0.62 |
| Years of Education | 15.87 | 2.32 | 16.02 | 2.14 | F = 2.47 | 0.33 |
| MMSE | 27.3 | 1.3 | 26.7 | 1.5 | F = 1.03 | 0.51 |
| RAVLT trials 1–5 total | 49.74 | 10.74 | 47.04 | 10.62 | F = 3.12 | 0.47 |
ApoE ε4 apolipoprotein E ε4 allele, RAVLT Rey Auditory Verbal Learning Test, MMSE mini-mental state examination, SD standard deviation
Fig. 1.
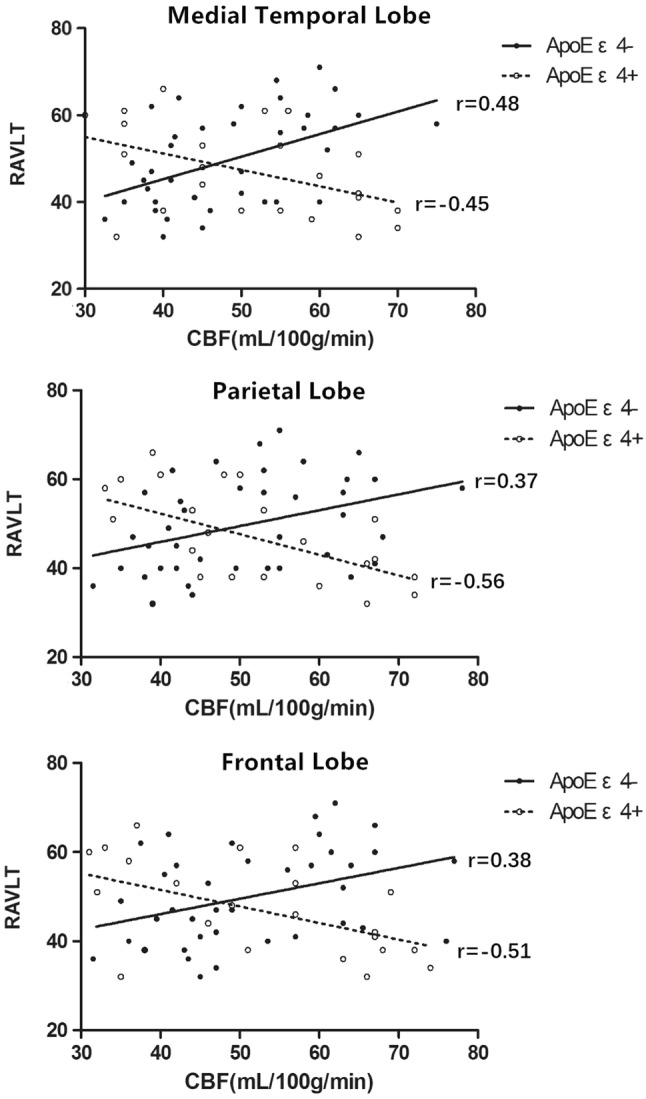
Scatterplots of interaction of ApoE ε4 and CBF on memory performance (RAVLT trials 1–5 total) for 3 regions of interest. Medial temporal lobe includes: hippocampus, parahippocampal gyrus and uncus; parietal lobe includes: supramarginal gyrus, angular gyrus, precuneus and posterior cingulate; frontal lobe includes: anterior cingulate gyrus, middle and medial frontal gyrus. Solid line represents the ApoE ε4+ group, dotted line represents the ApoE ε4− group. ApoE ε4 apolipoprotein E ε4 allele, CBF cerebral blood flow, RAVLT Rey Auditory Verbal Learning Test
Discussion
Our study explored whether there were interactions between regional CBF and ApoE genotype on memory function in older adults with normal cognition. In specific, results suggested that among ApoE ε4+ individuals, memory function was negatively associated with CBF of regions relating to AD and aging. Contrarily, among ApoE ε4− individuals, memory function was positively associated with CBF. The study indicated that for individuals who are without genetic risk of AD, hyperperfusion maintains memory performance, however, for individuals with the ApoE ε4, heightened CBF may not maintain memory performance any more.
