Abstract
Common wheat (Triticum aestivum L.) is one of the most important agricultural crop, which provides direct source of food for humans. Besides abiotic stresses, weeds pose a significant challenge to successful crop production. Avena fatua (wild oat) is one of the most common damaging grass weed, which causes 17–62% losses in yield of winter wheat. Excessive use of herbicides to control wild oat has resulted in serious environmental and human health hazards. Therefore, biological control of weeds is required to cope up with the increasing food demand and to attain self-sustainability. In this study, eighty eight rhizobacterial isolates were isolated from rhizosphere soil samples collected from Rewari and Hisar districts. After screening of the isolates, only thirty isolates showed in vitro antagonistic and herbicidal activities. The selected antagonistic isolates were further tested for production of IAA and ALA, and utilization of ACC. Bacterial isolates BWA18 and RWA52 produced 53.80 and 19.18 ug ml−1 IAA, respectively and high ALA production was shown by isolates HCA3 and RCA3. Five isolates i.e., BWA20, BWA23, BWA29, BWA38 and RCA3 showed significant ACC utilization. Inoculation of selected bacterial isolates BWA18, RWA69 and SYB101 showed significant increase in root dry weight (RDW) and shoot dry weight (SDW) of wheat plants under pot house conditions, and decreased RDW and SDW of A. fatua weed as compared to RDF-amended uninoculated soil at 25 DAS (days after sowing). Bacterial isolates RWA69 and SYB101 caused significant increase in RDW and SDW of wheat growth at 50 DAS, whereas their inoculation decreased RDW and SDW of A. fatua. Thus, seed bacterization with bacterial isolates RWA52, RWA69 and SYB101 caused significant increase in RDW and SDW of wheat, whereas their inoculation caused significant decrease in RDW and SDW of A. fatua. The best performing bacterial isolates RWA52 and RWA69 were identified as Bacillus siamensis and Bacillus endophyticus using 16S rRNA analysis. The promising rhizobacterial isolates could further be tested for the bioherbicidal activity and plant growth promotion effects under field conditions before their use as bioherbicides.
Keywords: Wheat, Avena fatua, Resource partitioning, Rhizosphere bacteria, Bacillus sp., Bioherbicides
Introduction
More than 50% of the world energy intake is provided by cereal crops such as wheat, rice and maize. Among these crops, wheat (Triticum aestivum L.) is the most important source of staple food for ever-increasing human population in the world, which occupies more than 41% of crop area worldwide (Gooding and Davis 1997; Curtis 2002). The limited nutrient resources essential for crop growth are depleted by the weeds, resulting in significant loss in the biomass and yield of different crops. Moreover, weed’s interference also increases the cost of production along with reduction in the quality of produce. Type of weed species, weed density and the time of emergence of weeds affect the competition of crop with the weeds for the resources i.e., space, light, moisture and nutrients under field conditions.
Avena fatua L. (wild oat) is one of the most common and economically damaging herbicide-resistant grasssy weed that infests a large number of crops around the world (Sharama and Vanden 2008). This weed is widely distributed in diverse agro-ecosystems (Holm et al. 1977) and represents a serious economic threat to crop yields due to their unique seed traits, high competitiveness, staggered germination, allelopathic potential and ability to persist in the soil seed bank. Weed infestation in wheat causes about 21% yield loss depending on crop species and weed density. Herbicides such as isoproturon, clodinafop-propargyl, fenoxaprop, pinoxaden, accord plus (fenoxaprop + metribuzin), sulfosulfuron and atlantis (meso + iodosulfuron) are applied for control of wild oat, but resistance to several commonly used herbicides is becoming more prevalent recently. Moreover, excessive spraying of herbicides and agrochemicals for weed control is environmentally unsafe and uneconomical, and has resulted into considerable pollution of soil, water and air. Therefore, it is necessary to develop cost effective, eco-friendly and sustainable weed management biocontrol practices in which microorganisms or their products could be used for partitioning of bioresources (nutrients, light, water and space) to promote the growth of crops and simultaneously to suppress the growth of weed species (Weller 1988; Lakshmi et al. 2015; Sindhu et al. 2018; Zorner et al. 2018).
Several rhizosphere bacteria have been identified which possess the bioherbicidal activity and these genera include species of Acinetobacter, Achromobacter, Alcaligenes, Azospirillum, Bacillus, Burkholderia, Enterobacter, Pseudomonas, Ralstonia, Serratia and Rhizobium etc. (Kennedy et al. 1991; Ahemad and Kibret 2014; Sindhu et al. 2018; Phour and Sindhu 2019). Some of these rhizosphere bacteria were found to suppress the growth of weed species by producing phytohormones such as indole acetic acid (IAA) and δ-aminolevulinic acid (ALA), which affect different plant developmental processes in plants. The high concentration of phytohormones has been reported to adversely affect the germination and growth of weeds (Barazani and Friedman 1999; Mohan Babu et al. 2003; Sindhu and Sehrawat 2017). ALA produced by rhizobacteria has also been reported as an effective natural biodegradable herbicide (Sasikala et al. 1994) along with its growth-promoting effect on different crops and vegetables (Sasaki et al. 1993; Phour et al. 2018). In addition, rhizosphere bacteria exhibiting ACC (1-aminocyclopropane-1-carboxylate) deaminase activity have been found quite beneficial under abiotic and biotic stress conditions, leading to promotion of plant growth by reducing production of stress-induced ethylene (Glick 2005; Chaudhary and Sindhu 2017; Saikia et al. 2018).
These biocontrol strategies are highly compatible with the sustainable agriculture and for conservation of natural resources. In earlier studies, rhizobacterial isolates were obtained that showed suppressive properties against green foxtail [Setaria virdis (L.) Beauv.] and wild oat (Avena fatua L.) and their effectiveness was studied using various weed/crop densities (Boyetchko 1994, 1997). Literature shows that no efforts were further made to understand the mechanism involved in growth suppression of the wild oats and no field trial or pot house studies were conducted. In this study, rhizosphere bacteria belonging to Bacillus siamensis and Bacillus endophyticus were characterized, which suppressed the growth of wild oat under pot house conditions. Moreover, seed bacterization of wheat and wild oat with promising rhizobacterial isolates RWA52, RWA69 and SYB101 caused significant reduction in RDW and SDW of A. fatua, whereas inoculation of these rhizobacterial isolates showed significant increase in RDW and SDW of wheat plants under pot house conditions.
