Abstract
Plasma cell leukemia (PCL) is an aggressive rare leukemic variant of multiple myeloma (MM). We aim to present 4 years data on clinical profile and treatment outcomes of Primary PCL (PPCL) patients treated at tertiary care cancer centre from Northern India. To analyse response and safety profile of a PPCL with or without stem cell transplantation. Retrospectively reviewed and analysed PPCL patient’s data at our centre from January-2013 to June-2017. Total 11 PPCL patients diagnosed among 240 MM patients during study period. Eight were males. Only 10 patients were started on treatment. Four (n = 4/10) patients underwent stem cell transplantation. Overall response rate was 70% (n = 7). Eleven culture positive bacterial infections (bloodstream = 2, urinary tract = 3; pulmonary = 6) were recorded. Four patients had fungal infections. One patient had Herpes Zoster infection. Relapse rate of entire cohort was 50% (n = 5). Median PFS and OS of entire cohort was 11 months (95% confidence interval 6.3–15.6) and 21 months (95% C.I. 1–49.8) respectively. The estimated PFS and OS at 1 year of transplanted versus nontransplanted patients were 71% + 24% versus 0% (P = 0.96) and 71% + 24% versus 15% + 19% (P = 0.234) respectively. Treatment with PIs + IMAs followed by transplants (single/double) might improve depth and duration of remission and OS. Patients should be treated with indefinite maintenance therapy to control disease.
Keywords: Primary plasma cell leukemia, Stem cell transplantation, Multiple myeloma, Overall survival
Introduction
Plasma cell leukemia (PCL) is a rare aggressive leukemic variant of multiple myeloma (MM) with poor prognosis. The International Myeloma Working Group (IMWG) in 2013 recommended presence of either circulating plasma cells as ≥ 20% or increased absolute number (2 × 109 L) of plasma cells in peripheral blood [1]. However, IMWG suggests to lower peripheral blood plasma cells ≥ 5% and/or absolute plasma cell count ≥ 0.5 × 109/L for future reference. Atypical plasma cells can be found infiltrating extramedullary tissues such as liver, spleen, peritoneal fluid and cerebrospinal fluid. PCL accounts for 2–4% of patients with MM [2, 3]. If PCL arises de novo without prior evidence of MM, it is termed as primary PCL (PPCL) and PCL evolving from a pre-existing MM or relapsed/refractory MM is termed as a secondary PCL (SPCL) [1]. Incidence of PPCL is 60–70%, remaining are SPCL. Clinically, hepatosplenomegaly, lymphadenopathy and osteolytic lesions are more common with PPCL, along with higher LDH and β-2 microglobulin. The introduction of immunomodulatory drugs (IMiDs) and proteasome inhibitors (PI) (bortezomib) have improved the overall survival and prognosis for PCL patients over the past decade, with more patients undergoing autologous stem cell transplant (ASCT) [4].
Because of rarity of this disease, few case series are available [4–9]. There is paucity of literature from India, especially the impact of the above therapies in resource poor countries. We present our 4 years data on the clinical profile, treatment and survival outcomes of PPCL patients treated at our tertiary care cancer centre from Northern India.
Materials and Methods
This is a retrospective analysis of patients diagnosed with PPCL at Hemato-Oncology department of our centre from year Jan-2013 to June 2017. Primary aim was to study response rate with novel agent based chemotherapies with and without ASCT, progression free survival (PFS) and overall survival (OS) in primary PCL patients. Study protocol was approved by institutional review board (IRB).
After diagnosis & ISS staging, a 3 drug induction therapy was given with IMiDs and PIs for 3–4 cycles. Chemotherapy responsive and fit patients were offered ASCT. Peripheral blood stem cell mobilization was done with GCSF ± Plerixafor. Conditioning regimen used was Melphalan 200 mg/m2. All patients were given maintenance chemotherapy once response achieved, 3 months after ASCT or 6 months of chemotherapy. The follow-up was censored at 31 December 2017.
Clinical and laboratory parameters, response to therapy, time to transplant and OS were recorded as per criteria laid down by IMWG guidelines, 2013 [10]. Descriptive statistics such as median/range was calculated for all the variables using SPSS version 21 (IBM, Armonk, NY, USA). Probabilities of overall survival (OS) and progression free survival (PFS) were plotted as per Kaplan-Meier estimates.
