Abstract
Quantifying brain dynamics during anesthesia is an important challenge for understanding the neurophysiological mechanisms of anesthetic drug effect. Several single channel Electroencephalogram (EEG) indices have been proposed for monitoring anesthetic drug effect. The most commonly used single channel commercial index is the Bispectral index (BIS). However, this monitor has shown some drawbacks. In this study, a nonlinear functional connectivity measure named Standardized Permutation Mutual Information (SPMI) is proposed to describe communication between two-channel EEG signals at frontal and temporal brain regions during a controlled propofol-induced anesthesia and recovery design from eight subjects. The SPMI index has higher correlation with estimated propofol effect-site concentration and has better ability to distinguish three anesthetic states of patient than the other functional connectivity indexes (cross-correlation, coherence, phase analysis) and also the BIS index. Moreover, the SPMI index has a faster reaction to the effect of drug concentration, less variability at the consciousness state and better robustness to noise than BIS.
Keywords: Electroencephalogram, Depth of anesthesia, Mutual information, Bispectral index
Introduction
Monitoring depth of anesthesia (DOA) is a major ongoing challenge for anesthetists to reduce anesthetic drug consumption, avoiding intraoperative awareness (Sebel et al. 2004; Gugino et al. 2001) and prolonged recovery (Monk et al. 2005). Because the main target of anesthetic drug effect is in central nervous system, electroencephalogram (EEG) signal processing is useful for monitoring DOA. EEG is well accepted due to high temporal resolution and widely used in clinical application (Mumtaz et al. 2017; Hou et al. 2017; Talebi et al. 2018; Mohammadpoory et al. 2019; Hejazi and Nasrabadi 2019).
In recent years, a number of single channel EEG-based non-linear approaches such as fractal (Gifani et al. 2007; Nguyen-Ky et al. 2010; Li et al. 2017a, b), Bayesian analysis (Nguyen-Ky et al. 2013, 2014), relative power spectrum density (Liang et al. 2018; Saadeh et al. 2019; Shalbaf et al. 2019) Hilbert–Huang transform (Shalbaf et al. 2012a, b), Phase-rectified signal averaging (Liu et al. 2017a, b), distribution of auto-regressive moving average model parameters (Kuhlmann et al. 2017) and entropy (Shalbaf et al. 2012a, b, 2013, 2018; Liang et al. 2015; Li and Wen 2017; Mateos et al. 2018) have been proposed for DOA assessment. The most commonly used single channel EEG-based commercial monitor of DOA is the bispectral index (Rampil 1998) (BIS, Aspect Medical Systems, Newton, MA). But, this monitor has shown some drawbacks such as sensitivity to artifact (Nguyen-Ky et al. 2013), failure to respond to some anesthetic drugs (Johansen and Sebel 2000), and large time delays in response to EEG changes (Pilge et al. 2006; Shalbaf et al. 2013; Hagihira et al. 2001).
To better understand the effects of anesthetic drugs, it is important to study not only single channel EEG in one region, but also how the functional connectivity of different areas of the brain in multi-channel EEG signals are changed. Anesthetics disturb communication between cortical areas (Schrouff et al. 2011; Alkire et al. 2008; Nallasamy and Tsao 2011; Lee et al. 2009a, b), and consequently impair large-scale integration of information, hypothesized to be a prerequisite to proper brain function, particularly conscious perception (Schrouff et al. 2011; Alkire et al. 2008). Therefore, disconnection or suppression of communication between brain regions, especially frontal to parietal regions (Lee et al. 2009a, b) is hypothesized to be an important factor in causing unconsciousness. Functional connectivity methods have been proposed to assess relationships between simultaneous, but spatially separated, signals (Pereda et al. 2005; Breakspear 2004; Stam 2005).