Materials and methods
Isolation of bacteria from rhizosphere of wheat
Soil samples were collected from the rhizosphere of wheat grown in the field areas of Bawal (28°3′ 18.51″N; 76°35′ 52.60″E), Rewari (28°10′ 27″N; 76°38′ 44″E) and Hisar districts (29°09′ 51.66″N; 75°41′ 2.94″E) after 60 and 90 days of plant growth. The soils of the Hisar farm were sandy loam in texture (organic carbon, 0.44%; EC, 0.42; pH, 7.2, available N, P and K, 105, 12 and 186 kg ha−1, respectively), whereas soil of the Bawal region were loamy sand in texture (organic carbon, 0.42%; EC, 0.24; pH, 7.9, available N, P and K, 112, 21 and 334 kg ha−1, respectively). Dilution plating of composite rhizosphere soil samples was made on Luria–Bertani (LB) medium plates. After 3–4 days incubation of plates at 28 ± 2 °C, rhizobacterial colonies were selected based on typical morphological and pigment producing characteristics. A total of 88 rhizobacterial isolates selected from rhizosphere soil were transferred on LB agar medium slopes (Sindhu et al. 1999). These selected isolates were screened for growth retardation effects on weed and plant growth-promoting attributes for wheat crop. Bacillus subtilis strain SYB101 with bioherbicidal effect on management of Phalaris minor weed was used as reference strain (Phour and Sindhu 2018).
Identification of promising bacterial isolates
Preliminary characteristics of the promising isolates included colony morphology, Gram staining, physiological and biochemical tests. Two best performing rhizobacterial isolates were then identified using 16S ribosomal RNA (rRNA) gene sequencing. Total genomic DNA was extracted using a DNA isolation kit and the 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the universal primers 27F 5′ (AGA GTT TGA TCC TGG CTC AG) 3′ and 1492R 5′ (TAC GGY TAC CTT GTT ACG ACT T) 3′, which are designed to amplify universally conserved regions of the 16S rRNA gene and resulted in the amplification of an approximately 1500 base pair PCR product. Amplification and purification of the product was done as described previously (Pandey et al. 2002). The phylogenetic tree was constructed using ClustalX, DAMBE and Mega7 software.
Screening of rhizobacterial isolates for production of indole acetic acid and δ-aminolevulinic acid
All the 88 rhizobacterial isolates were screened for in vitro antagonistic and herbicidal activities, and thirty isolates were finally evaluated for plant growth promoting beneficial activities. For IAA estimation, selected bacterial cultures were inoculated (in triplicate) in 30 ml LB broth flasks supplemented with DL-tryptophan @ 100 μg ml−1 and were incubated at 28 ± 2 °C for 72 h under stationary conditions of growth. Culture samples were centrifuged at 10,000 rpm for 15 min (Remi Instruments, Mumbai, India) and IAA was determined in the culture supernatant by the method as described by Malik and Sindhu (2011).
For ALA determination, selected antagonistic bacterial cultures were inoculated (in triplicate) in 10 ml LB broth tubes supplemented with 15 mM glycine and succinate. Culture samples were withdrawn after incubation at 28 ± 2 °C for 48 h and centrifuged at 10,000 rpm for 15 min (Remi Instruments, Mumbai, India). ALA was determined in the culture supernatant by the method as described by Mauzerall and Granick (1955).
Screening of rhizobacterial isolates for utilization of 1-aminocyclopropane-1-carboxylate
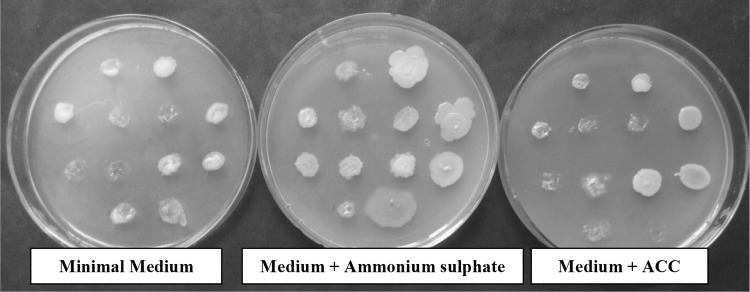
Minimal medium (Dworkin and Foster 1958) supplemented with 3 mM ACC was used for ACC utilization (Penrose and Glick 2003). 48 h old growth of rhizobacterial isolate was spotted on medium plates and growth was recorded after 5 days of incubation at 28 ± 2 °C. The bacterial isolates showing good growth on ACC supplemented medium plates, indicating high efficiency of ACC utilization as nitrogen source. Minimal medium plates containing ammonium sulfate as nitrogen source were used as control for growth comparison of different bacterial isolates.
Efficacy of rhizobacterial inoculation on growth of weed and wheat
Selected rhizobacterial isolates were checked for suppression of growth of Avena fatua weed and for growth stimulation effects on growth of wheat (Triticum aestivum) variety WH1105 under pot house conditions. Seeds of wheat/weed were inoculated with 10 ml culture suspension of bacteria (107–108 cells ml−1 of growth suspension). Inoculated seeds were sown in pots (with three replications for each treatment) and uninoculated seeds were sown as control (Chaudhary and Sindhu 2016). The soil used for growth of plants in greenhouse study was collected from Ludas village (Hisar district) from two to ten cm depth of soil after harvesting of the pearl millet crop. The soil used was sandy soil in texture with the following characteristics i.e., organic carbon, 0.26%; electrical conductivity, 0.48 dSm−1; pH, 7.8; available phosphorus, 10 kg ha−1, available K2O, 228 kg ha−1; available nitrogen, 78 kg ha−1. Soil was supplemented with required doses of nitrogen and phosphorus fertilizers. After germination, eight healthy seedlings were maintained in each pot. The observations for root and shoot growth of wheat and weeds were recorded at 25, 50 and 75 days of plant growth.
Statistical analysis
Completely Randomized Design (CRD) was used for experimental data analysis. All determinations in the experiment were carried out in triplicate and data represented are average values of three replications. The C.D. values represent coefficient of deviation.
Results
Microbial communities inhabiting the rhizosphere of different crop plants have been found to suppress/inhibit the growth of weeds by reducing seed germination, weed density and biomass (Park et al. 2015; Sindhu and Sehrawat 2017). In this study, 30 rhizobacterial isolates were finally selected, out of 88 rhizobacterial isolates initially obtained, for plant growth promoting beneficial activities. Based on preliminary screening in relation to in vitro antagonistic and herbicidal activities, five rhizobacterial isolates were studied for growth suppression of wild oat weed under pot house conditions and the possible mechanism of weed growth inhibition was explored.