Results
Total 11 patients were diagnosed with PPCL among the 240 MM patients diagnosed and treated at our centre during study period. Median duration of symptoms at presentation was 3 months (1–8 months). Most common presenting complaints were bodyache followed by weakness, weight loss, renal dysfunction and fever. Anemia was present in 10/11 patients, while renal insufficiency, hypercalcemia and bone lesions were present in 5, 4 and 8 patients respectively. Most patients were ISS stage III (n = 9/11) and IgG subtype (Table 1). Five patients had raised LDH. Fluorescence in-situ hybridization (FISH) was available for only three patients and it was positive for both deletion17p13.1 and t(4;14) in 2 patients.
Table 1.
Demographics details of eligible patients
| Characteristics (n = 11) | Median | N (%) |
|---|---|---|
| Age (years) | 57 (37–69) | – |
| Male | – | 8 (72.7) |
| Female | 3 (27.2) | |
| Hb (g/dl) | 7.8 (5.4–10.7) | – |
| TLC (/cumm) | 27,730 (11,500–98,200) | – |
| Platelets (/cumm) | 80,000 (8000–131,000) | – |
| Albumin (gm/dl) | 3.4 (2.2–4.4) | – |
| Creatinine (mg/dl) | 1.8 (0.8–8.1) | – |
| B2-microglobulin (mg/ml) | 9.3 (5.2–34.1) | – |
| Peripheral plasma cells (%) | 43 (22–91) | – |
| Bone marrow plasma CELLS (%) | 74 (15–100) | – |
| Serum protein electrophoresis (SPEP) (gm/dl) | 3.3 (0–7.3) | – |
| Performance status (ECOG) | ||
| Score-1 | 7 (63.6) | |
| Score-2 | 3 (27.2) | |
| Score-3 | 1 (09) | |
| 24 h Urine Protein (gm/24 h) |
0.4 (0.09–3.2) Nil in n = 2 patients |
– |
| Uric Acid (mg/dl) | 10.9 (5.7–21.4) | – |
| LDH (U/L) | 434 (106–1531) | – |
| Hepatomegaly | – | 2 (18.1) |
| Splenomegaly | – | 3 (27.2) |
| Extramedullary involvement | – | 2 (18.1) |
| Bony lesions present | – | 8 (72.7) |
| ISS staging | – | |
| ISS-1 | 0 | |
| ISS-2 | 1 (09) | |
| ISS-3 | 9 (81.1) | |
| Not available | 1 (09) | |
| Subtype | – | 3 (27.2) |
| IgG | 2 (18.1) | |
| Light chain | 6 (54.5) | |
| Not available | ||
| FISH (tested for del17p; t(14;20); t(14;16); t(4;14); del 13q) | 3 patients (27.2) | |
| Negative | 1/3 | |
| Positive for del 13q14.3, del 17p13.1, t(4;14) | 1/3 | |
| Positive for del 17p13.1, t(4;14) | 1/3 | |
| Response to chemotherapy (N = 10) (post 2-4 cycles of IMiDs + PIs chemotherapy) | – | |
| Complete response (CR)/very good partial response (VGPR) | 6 (60) | |
| Partial response (PR) | 1 (10) | |
| Stable disease (SD) | 1 (10) | |
| Progressive disease (PD) | 2 (20) | |
Out of 11 patients, 10 patients were started on treatment. Most commonly used regimen for first line chemotherapy was Bortezomib-Thalidomide-Dexamethasone (VTD, n = 4) used in years 2013 to 2015 followed by Bortezomib-Cyclophosphamide-Dexamethasone (VCD, n = 3) in year 2015 and Bortezomib-Lenalidomide-Dexamethasone (VLD, n = 3) in year 2016–2017. Median number of chemotherapy cycles received was 4 (3–10). 4 patients underwent stem cell transplantation. Overall response rate (ORR) was 70% (n = 7).
Post Chemotherapy Therapy
Four (40%) patients underwent ASCT (Table 2) while 2/4 received tandem transplant. Pre-ASCT response of patients were CR/VGPR (n = 3), and PR (n = 1). Post-ASCT, all 4 patients achieved CR/VGPR at 3 months after which maintenance therapy was started. Post ASCT all patients received maintenance therapy (bortezomib + thalidomide = 1; bortezomib = 1; thalidomide = 1; lenalidomide = 1) whereas n = 3/6 patients from chemotherapy group only received maintenance therapy (bortezomib = 1; thalidomide = 1 and lenalidomide = 1).