Some of the traditional linear functional connectivity measures in the time and frequency domain such as Cross-correlation (COR) and Coherence (COH) have been proposed in multi-channel EEG signals during general anesthesia (Li et al. 2013; Hayashi et al. 2014; Akeju et al. 2014; Li et al. 2017a, b). But, these techniques encounter problems with nonlinear and nonstationary EEG signals. Another commonly used method of functional connectivity is the phase difference of multi-channel EEG signals named phase locking value (PLV) (Lachaux et al. 1999; Nicolaou and Georgiou 2014; Pal et al. 2017). But, this method is also sensitive to artifacts and nonlinear coupling in EEG signals. Considering the points mentioned above, nonlinear methods of functional connectivity might be more useful for studying interdependence of multi-channel EEG signals. Recently, nonlinear methods of functional connectivity such as mutual information and an improved method, Standard Permutation Mutual Information (SPMI) have been widely applied for neurological disease diagnosis, such as seizures, Alzheimers dementia, and autism, in multi-channel EEG analysis (Abásolo et al. 2008; Hall Jr. and Sarkar 2011; Langen et al. 2009). Therefore, characterizing brain dynamics using nonlinear functional connectivity methods might be an important tool for understanding and tracking the neurophysiological mechanisms of anesthetics.
In this work, SPMI index is utilized to investigate the nonlinear functional connectivity of long-range two-channel EEG signals from frontal and temporal montages during a controlled propofol-induced anesthesia and recovery. We want to evaluate the performance of this index for tracking anesthetic drug concentration and the ability to distinguish three different states (awake, unconscious and recovery states) and finally to compare with the BIS index as single channel EEG-based commercial monitor of DOA.
Materials and methods
Subjects and data acquisition
With the approval of the Waikato Hospital Ethical Committee, EEG data of eight volunteers (five male and three female, weight 59–120 kg, age 18–42 years) under Propofol anesthetic were used in this study. The details have been previously published (Williams and Sleigh 1999). All subjects gave written informed consent. Two bipolar EEG signals were recorded at the position of Fp1-F7 and C3-T3 according to the 10–20 international system using scalp electrodes. The reference electrode was positioned at FpZ. By these montages, EEG activity of the left prefrontal and left temporal was acquired. The EEG signals and also BIS index (extracted from the pre-frontal region) were recorded using the A-1000 BIS monitor (Aspect Medical Systems, Natick, MA, USA) with the sampling rate 256 Hz and 0.2 Hz, respectively. The EEG signal pre-processing consists of three steps: Low and high frequency filters of EEG signal set at 0.5 and 70 Hz, respectively. Also, the notch filter is set at 50 Hz. Finally, raw EEG data exceeding amplitude of 200 µV were removed as outlier points. The range of the value of BIS index is between 0 and 100, but is transformed to 0–1 for comparison with other indexes.
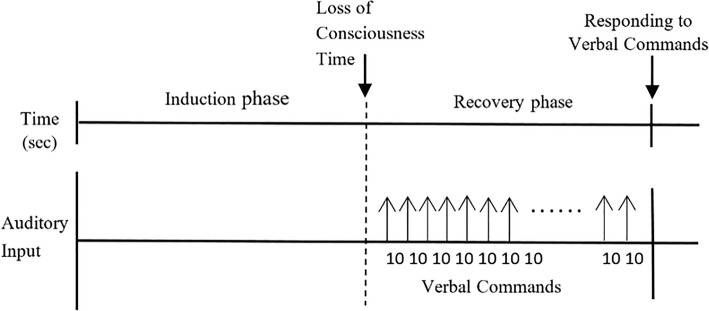
All subjects had a continuous infusion of Propofol at fixed concentration of 150 ml/h (1500 mg/h) via a syringe pump after intravenous access, and simultaneously the BIS index and the two mentioned channel EEG signals were recorded. Induction was stopped, when the subjects lost consciousness. Loss of consciousness (LOC) time was defined the time of dropping a syringe filled with water, held between forefinger and thumb. Then, the subjects were allowed to awake and a pre-recorded tape of random numbers and some verbal commands such as “move your left foot” at 10-s intervals was played. When the subjects were able to respond the verbal commands correctly, the recovery time or recovery of consciousness time was started. 60 s after this time, the study was terminated. So in this study, three states named awake (10 s after beginning of study), unconscious (after LOC time) and recovery (after responding to verbal commands) are assessed. The sequence diagram of the experimental design over the whole experiment period is demonstrated in Fig. 1.
Fig. 1.
The experimental sequence diagram with a fixed propofol concentration
Cross-correlation (COR)
The cross-correlation is one of the classical measures of linear interdependence between the magnitude two time series x(t) and y(t) in time domain (Pereda et al. 2005):
| 1 |
Where N is the number of samples and τ is time delay between two time series. This index ranges from − 1 (complete linear inverse correlation) to + 1 (complete linear direct correlation) and 0 means no linear interdependence between the two signals.