In vitro screening for beneficial attributes of rhizobacteria
Thirty selected rhizobacterial isolates were tested for production of indole acetic acid, δ-aminolevulinic acid and utilization of 1-aminocyclopropane-1-carboxylate. Four isolates i.e., BWA1, RWA55 and RWA72 produced 16.73, 16.24 and 12.12 μg ml−1 IAA, respectively (Table 1). Maximum production of IAA was observed in isolate RWA52 (19.18 μg ml−1), whereas isolate BWA18 produced the highest amount of IAA (53.80 μg ml−1). Only nineteen percent isolates showed medium to high amount of IAA production (Fig. 1). Ten bacterial isolates i.e., BWA1, BWA6, BWA19, BWA25, BWA27, BWA36, RWA48, RWA63, SYB101 and HMM97 showed more than 5 μg ml−1 ALA production (Table 1; Fig. 2). Other nineteen bacterial isolates showed less production of ALA (< 5 μg ml−1). Screening of rhizobacterial isolates for AAC utilization showed that only four isolates i.e., BWA20, BWA23, BWA29 and BWA38 showed significant growth on ACC supplemented plates (Table 1). Fourteen rhizobacterial isolates showed little growth, whereas seven isolates showed moderate growth on ACC supplemented plates. Five isolates lacked the ability to utilize ACC and did not grow on ACC supplemented plates. Overall, ACC utilization ability was observed with only 52.94% rhizobacterial isolates (Figs. 3, 4).
Table 1.
Plant growth promoting characteristics of selected rhizobacterial isolates
| S. No. | Rhizobacterial isolates | IAA concentration (μg ml−1) | ALA concentration (μg ml−1) | Category of ACC utilizing isolates |
|---|---|---|---|---|
| 1. | BWA1 | 16.73 | 5.70 | + |
| 2. | BWA2 | 3.49 | 4.29 | +++ |
| 3. | BWA6 | 4.07 | 5.11 | ++ |
| 4. | BWA7 | 10.85 | 4.58 | − |
| 5. | BWA8 | 6.21 | 4.16 | + |
| 6. | BWA14 | 6.15 | 4.33 | ++++ |
| 7. | BWA18 | 53.80 | 4.79 | + |
| 8. | BWA19 | 3.55 | 5.00 | +++ |
| 9. | BWA20 | 4.52 | 4.37 | +++++ |
| 10. | BWA23 | 6.75 | 3.70 | +++++ |
| 11. | BWA25 | 6.78 | 7.12 | ++ |
| 12. | BWA27 | 12.00 | 5.70 | + |
| 13. | BWA29 | 6.12 | 4.70 | +++++ |
| 14. | BWA36 | 5.33 | 5.04 | + |
| 15. | BWA38 | 6.81 | 4.79 | +++++ |
| 16. | BWA40 | 6.96 | 4.29 | ++++ |
| 17. | RWA42 | 7.78 | 4.37 | − |
| 18. | RWA48 | 4.52 | 5.79 | − |
| 19. | RWA52 | 19.18 | 4.58 | ++ |
| 20. | RWA53 | 4.52 | 4.74 | − |
| 21. | RWA55 | 16.24 | 4.62 | + |
| 22. | RWA59 | 9.74 | 4.54 | + |
| 23. | RWA63 | 7.60 | 5.41 | + |
| 24. | RWA69 | 4.76 | 4.95 | ++++ |
| 25. | RWA71 | 3.89 | 4.08 | ++ |
| 26. | RWA72 | 12.12 | 4.87 | +++ |
| 27. | SYB101 | 8.62 | 9.14 | ++ |
| 28. | HMM97 | 7.66 | 5.58 | − |
| 29. | HMM8 | 5.39 | 4.95 | ++++ |
| 30. | SB153 | 5.42 | 3.87 | ++ |
Growth of rhizobacterial isolates on minimal medium supplemented with ACC is denoted as: +: less, ++: moderate, +++: good, ++++: very good, +++++: significant growth and, −: no growth
Fig. 1.
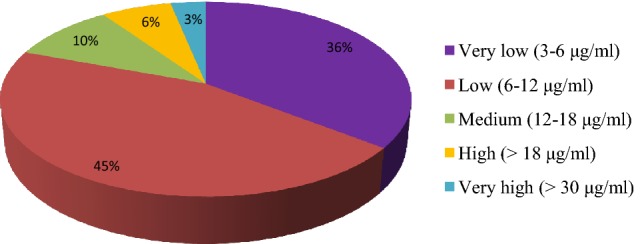
Percent frequency of selected rhizobacterial isolates for IAA production
Fig. 2.
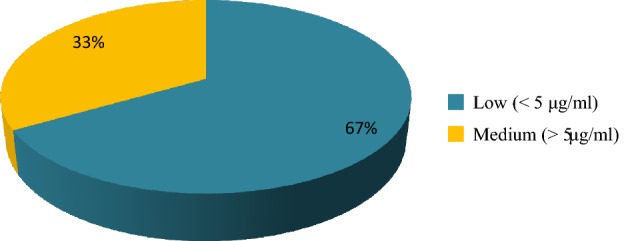
Percent frequency of selected rhizobacterial isolates for production of δ-aminolevulinic acid
Fig. 3.
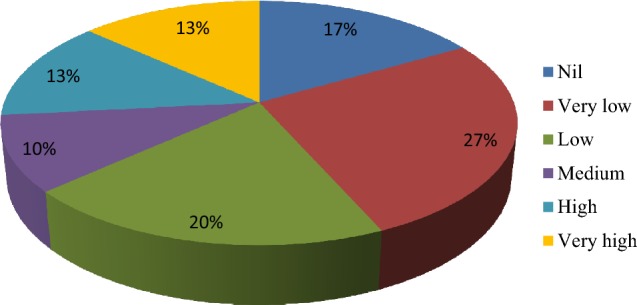
Percent frequency of rhizobacterial isolates for ACC utilization
Fig. 4.
Growth of different rhizobacterial isolates on minimal medium plates and on medium supplemented with ammonium sulphate and ACC
Inoculation effect of selected rhizobacteria on growth of wheat and weed
Five rhizobacterial isolates i.e., BWA18, BWA29, RWA52, RWA69 and SYB101 were finally selected for seed bacterization of wheat and weed, based on the variation in their beneficial attributes, under pot house conditions. At 25 days of growth, inoculation with bacterial isolate RWA69 caused 121 and 140% increase in RDW and SDW of wheat growth, respectively and its inoculation decreased the root dry weight of A. fatua by 11% and also decreased the shoot dry weight of the weed (Table 2; Fig. 5). Increase in root dry weight (171%) and shoot dry weight (110%) of wheat plants was observed by inoculation of Bacillus isolate SYB101, whereas its inoculation decreased RDW and SDW of A. fatua (Table 2; Fig. 6). At 50 days of observation, inoculation with bacterial isolates RWA69 and SYB101 caused significant increase in RDW and SDW of wheat growth, whereas their inoculation decreased RDW and SDW of A. fatua (Table 2; Figs. 7, 8). Inoculation with bacterial isolate RWA52 showed 48 and 12% gain in root and shoot dry weight shoot of wheat growth, respectively, whereas its inoculation improved the root dry weight by 24% but decreased the shoot dry weight of A. fatua by 12% (Table 2).