Table 2.
ASCT characteristics
| Characteristic (N = 4) | Median | N (%) |
|---|---|---|
| Pre-ASCT response | – | |
| CR/VGPR | 3 (75) | |
| PR | 1 (25) | |
| Time to transplant from diagnosis (months) | 3.5 (3–5) | – |
| Dose of melphalan (mg/m2) | 200 (190–200) | – |
| Dose of CD34 cells (× 106 /cumm) | 4 (3.28–4.6) | – |
| Days of neutrophil engraftment (days) | 10 days (8–10) | – |
| Days of platelet engraftment (days) | 11 days (10–12) | – |
| Febrile neutropenia | – | 4 (100) |
| Mucositis | – | 4 (100) |
| Oral Mucositis Grade-3/4 | 3/4 | |
| Lower G.I. Mucositis | 3/4 | |
| PRBC units | 2 (0–2) | – |
| PRP units | 10 (6–18) | – |
| Post-ASCT response (at 3 months interval) | – | |
| CR/VGPR | 4 (100) |
Toxicities and Infections (Table 3)
Table 3.
Toxicities
| Characteristics | Total (n = 10) | Post Chemotherapy (n = 6) | Post ASCT (n = 4) |
|---|---|---|---|
| Total number of Culture Positive Bacterial Infections | N = 5/10 | ||
| Gram Positive | 01 | 00 | 03 |
| Gram Negative | 10 | 07 | 01 |
| Bloodstream Infections | 02 | 01 | 01 |
| Urinary Tract Infections | 03 | 02 | 01 |
| PulmonaryInfections | 06 | 04 | 02 |
| Viral Infections | 02 | 01 | – |
|
Upper Respiratory Tract Infection |
01 | – | 01 |
| Herpes Zoster | 01 | – | 01 |
| Invasive Fungal infections | 04 | ||
| Possible | 01 | 03 | – |
| Probable | 03 | ||
| Cryptosporodium diarrhoea | 01 | – | 01 |
| Peripheral neuropathy | 05 | 05 | – |
| Diarrhoea | 03 | 02 | 01 |
| Thrombocytopenia | 07 | 03 | 4 |
| Deranged renal function | 01 | 01 | – |
| Deranged hepatic function | 02 | 02 | – |
| Mucositis | 04 | – | 04 |
Infections were the most common toxicities observed during entire course of treatment. Bacterial, fungal and viral infections were commonly seen both after chemotherapy or transplant. Patients on chemotherapy also developed deranged liver and kidney function tests, while transplant patients had mucositis (grade 3–4) and cryptococcal diarrhoea.
Outcome Survival
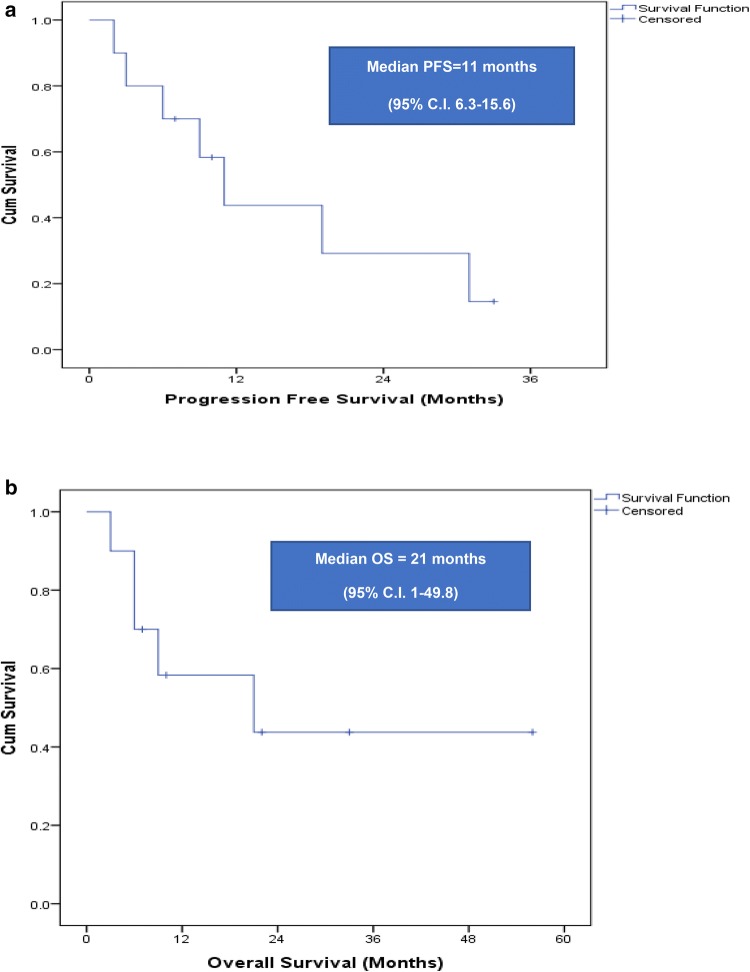
At a median follow up period of 22 months (95% C.I. 1–44.1), relapse rate of entire cohort was 70% (n = 7) including 2 patients who relapsed post ASCT. Median PFS of entire cohort was 11 months (95% C.I. 6.3–15.6) (Fig. 1a). The estimated PFS at 1 year of transplanted versus non-transplanted patients were 71% ± 24% versus 0% (P = 0.96).