Coherence (COH)
Coherence is a linear method for assessment of the interdependence between two time series x(t) and y(t) in frequency domain. It can evaluate the relationship of time series at different frequency bands. It is calculated as magnitude squared coherence based on Fourier transform, which is ratio between the cross power spectral density Sxy(f) and their individual auto spectral densities Sxx(f), Syy(f).
| 2 |
The coherence index ranges between 0 (minimum linear correlation) and 1(maximum linear correlation).
Phase locking value (PLV)
Phase difference of time series is independent of the amplitude of the signals. The most commonly used phase analysis is the PLV (Lachaux et al. 1999). This method is independent of the amplitude of the signals and characterizes relative phase difference of time series defined as:
| 3 |
Where N is the total number of samples of the data set and Δφxy(t) is the relative phase difference between the two time series. The PLV takes value between 0 (minimum phase synchrony) and 1 (maximum phase synchrony).
Standardized permutation mutual information (SPMI)
The mutual information (MI) is an information theory method and is used to evaluate the interdependence between two time series based on Shannon’s information theory (Palus 1996). This method has been proposed to quantify strength of functional connectivity between time series. For a pair of discrete random variables x and y which are recorded from two time series with the probability distribution functions P(x) and P(y), respectively and their joint probability function P(x,y), the MI between x and y defined as:
| 4 |
The MI of two random variables is a method of estimating mutual dependence between them and quantifies the amount of information that obtained from observations of the effect of one random variable on another variable and vice versa.
Recently, a new measure called Permutation mutual information (PMI) is defined based on the probability distribution of the ordinal patterns. Order pattern analysis has proposed to evaluate dynamical characteristics of nonlinear time series (Bandt & Pompe 2002). Given the time series X = x(t) and Y = y(t) (t = 1, 2, …, N), we can obtain the embedding vector xi = [x(i), x(i + τ), …, x(i + (m + 1)τ)] (i = 1, 2, …, N − (m − 1)τ) and yi with the embedding dimension m and time lag τ. Then, sorting the elements of vectors xi and yi in ascending order respectively and there are m! possible order patterns in each vectors, which is also called permutations. Next, counting the number of each order pattern of all vectors and we can obtain the probability of each permutation.
The permutation entropy (PE) of the two time series X and Y are defined as:
| 5 |
where PX(i), PY(i) are the probability of permutation of X and Y respectively and m is embedding dimension. Also, the joint permutation entropy of X and Y is:
| 6 |
where PXY(i, j) is the joint probability of permutation of X and Y. Then, the PMI which is based on the probability distribution of permutation pattern is defined as:
| 7 |
According to the standardized mutual information, we have introduced the standardized permutation mutual information (SPMI) as follows:
| 8 |
Where the range of SPMI is between 0 (X and Y are independent) and 1(X and Y are fully correlated).
Statistical analysis
Prediction Probability (PK) statistic (Smith et al. 1996) is used to evaluate the correlation between the EEG measured functional connectivity index and Propofol effect-site concentration (Ceff) (derived from pharmacokinetic/pharmacodynamic (PK/PD) modeling (Mckay et al. 2006)) with the 2003 Excel software. PK value of 1 means that the index predicts Ceff correctly 100% of the time. This is done to assess the efficiency of new index for tracking the changes in Propofol drug effect. Furthermore, linear regression and the Pearson correlation coefficient are used for assessing the relationship between measured connectivity index and BIS.
Statistical significant differences of the index values of connectivity measures between awake and unconscious states and between unconscious and recovery states of all subjects are determined using 1-way analysis of variance (ANOVA). The normal distribution of the index values is investigated before performing analyses. Also, Box plots are used to evaluate the performance of each connectivity index in distinguishing different anesthetic states. Finally, the coefficient of variation (the standard deviation (SD)/mean) of each index is calculated to describe the index stability and its robustness to noise during anesthesia process.