Table 2.
Inoculation effect of rhizobacterial isolates on plant biomass of wheat and weed at different stages of plant growth
| Treatments | Days of growth | Wheat | Weed | ||
|---|---|---|---|---|---|
| RDW (g plant−1) | SDW (g plant−1) | RDW (g plant−1) | SDW (g plant−1) | ||
| Control (uninoculated soil) + Wheat | 25 | 0.14 | 0.19 | – | – |
| 50 | 0.21 | 0.52 | – | – | |
| 75 | 0.53 | 0.76 | – | – | |
| Control (uninoculated soil + N, P fertilizers) + Wheat | 25 | 0.28 | 0.40 | – | – |
| 50 | 0.46 | 0.68 | – | – | |
| 75 | 0.75 | 0.94 | – | – | |
| T1 + Weed (Avena fatua) | 25 | – | – | 0.03 | 0.06 |
| 50 | – | – | 0.07 | 0.22 | |
| 75 | – | – | 0.12 | 0.41 | |
| T2 + Weed (Avena fatua) | 25 | – | – | 0.18 | 0.28 |
| 50 | – | – | 0.34 | 0.64 | |
| 75 | – | – | 0.62 | 1.04 | |
| T1 + Wheat + Weed | 25 | 0.18 | 0.18 | 0.05 | 0.14 |
| 50 | 0.30 | 0.46 | 0.12 | 0.30 | |
| 75 | 0.60 | 0.68 | 0.18 | 0.36 | |
| T2 + Wheat + Weed | 25 | 0.32 | 0.36 | 0.14 | 0.30 |
| 50 | 0.48 | 0.64 | 0.29 | 0.52 | |
| 75 | 0.72 | 0.83 | 0.44 | 0.84 | |
| T2 + Wheat + BWA18 | 25 | 0.38 | 0.46 | – | – |
| 50 | 0.50 | 0.72 | – | – | |
| 75 | 0.68 | 0.98 | – | – | |
| T2 + Weed + BWA18 | 25 | – | – | 0.22 | 0.34 |
| 50 | – | – | 0.38 | 0.74 | |
| 75 | – | – | 0.53 | 1.06 | |
| T2 + Wheat + Weed + BWA18 | 25 | 0.30 | 0.32 | 0.15 | 0.28 |
| 50 | 0.42 | 0.64 | 0.25 | 0.52 | |
| 75 | 0.62 | 1.32 | 0.39 | 0.68 | |
| T2 + Wheat + BWA29 | 25 | 0.36 | 0.50 | – | – |
| 50 | 0.48 | 0.82 | – | – | |
| 75 | 0.58 | 1.60 | – | – | |
| T2 + Weed + BWA29 | 25 | – | – | 0.36 | 0.62 |
| 50 | – | – | 0.74 | 1.12 | |
| 75 | – | – | 1.02 | 1.42 | |
| T2 + Wheat + Weed + BWA29 | 25 | 0.22 | 0.36 | 0.22 | 0.40 |
| 50 | 0.40 | 0.64 | 0.52 | 0.84 | |
| 75 | 0.64 | 1.20 | 0.74 | 1.08 | |
| T2 + Wheat + RWA52 | 25 | 0.40 | 0.50 | – | – |
| 50 | 0.68 | 0.76 | – | – | |
| 75 | 0.98 | 1.60 | – | – | |
| T2 + Weed + RWA52 | 25 | – | – | 0.20 | 0.30 |
| 50 | – | – | 0.42 | 0.56 | |
| 75 | – | – | 0.67 | 0.88 | |
| T2 + Wheat + Weed + RWA52 | 25 | 0.32 | 0.32 | 0.16 | 0.22 |
| 50 | 0.62 | 0.64 | 0.30 | 0.38 | |
| 75 | 0.76 | 1.36 | 0.42 | 0.54 | |
| T2 + Wheat + RWA69 | 25 | 0.62 | 0.96 | – | – |
| 50 | 0.86 | 1.22 | – | – | |
| 75 | 1.04 | 2.18 | – | – | |
| T2 + Weed + RWA69 | 25 | – | – | 0.21 | 0.42 |
| 50 | – | – | 0.30 | 0.56 | |
| 75 | – | – | 0.52 | 0.90 | |
| T2 + Wheat + Weed + RWA69 | 25 | 0.52 | 0.82 | 0.10 | 0.22 |
| 50 | 0.74 | 1.04 | 0.25 | 0.44 | |
| 75 | 0.92 | 1.86 | 0.58 | 0.72 | |
| T2 + Wheat + SYB101 | 25 | 0.76 | 0.84 | – | – |
| 50 | 1.02 | 1.52 | – | – | |
| 75 | 1.34 | 2.35 | – | – | |
| T2 + Weed + SYB101 | 25 | – | – | 0.20 | 0.20 |
| 50 | – | – | 0.28 | 0.52 | |
| 75 | – | – | 0.50 | 0.88 | |
| T2 + Wheat + Weed + SYB101 | 25 | 0.58 | 0.68 | 0.11 | 0.24 |
| 50 | 0.94 | 1.40 | 0.22 | 0.46 | |
| 75 | 1.16 | 2.56 | 0.36 | 0.74 | |
| CD at 5% | 25 | 0.032 | 0.030 | 0.040 | 0.042 |
| 50 | 0.030 | 0.038 | 0.041 | 0.036 | |
| 75 | 0.040 | 0.184 | 0.037 | 0.182 | |
Treatment T1 represents uninoculated soil, whereas T2 represents uninoculated soil amended with N and P fertilizer doses. Data represented are average values of three replications. The C.D. values represent coefficient of deviation. RDW root dry weight, SDW shoot dry weight
Fig. 5.
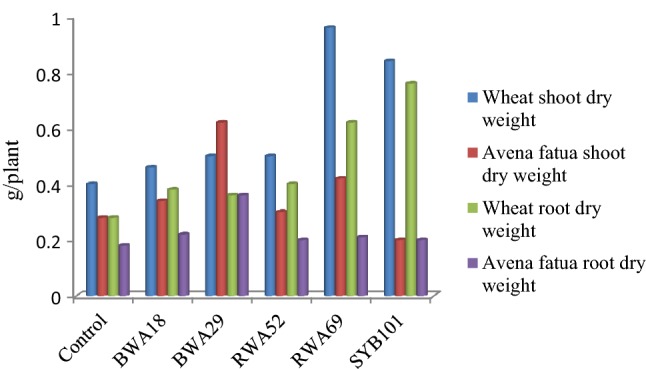
Inoculation effect of bacterial isolates on biomass of wheat and Avena fatua at 25 days of plant growth
Fig. 6.