Fig. 1.
a Kaplan meier curve is depicting progression free survival of cohort. b Kaplan meier curve is depicting overall survival of cohort
Median OS of entire cohort was 21 months (95% of C.I. 1–49.8) (Fig. 1b). The estimated OS at 1 year of transplanted versus non-transplanted patients were 71% ±24% versus 15% ± 19% (P = 0.234). Mortality rate was 50% and cause of deaths were PD in all patients.
Discussion
PCL is a challenging disease with poor prognosis with no definite treatment options that can lead to long term PFS or OS. Available literature is very limited, with few prospective randomized trials. In the past decades IMiDs such as thalidomide/lenalidomide and PIs such as bortezomib have significantly improved clinical scenario of MM in terms of deeper responses, PFS, OS and quality of life. As a result these therapies have been also used in PCL. Stem cell transplantation (Autologous/Allogeneic) is currently favourably suggested in PPCL patients [11–13]. ASCT is a feasible option in PCL patients which may improve PFS and OS as compared to patients treated with chemotherapy alone. Maintenance chemotherapy should be started at the earliest once response achieved.
Our study highlights the key clinical features of PCL. Most of the patients tend to present with ISS-3, raised LDH and tend to have poor cytogenetic abnormalities. Extramedullary disease was seen in occasional patients. Our study also highlights high incidence of infectious complications with bacterial and fungal infections being commonly seen. This warrants for improving supportive care for preventing and treating infections. Emergence of multi-drug resistant bugs contributes to high infection related mortality in our setting.
Reported results from available literature were contrasting in nature because of use different therapies, novel agents and SCT. Our study reported 70% ORR to initial therapy and at a median follow up period 22 months (95% C.I. 1–44.1), median OS was 21 months (95% of C.I. 1–49.8). Our results are comparable with the previously published literature [14–19] (Table 4).
Table 4.