Results
We calculated the functional connectivity between frontal and temporal lobe EEG signals using SPMI index over 10-s epochs for the whole experimental period, and for all subjects. In the calculation of this index, we have two adjustable parameters (m, the embedding dimension, and τ, the lag for the embedding). m is usually chosen as 4–6 because for m ≤ 3, there would be too few possible patterns. For a larger m, unrealistically long data are required to obtain a permutation entropy value (e.g., m = 7, there are m! = 5040 possible patterns, at least 20 s data will be required at the sample rate of 256). We investigated the influence of m from 4 to 6 in this study to avoid deviation of the permutation mutual information. Selecting too small or too large m leads to the incorrect estimation of ordinal patterns. Also, the time delay τ was tested. Too small or too large τ cause small estimated value of mutual information. After numerous exploratory calculations, we have found that the parameter selection m = 6 and τ = 1 resulted in the best SPMI performance to distinguish different anesthetic states. Based on the PK/PD modeling and prediction probability analysis, this index had the highest correlation with anesthetic drug effect.
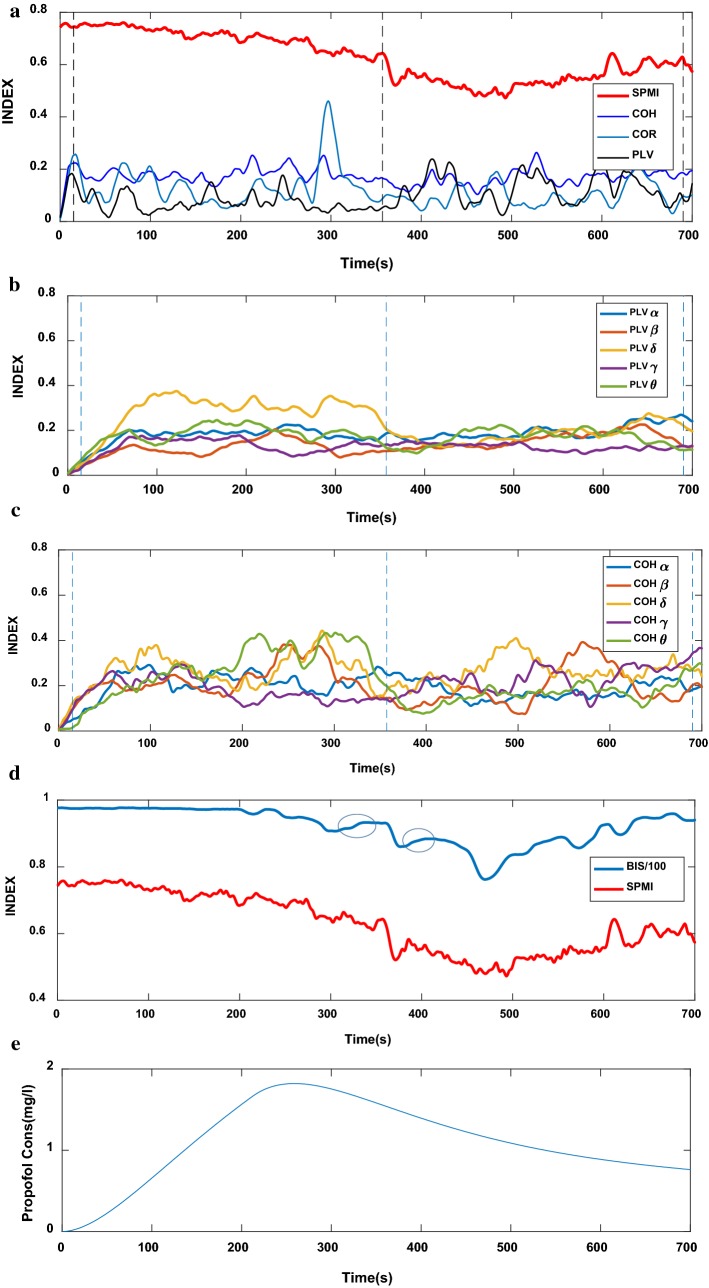
The SPMI index is compared with a range of commonly used methods of functional connectivity: COR, COH, and PLV. The functional connectivity indexes using COH and PLV are also calculated for each of five frequency bands: Delta δ(1–4 Hz), Theta θ(5–8 Hz), Alpha α(9–13 Hz), Beta β(14–30 Hz) and Gamma γ(31–40 Hz). The calculated values of SPMI, COR, COH and PLV indexes (Fig. 2a–c) for the one subject during the whole procedure and simultaneously the corresponding BIS (Fig. 2d) and effect-site propofol concentration (Ceff) (Fig. 2e) for the same subject are shown in Fig. 2. As can be seen from this Figure, the SPMI index follows the changes in Propofol drug effect in a usual clinical setting (Fig. 2a). While COR, COH and PLV indexes fail to track the changes. This may be due to the fact that EEG signals at frontal and temporal lobes have different amplitudes and noise, which distort the results of these measures. Also, COR, COH and PLV indexes, as linear analysis methods, may not have adequate ability to evaluate an EEG signal with dynamic and nonlinear behavior.