Growth of wheat and weed (A. fatua) plants after inoculation with bacterial isolate SYB101 at 25 days of plant growth. T1: Control (uninoculated soil) + Wheat; T2: Control (uninoculated soil + N, P Fertilizers) + Wheat; T3: Control (uninoculated soil) + Weed; T4: Control (uninoculated soil + N, P Fertilizers) + Weed; T28: T2 + Wheat + SYB101; T29: T2 + Weed + SYB101; T30: T2 + Wheat + Weed + SYB101
Fig. 7.
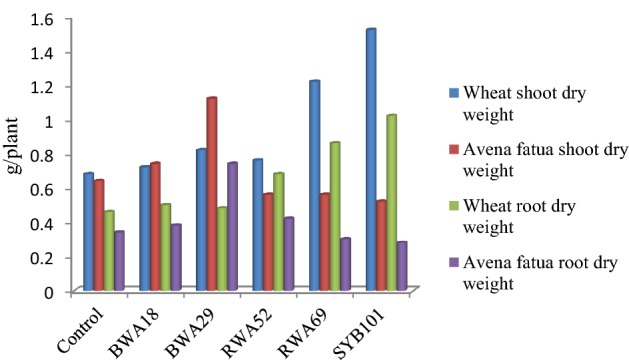
Inoculation effect of different bacterial isolates on biomass of wheat and Avena fatua at 50 days of plant growth
Fig. 8.
Growth of wheat and weed (A. fatua) plants after inoculation with bacterial isolate RWA69 at 50 days of plant growth. T1: Control (uninoculated soil) + Wheat; T2: Control (uninoculated soil + N, P Fertilizers) + Wheat; T3: Control (uninoculated soil) + Weed; T4: Control (uninoculated soil + N, P Fertilizers) + Weed; T25: T2 + Wheat + RWA69; T26: T2 + Weed + RWA69; T27: T2 + Wheat + Weed + RWA69
Inoculation of rhizobacterial isolate BWA18 decreased the root dry weight by 9% but showed 4% increase in SDW of wheat at 75 days of observation. Whereas, its inoculation decreased the root dry weight (14%) of weed and minor increase (2%) was observed in shoot dry weight of A. fatua in comparison to RDF amended uninoculated soil treatment (Table 2; Fig. 9). Inoculation of bacterial isolate RWA52 resulted in 31 and 70% gain in root and shoot dry weight of wheat, respectively, whereas its inoculation decreased the RDW and SDW of A. fatua weed by 32 and 15%, respectively (Fig. 10). Similarly, inoculation with rhizobacterial isolate RWA69 showed 38% gain in root dry weight and shoot dry weight of wheat was improved by 131%, and its inoculation caused 16 and 13% decrease in RDW and SDW of A. fatua, respectively. Inoculation with another promising rhizobacterial isolate SYB101 showed 79 and 150% increase in RDW and SDW of wheat, respectively and its inoculation decreased the root dry weight by 19% and caused 15% decrease in shoot dry weight of A. fatua.
Fig. 9.

Inoculation effect of different rhizobacterial isolates on biomass of wheat and Avena fatua at 75 days of plant growth
Fig. 10.
Growth of wheat and weed (A. fatua) plants after inoculation with bacterial isolate RWA52 at 75 days of plant growth. T1: Control (uninoculated soil) + Wheat; T2: Control (uninoculated soil + N, P Fertilizers) + Wheat; T3: Control (uninoculated soil) + Weed; T4: Control (uninoculated soil + N, P Fertilizers) + Weed; T22: T2 + Wheat + RWA52; T23: T2 + Weed + RWA52; T24: T2 + Wheat + Weed + RWA52
Identification of promising rhizobacterial isolates by the 16S rRNA sequencing
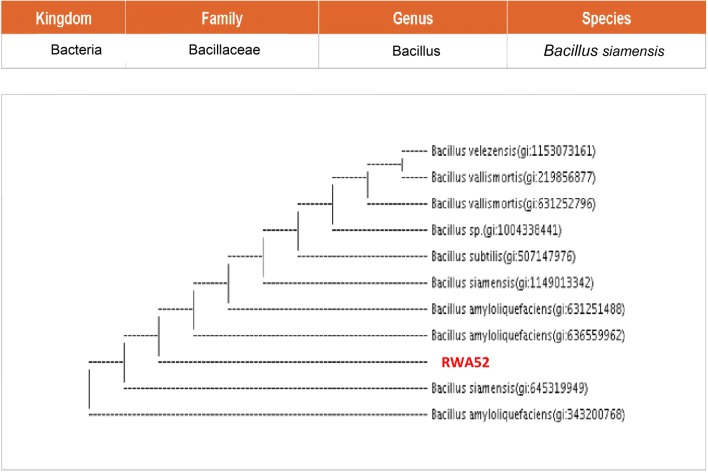
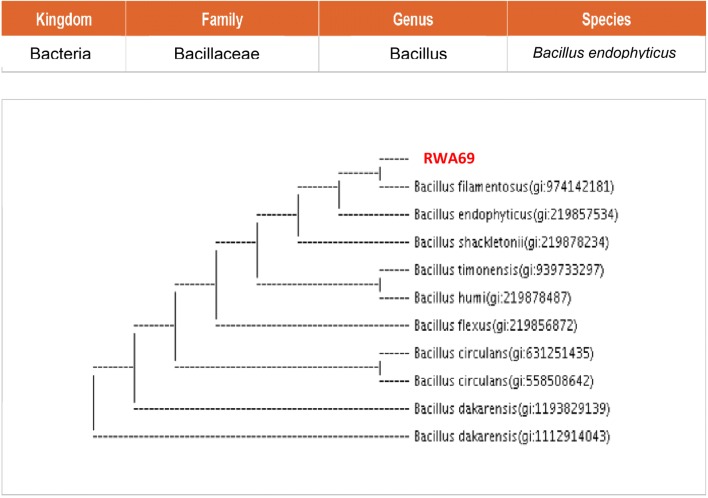
Two promising rhizobacterial isolates RWA52 and RWA69 were identified by using universal primers 8-27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′). Genomic DNA of rhizobacterial isolates was obtained according to the method described by Pitcher et al. (1989). The partial 16S rDNA gene sequence of the RWA52 and RWA69 bacterial isolates were then used as a query to search for similar sequences in GenBank database. The rhizobacterial isolates RWA52 and RWA69 were identified as Bacillus siamensis and Bacillus endophyticus, respectively (Figs. 11, 12).