Typically, decreased regional CBF are explained as representing decreases of cognitive function, while increased CBF in preclinical AD are often thought to reflect a compensatory strategy to pathologic process (Dai et al. 2009). Indeed, previous studies demonstrated that increased CBF was significantly correlated to better memory function in MCI with AD risk factors, and it was explained as a compensatory response since higher CBF can provide more oxygen and glucose to support neuronal activity (Fleisher et al. 2009; Bangen et al. 2012; Zlatar et al. 2014), as the case for the ApoE ε4− participants in this study. Contrarily, for cognitively normal older adults with ApoE ε4, heightened CBF may not support better cognitive function any more. Unlike previous studies that focused on MCI or AD patients, we chose cognitively normal older adults as participants and, crucially, there was no significant difference in cognitive function between the ApoE ε4+ and ApoE ε4− group. In ApoE ε4+ group, increased CBF may indicate that these participants’ RAVLT performance is decreasing (although still normal), and more CBF are needed to maintain the decreasing memory function. It has been showed that early MCI was characterized by hyperperfusion and later MCI by hypoperfusion when transiting to dementia (Wierenga et al. 2014) and it is possible that ApoE ε4+ participants in our study are more vulnerable to develop MCI. To further determine the role of increased CBF, a longitudinal study is in need to explore CBF differences across the trajectory of AD.
Normal neuronal computation and information processing requires sophisticated regulation of the chemical composition of the neuronal environment maintained by the blood–brain barrier (BBB) (Iadecola 2004, 2013; Zlokovic 2008). BBB limits the entry of neurotoxic blood-derived products and macromolecules into the brain (Zlokovic 1995; Zlokovic et al. 1985, 1987). It also plays a critical role in removing neurotoxic products from brain such as amyloid β (Aβ). BBB is composed of endothelial cells, perivascular mural cells and pericytes, of which pericytes plays a key role in maintaining the integrity of BBB. ApoE4, the corresponding protein of ApoE ε4, is associated with neurovascular dysfunction in patients with neurological disorders (Kim et al. 2009; Verghese et al. 2011) (e.g. AD, traumatic brain injury and haemorrhage) as well as in individuals with normal cognition (Reiman et al. 2004; Thambisetty et al. 2010; Sheline et al. 2010). The mechanisms may be due to toxic effects of ApoE4 on cerebrovascular and/or neurons. First, ApoE4 can lead to pericyte loss via cyclophilin A (Bell et al. 2012), and reduction of pericytes can result in a long-term BBB leakage and microvascular changes contributing to neurodegenerative diseases (Armulik et al. 2010; Bell et al. 2010; Daneman et al. 2010). For instance, accelerated pericyte degeneration and BBB breakdown have been reported in ApoE ε4 carriers with AD (Halliday et al. 2016). Moreover, using contrast MRI, subtle BBB leakages can be detected during normal aging in the medial temporal lobe, which worsens with MCI (Montagne et al. 2015a). Second, it is well established that ApoE4 is associated with increase of Aβ in brain (Kim et al. 2009; Zlokovic 2013) and impairment of Aβ removal across the BBB (Bell et al. 2007; Castellano et al. 2011). Finally, ApoE4 has direct toxic effects on neurons which may be mediated by its role in tau phosphorylation, synaptic plasticity and neuroinflammation (Zlokovic 2013; Kim et al. 2009; Mahley et al. 2009). Therefore, for ApoE ε4+ individuals, although the CBF does increase during the preclinical phase of cognitive function disorder, this increase can no longer help to maintain their memory function because of the various pathological neurovascular changes caused by ApoE4. Taken together, the discussion above may help to interpret why heightened CBF in ApoE ε4+ group was correlated with worse memory function compared to that in the APOE ε4− group.
An increasing number of studies have demonstrated that ASL CBF can be considered as a useful biomarker in individuals with AD risks as this technique can distinguish the vulnerable from normal participants (Fleisher et al. 2009; Bangen et al. 2012). Furthermore, ASL CBF can predict progression from the preclinical phase to AD sensitively (Beason-Held et al. 2013; Chao et al. 2010). Our present research underlined the vital correlation between CBF and memory performance. Moreover, it further demonstrated that ASL CBF is a reliable biomarker of genetic risk of AD (i.e. ApoE ε4) and is associated with memory function in cognitively normal older adults. Additionally, our research highlighted the potential value of detecting vascular factors in the pathogenesis of AD.