Comparison with previously published literature
| Published studies | ORR | Median PFS | Median OS |
|---|---|---|---|
| Pagano et al. [14] | 55% (median DOR = 16.4 months) | – | 12.6 months (range, 0.5–75.8), with a survival at 5 years was 9.3% |
| Musto et al. [15] | 60.8% (post 4 cycles) | 52.1% (15 months) | 65.2% (15 months) |
| Musto et al. [16] | 92% | 8 months | 12 months |
| D Arena et al. [17] | 79% | – | 55% (2 years) |
| Colovic et al. [18] | 7/30 patients achieved remission | – | 4.5 months |
| García-Sanz et al. [19] | 37% (ORR = 29% + PR = 8%) | – | 8 months |
| Mahindra et al. [20] | |||
| Autologous SCT | 76% (at transplant) | 34% at 3 years | 64% at 3 years |
| Allogeneic SCT | 64% (at transplant) | 20% at 3 years | 39% at 3 years |
|
Drake et al. [21] Autologous SCT |
41.2% (at day 100 post SCT) |
– | 25.7 (19.5–31.9 months) |
|
Pagano et al. [14] SCT |
– |
HR for relapse 0.13, (95% CI 0.04–0.41) |
38.1 months (range 4.8–75.8 months) |
|
Our study Median follow up-22 months |
70% (post induction chemotherapy) |
11 months (95% C.I. 6.3–15.6) |
21 months (95% C.I. 1–49.8) |
In view of aggressive nature of PCL, the need for therapy with a relatively early and deeper response is desirable. Therefore, high dose therapy followed by SCT was offered to all the patients with PCL but only 40% (n = 4) patients underwent ASCT at our centre. Two out of four patients received tandem ASCT for improved and deeper duration of response. The estimated 1 year OS of transplanted versus non-transplanted PPCL patients were 71% ± 24% versus 15% ± 19%. None of our patients underwent allogenic SCT. The Center for International Blood & Marrow Transplant Research (CIBMTR) reported outcomes of 147 patients with PPCL (ASCT = 97; Allogeneic SCT = 50) between 1995 and 2006. Median follow-up was 38 months of ASCT and 68/97 received tandem ASCT. PFS at 3 year was 34% (95% C.I. 23–46%) versus 20% (95% C.I. 10–34%) in ASCT versus Allogeneic SCT group. OS at 3 year of ASCT versus Allogeneic SCT group was 64% (95% C.I. 52–75%) and 39% (95% C.I. 1–11%) respectively [20]. Pagano et. al [14] showed longer OS (38.1 months) and duration of response (25.8 months) for hematopoietic stem cell transplantation received versus non-transplanted PPCL patients[9.1 months versus 7.3 months (P = 0.001)]. European Group for Blood and Marrow Transplantation (EBMT) registry reported outcomes of ASCT in PPCL patients. A total of 272 patients of PPCL was included and 88/272 underwent ASCT. They demonstrated less survival post ASCT in PPCL patient than MM. Post ASCT, 41.2% of patients achieved CR at 100 days with median OS was 25.7 months. (95% C.I. 19.5–31.9 months) [21].
Early start of maintenance therapy with novel agents alone or in combination could prolong depth and duration of response, like in multiple myeloma. However, very early relapses may occur after ASCT hence consolidation or maintenance therapy may be needed early within 30–60 days after engraftment. Royer et al showed improved OS (36.3%) in patients who received consolidation or maintenance therapy post 2nd ASCT [22].
With aim to improve PFS and OS of PCL patients, other therapeutic approaches should be explored. The use of newer drugs (carfilzomib, pomalidomide, daratumumab, elotuzumabetc) in induction, consolidation or maintenance in various combinations could prove to be a game changer for PPCL patients. Recommendation of cytogenetics and FISH studies tests to be mandatory for diagnosis in all suspected PCL cases. Role of Allogeneic SCT (potentially curative) and tandem—ASCT still needs to be defined.
Limitations of our study were retrospective nature of study, less number of patients, non- availability of immunofixation (IFE) subtyping of clonal immunoglobulins, short duration follow-up and lack of cytogenetics/FISH profile information of all patients.
To conclude, PCLs are rare and aggressive in nature, presenting with high risk features. Treatment with PIs + IMiDs followed by autologous transplant likely improve depth and duration of remission and OS. PD and early relapses are common. All PCL patients should be encouraged to participate in prospective multicenter clinical trials.
Acknowledgements
We would like to acknowledge an entire team of hemato-oncology and bone marrow transplant unit, Rajiv Gandhi Cancer Institute & Research Centre for their continuous support for retrieving records of eligible patients.
Compliance with Ethical Standards
Conflict of interest
All authors have declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by our Institutional Review Board (IRB).