Fig. 11.
Phylogenetic (neighbour joining) tree based on 16S rRNA sequence analysis indicating phylogenetic position of bacterial isolate RWA52
Fig. 12.
Phylogenetic (neighbour joining) tree based on 16S rRNA sequence analysis indicating phylogenetic position of bacterial isolate RWA69
Discussion
Besides climate change and abiotic stresses, harmful phytopathogens, insects and weeds are major constraints to enhance food productivity. Recently, significant efforts are being made worldwide to characterize effective biocontrol agents for control of plant diseases as well as for suppressing the growth of weeds (Kennedy and Stubbs 2007; Mejri et al. 2010; Park et al. 2015; Sindhu et al. 2016). The production of phytotoxins, phytohormones (IAA, ALA) and antibiotics by rhizosphere bacteria residing in the vicinity of the weed roots have been implicated in exerting deleterious effects on the growth of weeds. Interestingly, many rhizospheric bacteria showing weed growth inhibiting properties (Kremer and Kennedy 1996; Sindhu et al. 2018), were found to stimulate the growth of crop plants (Li and Kremer 2006; Mejri et al. 2010). Production of phytohormones and other secondary metabolites have been reported as the predominant mechanisms of biocontrol of weeds/phytopathogens (Saraf et al. 2014; Sindhu et al. 2018; Phour and Sindhu 2019).
In this study, 30 rhizobacterial isolates (selected out of total 88 rhizobacterial isolates) were screened for production of IAA, ALA and ACC utilization ability. Maximum production of IAA was observed in bacterial isolate BWA18 (53.80 μg ml−1) followed by isolate RWA52 (> 18 μg ml−1) (Table 1). Bacterial isolate RWA52 exhibiting significant production of IAA significantly inhibited the growth of Avena fatua, whereas its inoculation significantly improved the wheat growth at different stages of observations (Table 2). Similar suppressive effect on the growth of weed morning glory was correlated with the IAA production by Bradyrhizobium japonicum isolate GD3 (Kim and Kremer 2005). Sachdev et al. (2009) isolated nine Klebsiella pneumoniae strains from rhizosphere of wheat and six strains showed variable amounts of IAA production. Seed bacterization with these six strains significantly increased root length and shoot height of inoculated wheat seedlings over the uninoculated control plants under pot house studies. Meliani et al. (2017) reported that plant growth promoting bacteria Pseudomonas fluorescens and P. putida produced high levels of IAA i.e., 89 and 116 µg ml−1, respectively and significantly enhanced the plant growth. Similarly, the amount of IAA produced by P. trivialis strain X33d was correlated with growth suppression of weed great brome and stimulation of the growth of durum wheat (Mejri et al. 2010).
ALA has been reported to act as herbicide and growth promoting factor in agriculture applications and it also contribute towards tolerance of salt and cold temperature in plants (Watanabe et al. 2000). In this study, ten bacterial isolates BWA1, BWA6, BWA19, BWA25, BWA27, BWA36, RWA48, RWA63, HMM97 and SYB101 showed more than 5 μg ml−1 ALA production (Table 1; Fig. 2). Zhang et al. (2008) reported that the combined treatment of oil seed rape plants with ALA and post emergence herbicide, propyl 4-(2-(4,6-dimethoxypyrimidine-2-yloxy benzylamino) benzoate (ZJ0273) improved the growth and development of oilseed rape seedlings (Brassica napus cv. ZS758) and also improved weed control efficacy. Khandelwal et al. (2018) observed that 80% of the rhizobacterial isolates produced ALA. Out of these isolates, four rhizobacterial isolates showed root growth inhibition of Chenopodium album weed at both 5th and 10th day of seed germination. Phour and Sindhu (2019) reported that Bacillus flexus strain JMM24 produced 11.70 µg ml−1 of ALA and its inoculation on Lathyrus aphaca weed caused 92% decrease in RDW and SDW at 75 days of growth under pot house conditions.
Some rhizobacterial strains possessing ACC deaminase activity have recently been found to improve the biomass and yield of different crops (Glick 2003; Chaudhary and Sindhu 2017). Out of 30 isolates tested in this study, 52.94% rhizobacterial isolates showed ACC utilization ability (Table 1; Fig. 3). B. endophyticus strain RWA69, which showed significant growth on ACC supplemented plates, showed significant decrease in the RDW and SDW of weed and promoted the growth of wheat at 50 days of plant growth (Fig. 8). Stearns et al. (2012) reported that the ACC deaminase-containing bacterium Pseudomonas putida strain UW4 significantly promoted the growth of Brassica napus. Magnucka and Pietr (2015) selected three fluorescent pseudomonads containing ACC deaminase activity from rhizosphere of wheat (Triticum aestivum L.) and rape (Brassica napus L.), and Pseudomonas brassicacearum strain RZ310 posessing highest ACC deaminase activity showed beneficial effect on the growth of wheat seedlings even at low cell densities. Ten bacterial strains belonging to Enterobacter, Serratia, Klebsiella and Escherichia genera were isolated (Carlos et al. 2016) and Serratia strain showed the positive correlation between ACC deaminase and IAA production in the presence of heavy metals, and its inoculation also promoted the growth of Helianthus annuus.