The strength of our study was that we included a well-matched sample of cognitively normal older adults who have completed ASL MRI and multiple cognitive assessments. Our research has several limitations. First, the sample size was small and the distribution of ApoE groups was unbalanced (62.9% ApoE ε4− versus 37.1% ApoE ε4+). Second, A longitudinal study should be conducted in the future to richen the research. It is possible that some of the ApoE ε4+ participants remain cognitively normal and, likewise, some of the ApoE ε4− participants may develop cognitive impairment over time. Despite these limitations, ASL CBF may prove to be a sensitive biomarker in terms of very early AD.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Inc., Biogen Idec Inc., BristolMyers Squibb Company, Eisai Inc., Elan Pharmaceuticals, Inc., Eli Lilly and Company, EuroImmun, F. HoffmannLa Roche Ltd and its affiliated company Genentech, Inc., Fujirebio; GE Healthcare, IXICO Ltd., Janssen Alzheimer Immunotherapy Research & Development, LLC., Johnson & Johnson Pharmaceutical Research & Development LLC., Medpace, Inc., Merck & Co., Inc., Meso Scale Diagnostics, LLC., NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc., Piramal Imaging, Servier, Synarc Inc., and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author contributions
JYW designed the experiment, analyzed the data and drafted this manuscript. GPP, PL, XFT are responsible for revising this manuscript. BYL is responsible for designing this experiment, revising and finalizing this manuscript.
Funding
This study was funded by the Ministry of Science and Technology of the People’s Republic of China (2016YFC1306402), the Science Technology Department of Zhejiang Province (2017C03011), and the Medical Science and Technology Project co-founded by Zhejiang Province and the Ministry of Health of China (WKJ-ZJ-1612).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Armulik A, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, Bondi MW. Assessment of Alzheimer’s disease risk with functional magnetic resonance imaging: an arterial spin labeling study. J Alzheimer’s Dis: JAD. 2012;31(Suppl 3):S59–S74. doi: 10.3233/JAD-2012-120292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held LL, Goh JO, An Y, Kraut MA, O’Brien RJ, Ferrucci L, Resnick SM. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. 2013;33:18008–18014. doi: 10.1523/JNEUROSCI.1402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Res. 2009;1267:77–88. doi: 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Bondi MW, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimer’s Dis: JAD. 2014;42:275–289. doi: 10.3233/jad-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord. 2010;24:19–27. doi: 10.1097/WAD.0b013e3181b4f736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s Dement. 2015;11:415–424. doi: 10.1016/j.jalz.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, et al. Cerebral perfusion and oxygenation differences in Alzheimer’s disease risk. Neurobiol Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab. 2016;36:216–227. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010;1315:119–127. doi: 10.1016/j.brainres.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, Soiza RL. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: is Alzheimer’s a vascular disorder? Am J Cardiovasc Dis. 2013;3:197–226. [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Kim MJ, Rhee HY, Ryu CW, Kim EJ, Petersen ET, Jahng GH. Regional cerebral perfusion in patients with Alzheimer’s disease and mild cognitive impairment: effect of APOE epsilon4 allele. Neuroradiology. 2013;55:25–34. doi: 10.1007/s00234-012-1077-x. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Roberts R. Vascular risk factors: imaging and neuropathologic correlates. J Alzheimer’s Dis: JAD. 2010;20:699–709. doi: 10.3233/JAD-2010-091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–1254. doi: 10.1002/(SICI)1522-2594(199906)41:6<1246::AID-MRM22>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50(Suppl):S183–S188. doi: 10.1194/jlr.r800069-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Pa J, Zlokovic BV. Vascular plasticity and cognition during normal aging and dementia. JAMA Neurol. 2015;72:495–496. doi: 10.1001/jamaneurol.2014.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, Pendleton N. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology. 2006;20:549–557. doi: 10.1037/0894-4105.20.5.549. [DOI] [PubMed] [Google Scholar]
- Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Saunders AM, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J, Brickman AM, Habeck CG, Salthouse TA, Stern Y. Cerebral blood flow and gray matter volume covariance patterns of cognition in aging. Hum Brain Mapp. 2013;34:3267–3279. doi: 10.1002/hbm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol. 2010;67:93–98. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, et al. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimer’s Dis: JAD. 2013;34:921–935. doi: 10.3233/JAD-121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimer’s Dis: JAD. 2014;42(Suppl 4):S411–S419. doi: 10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<237::AID-NBM475>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zlatar ZZ, Wierenga CE, Bangen KJ, Liu TT, Jak AJ. Increased hippocampal blood flow in sedentary older adults at genetic risk for Alzheimer’s disease. J Alzheimer’s Dis: JAD. 2014;41:809–817. doi: 10.3233/JAD-132252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood–brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/A:1016254514167. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood–brain barrier permeability to leucine-enkephalin, d-alanine2-d-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Transport of leucine-enkephalin across the blood–brain barrier in the perfused guinea pig brain. J Neurochem. 1987;49:310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]