Informed Consent
This is a retrospective study hence for this type of study formal consent is not required. IRB waived off the need to take consent for the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernández de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DF, et al. International Myeloma Working Group. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasqualethi P, Festuccia V, Collacciani A, Acitelli P, Casale R. Plasma cell leukemia, a report on 11 cases and review of the literature. Panminerra Med. 1996;38:179–184. [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Plasma cell leukemia. Br J Hematol. 1994;88:754–759. doi: 10.1111/j.1365-2141.1994.tb05114.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Kim J, Cho Y, Seo BK, Kim BS. Combination chemotherapy with bortezomib, cyclophosphamide and dexamethasone may be effective for plasma cell leukemia. Jpn J Clin Oncol. 2007;37:382–384. doi: 10.1093/jjco/hym037. [DOI] [PubMed] [Google Scholar]
- 5.Bladé J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13:1259–1272. doi: 10.1016/S0889-8588(05)70125-8. [DOI] [PubMed] [Google Scholar]
- 6.Costello R, Sainty D, Bouabdallah R, Fermand JP, Delmer A, Divine M, et al. Primary plasma cell leukaemia: a report of 18 cases. Leuk Res. 2001;25:103–107. doi: 10.1016/S0145-2126(00)00102-8. [DOI] [PubMed] [Google Scholar]
- 7.Majumdar N, Kumar R, Anand M, Kalita D, Ghara N, Chopra A, et al. Plasma cell leukemia-a study of 28 cases from India. Hematology. 2009;14:198–203. doi: 10.1179/102453309X426191. [DOI] [PubMed] [Google Scholar]
- 8.Naseem S, Kaur S, Gupta R, Kashyap R, Nityanand S. Plasma cell leukemia: case series from a tertiary center with review of literature. Indian J Hematol Blood Transfus. 2012;28:10–14. doi: 10.1007/s12288-011-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kar R, Priyadarshini SG, Niraimathi M, Basu D, Badhe BA. Clinico-pathological spectrum of primary plasma cell leukemia diagnosed at a tertiary care centre in South India over 5 year period. Indian J Hematol Blood Transfus. 2012;28:170–174. doi: 10.1007/s12288-011-0133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Myeloma Working (IMW) (http://imwg.myeloma.org/)
- 11.Sher T, Miller KC, Deeb G, et al. Plasma Cell leukemia and other aggressive plasma cell malignancies. Br J Haemtol. 2010;150:418–427. doi: 10.1111/j.1365-2141.2010.08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albarracin F, Fonseca R. Plasma cell leukemia. Blood Rev. 2011;25:107–112. doi: 10.1016/j.blre.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musto P, Pagano L, Petrucci MT, et al. Primary plasma cell leukemia in the era of new drugs: has something changed? Crit Rev Oncol Hematol. 2012;82(2):141–149. doi: 10.1016/j.critrevonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Pagano L, Valentini CG, De Stefano V, Venditti A, Visnaie G, Petrucci, et al. Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011;22:1628–1635. doi: 10.1093/annonc/mdq646. [DOI] [PubMed] [Google Scholar]
- 15.Musto P, D’Auria F, Petrucci MT, Levi A, Cascavilla N, Falcone A et al. (2011) A: final results of a phase II study evaluating lenalidomide in combination with low dose dexamethasone as first line therapy for primary plasma cell leukemia. Blood (ASH Annual Meeting Abstracts). p 118 (Abstract 2925)
- 16.Musto P, Rossini F, Gay F, Pitini V, Guglielmelli T, D’Arena G, et al. Efficacy and safety of bortezomib in patients with plasma cell leukemia. Cancer. 2007;109:2285–2290. doi: 10.1002/cncr.22700. [DOI] [PubMed] [Google Scholar]
- 17.D’Arena G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, Mansueto G, et al. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol. 2012;23:1499–1502. doi: 10.1093/annonc/mdr480. [DOI] [PubMed] [Google Scholar]
- 18.Colović M, Janković G, Suvajdzić N, Milić N, Dordević V, Janković S. Thirty patients with primary plasma cell leukemia: a single center experience. Med Oncol. 2008;25(2):154–160. doi: 10.1007/s12032-007-9011-5. [DOI] [PubMed] [Google Scholar]
- 19.García-Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, Fernández-Calvo J, Sanz MA, Pérez-Simón JA, Rasillo A, Miguel JF. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93(3):1032–1037. doi: 10.1182/blood.V93.3.1032. [DOI] [PubMed] [Google Scholar]
- 20.Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole D, Zhang MJ, Li P, et al. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia. 2012;26:1091–1097. doi: 10.1038/leu.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, Niederwieser D, et al. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010;95:804–809. doi: 10.3324/haematol.2009.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C, et al. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the Intergroupe Francophone du Myélome. J ClinOncol. 2016;34:2125–2132. doi: 10.1200/JCO.2015.63.1929. [DOI] [PubMed] [Google Scholar]