In this study, four rhizobacterial isolates BWA18, RWA52, RWA69 and SYB101 stimulated the growth of wheat, and rhizobacterial isolates i.e., BWA18, BWA29 and RWA52 inhibited the growth of A. fatua in comparison to RDF amended uninoculated soil treatments (Table 2; Figs. 5, 6, 7, 8, 9, 10). Rhizobacterial isolates having inhibitory effect on the growth of weed A. fatua and growth-promoting effect on wheat could further be tested in controlled experimental designs in particular soil type, plant species and the environmental factors. In earlier studies, plant genotypes/varieties have been found to affect the colonization ability of the inoculated strains on the roots (Sheng 2005; Phour and Sindhu 2018). Therefore, the selection of competitive and effective bacterial strains is desirable among the indigenous soil bacteria, which could easily adapt and establish in the particular soil conditions at the inoculation site (Paau 1989; Sindhu and Dadarwal 2000). In similar studies, Kennedy et al. (1991) screened 1000 pseudomonads isolates for differential inhibition of downy brome (Bromus tectorum) and winter wheat. Out of these isolates, bacteria free culture filtrates obtained from only 8% isolates showed inhibitory effect on the root growth of downy brome weed, but no effect was observed on the root growth of winter wheat on agar. When these isolates were inoculated under field conditions, only two isolates (0.2%) suppressed the growth of downy brome by ~ 31–53% whereas, this treatment showed ~ 18–35% increase in the yield of winter wheat. Similarly, Li and Kremer (2006) reported that inoculation of Pseudomonas fluorescens strain G2-11 onto wheat and soybean crops suppressed the growth of Ipomea sp. and Convolvolus arvensis weeds, while its inoculation promoted the growth of wheat and soybean crops. Amara et al. (2015) reported significant increase in length of root and shoot as well as dry weight of root and shoot in the wheat crop in comparison to uninoculated control by coinoculation of Psychrobacter meritimus sp. strain A18, Serratia proteamaculans sp. and Bacillus anthracis sp. strain A29, along with nitrogenous and phosphatic fertilizers under pot and field conditions. These results showed that inoculation of rhizobacterial strains may influence the resource partitioning towards the growth suppression of weed and promotion of wheat ultimately leading to improved crop productivity in sustainable agriculture.
Conclusions
Until now, increase in wheat production using high yielding varieties has provided sufficient crop yield to keep pace with ever-increasing world population. Recently, the application of chemical fertilizers even at maximum dose is not further increasing the yield of various crops. To further improve wheat productivity, promising rhizosphere bacteria could be used for growth stimulation of wheat along with their suppressive effect on growth of phytopathogens and weeds. In this study, bacterial isolate BWA18 with high IAA (53.80 μg ml−1) production ability showed growth inhibition of weed and stimulated the growth of wheat at 25, 50 and 75 days of observation. Another isolate RWA52 (with IAA production, 19.18 μg ml−1) showed growth stimulation of wheat and growth inhibition of weed as compared to RDF amended uninoculated soil treatment. Another bacterial isolate RWA69 selected on the basis of ACC utilization ability, showed maximum growth stimulation of wheat at 25, 50 and 75 days of observation. Two promising rhizobacterial isolates RWA52 and RWA69 were identified by the 16S rRNA sequence analysis as Bacillus siamensis and Bacillus endophyticus, respectively. Results suggested that rhizobacterial isolates with growth inhibiting properties of A. fatua weed and growth promoting properties of wheat could be further explored under field conditions. The use of promising rhizobacterial isolates as bioherbicides will be more useful in the fields, where herbicide resistant population of A. fatua exists. Thus, development of bioherbicides is need-based ecofriendly and cost effective technology for pollution free sustainable agriculture.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26:1–20. [Google Scholar]
- Amara U, Wang YX, Cui XL, Khalid R, Ali S, Shabbir G, Hayat R. Screening and identification of soil bacteria for growth promotion of wheat (Triticum aestivum L.) J Biodiv Environ Sci. 2015;7(3):87–99. [Google Scholar]
- Barazani O, Friedman J. Allelopathic bacteria and their impact on higher plants. Crit Rev Plant Sci. 1999;18:741–755. doi: 10.1080/20014091096693. [DOI] [PubMed] [Google Scholar]
- Boyetchko SM (1994) Biological control of grassy weeds with rhizobacteria. In: Proceedings of fifteenth annual meeting of plant pathology society of Alberta. p 15 (Abstract)
- Boyetchko SM. Principles of biological weed control with microorganism. Hort Sci. 1997;32(2):201–205. [Google Scholar]
- Carlos MHJ, Stefani PVY, Janette AM, Melani MSS, Gabriela PO. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol Res. 2016;188:53–61. doi: 10.1016/j.micres.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Chaudhary SR, Sindhu SS. Growth stimulation of clusterbean (Cyamopsis tetragonoloba) by coinoculation with rhizosphere bacteria and Rhizobium. Legume Res. 2016;39(6):1003–1012. [Google Scholar]
- Chaudhary D, Sindhu SS. Amelioration of salt stress in chickpea (Cicer arietinum L.) by coinculation of ACC deaminase-containing rhizospheric bacteria with Mesorhizobium strains. Legume Res. 2017;40(1):80–86. [Google Scholar]
- Curtis BC. Wheat in the world. In: Curtis BC, Rajaram S, Macpherson HG, editors. Bread wheat improvement and production. Rome: Food and Agriculture Organization of the United Nations; 2002. pp. 1–18. [Google Scholar]
- Dworkin M, Foster J. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75:592–601. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. Phytoremediation: synergistic use of plant and bacteria to clean up the environment. Biotechnol Adv. 2003;21:383–393. doi: 10.1016/s0734-9750(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Glick BR. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett. 2005;251:1–7. doi: 10.1016/j.femsle.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Gooding MJ, Davies WP. Wheat production and utilization: systems, quality and the environment. Wallingford: CAB International; 1997. [Google Scholar]
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. The world’s worst weeds. Distribution and biology. Honolulu: University Press of Hawaii; 1977. [Google Scholar]
- Kennedy AC, Stubbs T. Management effects on the incidence of jointed goatgrass inhibitory rhizobacteria. Biol Control. 2007;40:213–221. [Google Scholar]
- Kennedy AC, Elliott LF, Young FL, Douglas CL. Rhizobacteria suppressive to the weed downy brome. Am J Soil Sci Soc. 1991;55:722–727. [Google Scholar]
- Khandelwal A, Sehrawat A, Sindhu SS. Growth suppression of Chenopodium album weed and growth promotion effect on wheat (Triticum aestivum L.) by inoculation of δ-aminolevulinic acid producing rhizobacteria. Intern J Curr Microbiol Appl Sci. 2018;7(2):1958–1971. [Google Scholar]
- Kim SJ, Kremer RJ. Scanning and transmission electron microscopy of root colonization of morning glory (Ipomoea spp.) seedlings by rhizobacteria. Symbiosis. 2005;39:117–124. [Google Scholar]
- Kremer RJ, Kennedy AC. Rhizobacteria as biocontrol agents of weeds. Weed Technol. 1996;10:601–609. [Google Scholar]
- Lakshmi V, Kumari S, Singh A, Prabha C. Isolation and characterization of deleterious Pseudomonas aeruginosa KC1 from rhizospheric soils and its interactions with weed seedlings. J King Saud Univ Sci. 2015;27:113–119. [Google Scholar]
- Li J, Kremer RJ. Growth response of weed and crop seedlings to deleterious rhizobacteria. Biol Control. 2006;39:58–65. [Google Scholar]
- Magnucka EG, Pietr SJ. Various effects of fluorescent bacteria of the genus Pseudomonas containing ACC deaminase on wheat seedling growth. Microbiol Res. 2015;181:112–119. doi: 10.1016/j.micres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Malik DK, Sindhu SS. Production of indole acetic acid by Pseudomonas sp.: effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum) Physiol Mol Biol Plants. 2011;17(1):25–32. doi: 10.1007/s12298-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D, Granick S. The occurrence and determination of δ-aminolevulinic acid and porphobilinogen in urine. J Biol Chem. 1955;219:435–446. [PubMed] [Google Scholar]
- Mejri D, Gamalero E, Tombolini R, Musso C, Massa N, Berta G, Souissi T. Biological control of great brome (Bromus diandrus) in durum wheat (Triticum durum): specificity, physiological traits and impact on plant growth and root architecture of the fluorescent pseudomonad strain X33d. Biocontrol. 2010;55:561–572. [Google Scholar]
- Meliani A, Bensoltane A, Benidire L, Oufdou K. Plant growth-promotion and IAA secretion with Pseudomonas fluorescens and Pseudomonas putida. Res Rev J Bot Sci. 2017;6:16–24. [Google Scholar]
- Mohan Babu R, Sajeena A, Vidhyasekaran P, Seetharaman K, Reddy MS. Characterization of a phytotoxic glycoprotein produced by Phoma eupyrena—a pathogen on water lettuce. Phytoparasitica. 2003;31:265–274. [Google Scholar]
- Paau MA. Improvement of Rhizobium inoculants. Appl Environ Microbiol. 1989;55:862–865. doi: 10.1128/aem.55.4.862-865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KK, Mayilraj S, Chakrabarti T. Pseudomonas indica sp. nov., a novel butane-utilizing species. Intern J System Evol Microbiol. 2002;52(5):1559–1567. doi: 10.1099/00207713-52-5-1559. [DOI] [PubMed] [Google Scholar]
- Park JM, Radhakrishnan R, Kang SM, Lee IN. IAA producing Enterobacter sp. I-3 as a potent bioherbicide candidate for weed control: a special reference with lettuce growth inhibition. Indian J Microbiol. 2015;55(2):207–212. doi: 10.1007/s12088-015-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Phour M, Sindhu SS. Bioherbicidal potential of rhizosphere bacteria for the management of Phalaris minor weed. Res Crops. 2018;19(3):380–386. [Google Scholar]
- Phour M, Sindhu SS. Bio-herbicidal effect of 5-aminolevulinic acid producing rhizobacteria in suppression of Lathyrus aphaca weed growth. BioControl. 2019;64:221–232. doi: 10.1007/s10526-019-09925-5. [DOI] [Google Scholar]
- Phour M, Ghai A, Rose G, Dhull N, Sindhu SS. Role of aminolevulininic acid in stress adaptation and crop productivity. Int J Curr Microbiol Appl Sci. 2018;7(5):1516–1524. [Google Scholar]
- Pitcher DG, Saunders NA, Owen RJ. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8(4):151–156. [Google Scholar]
- Sachdev DP, Chaudhari HG, Kastur VM, Dhavale DD, Chopade BA. Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J Expt Biol. 2009;47(12):993–1000. [PubMed] [Google Scholar]
- Saikia J, Sarma RK, Dhandia R, Yadav A, Bharali R, Gupta VK, Saikia R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci Rep. 2018;8(1):35–60. doi: 10.1038/s41598-018-21921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf M, Pandya U, Thakka A. Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res. 2014;169(1):18–29. doi: 10.1016/j.micres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Tanaka T, Nishio N, Nagai S. Effect of culture pH on the extracellular production of 5-aminolevulinic acid by Rhodobacter sphaeroides from volatile fatty acid. Biotechnol Lett. 1993;15:859–864. [Google Scholar]
- Sasikala C, Ramana CV, Rao PR. 5-aminolevulinic acid: a potential herbicide/insecticide from microorganisms. Biotechnol Program. 1994;10:451–459. [Google Scholar]
- Sharama MP, Vanden BWH. The biology of Canadian weeds: Avena fatua L. Plant Sci. 2008;58:141–157. [Google Scholar]
- Sheng XF. Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus. Soil Biol Biochem. 2005;37:1918–1922. [Google Scholar]
- Sindhu SS, Dadarwal KR. Competition for nodulation among rhizobia in legume- Rhizobium symbiosis. Indian J Microbiol. 2000;40:211–246. [Google Scholar]
- Sindhu SS, Sehrawat A. Rhizosphere microorganisms: application of plant beneficial microbes in biological control of weeds. In: Panpatte DG, Jhala VK, Vyas RV, Shelat HN, editors. Microorganisms for green revolution; microorganisms for sustainability. Singapore Pte Ltd: Springer Nature; 2017. pp. 391–430. [Google Scholar]
- Sindhu SS, Gupta SK, Dadarwal KR. Antagonistic effect of Pseudomonas spp. on pathogenic fungi and enhancement of growth of green gram (Vigna radiata) Biol Fertil Soils. 1999;29:62–68. [Google Scholar]
- Sindhu SS, Sehrawat A, Sharma R, Dahiya A. Biopesticides: use of rhizosphere bacteria for biological control of plant pathogens. Def Life Sci J. 2016;1:135–148. [Google Scholar]
- Sindhu SS, Khandelwal A, Phour M, Sehrawat A. Bioherbicidal potential of rhizosphere microorganisms for ecofriendly weeds management. In: Meena VS, Mishra PK, Bisht JK, Pattanayak A, editors. Agriculturally important microbes for sustainable agriculture; Applications in crop production and protection. Singapore Pte Ltd: Springer Nature; 2018. pp. 331–376. [Google Scholar]
- Stearns JC, Woody OZ, McConkey BJ, Glick BR. Effects of bacterial ACC deaminase on Brassica napus gene expression. Mol Plant-Microbe Interact. 2012;25(5):668–676. doi: 10.1094/MPMI-08-11-0213. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Tanaka T, Hotta Y, Kuramochi H, Takeuchi Y. Improving salt tolerance of cotton seedlings with 5-aminolevulinic acid. Plant Growth Regul. 2000;32:99–103. [Google Scholar]
- Weller DM. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol. 1988;26:379–407. [Google Scholar]
- Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L, Zhou WJ. Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul. 2008;27(2):159–169. [Google Scholar]
- Zorner P, Farmer S, Alibek K. Quantifying crop rhizosphere microbiome ecology: the next frontier in enhancing the commercial utility of agricultural microbes. Ind Biotechnol (New Rochelle NY) 2018;14(3):116–119. doi: 10.1089/ind.2018.29132.pzo. [DOI] [PMC free article] [PubMed] [Google Scholar]