Fig. 2.
An example of the changes with time for the EEG measures for one subject (a–d) and simultaneously the propofol effect site concentration Ceff (e). a The functional connectivity indexes between two-channel EEG signals using SPMI, COR, COH (all frequency bands) and PLV (all frequency bands). Three dashed vertical lines from the left to the right show times of baseline (wakefulness), loss of consciousness (LOC) and recovery, respectively. b, c The functional connectivity indexes between two-channel EEG signals using COH and PLV for the five frequency bands: Delta δ, Theta θ, Alpha α, Beta β and Gamma γ. d The functional connectivity indexes between two-channel EEG signals using SPMI and simultaneously the corresponding BIS. e simultaneously the propofol effect site concentration Ceff. The irregular rises of BIS index are identified in (d) with a circle
The values of SPMI and BIS index decrease from the awake to the unconscious state and then increase at recovery state. But, SPMI index tracks the effect of drug concentration more quickly (after 80th s) than BIS particularly about the change from awake to unconscious (Fig. 2d). In other words, SPMI has a shorter delay to effect of drug concentration than BIS index. Also, the BIS index demonstrates some irregular upward trends about 310–360th s and 380–420ths in unconscious state because of substantial loss in the amount of background EEG activity, while this deviation cannot be seen in SPMI. Thus, the SPMI index is more robust to artifacts than BIS.
To assess the ability of predicting Ceff using the proposed connectivity index, PK is computed for each subject. The PK values of COR, COH, PLV, BIS and SPMI indexes with Ceff are 0.63 ± 0.06, 0.62 ± 0.09, 0.61 ± 0.09, 0.77 ± 0.05 and 0.83 ± 0.05, respectively. SPMI has the highest PK value among connectivity indexes which demonstrate the ability of the index to predict Ceff strongly. Also, PK value between the SPMI index and the Ceff (0.83) is higher than between BIS and Ceff (0.77). So, the results show that SPMI has good ability in tracking the changes in Propofol drug effect during the whole experimental procedure.
Figure 3 illustrates the scatter plot of the SPMI index and BIS from 8 subjects during the whole procedure (1072 data points). Data points are fitted by a linear line with the equation as SPMI = 0.98*BIS-0.05. Thin lines show 95% confidence boundaries around the linear black bold line. According to this figure, few data points are beyond the thin lines. Furthermore, the Pearson correlation coefficient between SPMI and BIS is 0.84 that indicates the high similarity of these two methods in a usual clinical setting.
Fig. 3.
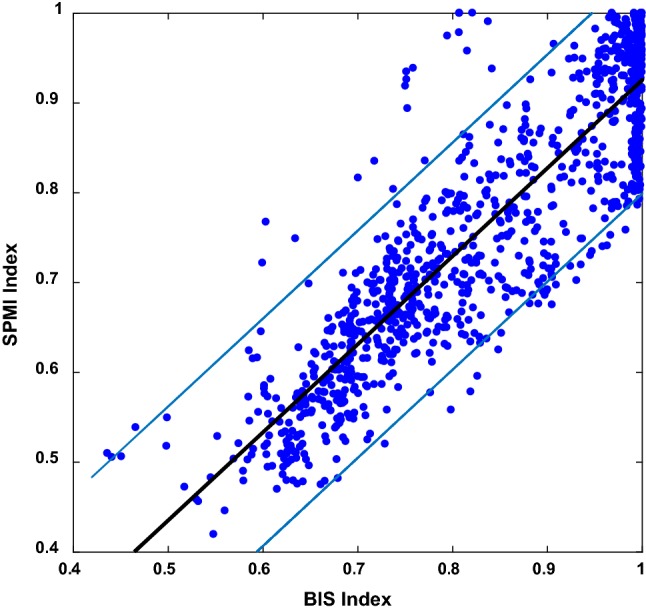
Scatter plot for the SPMI and BIS for all data points of 8 subjects during whole procedure. The best-fit line is bold and thin lines correspond to the 95% confidence boundaries
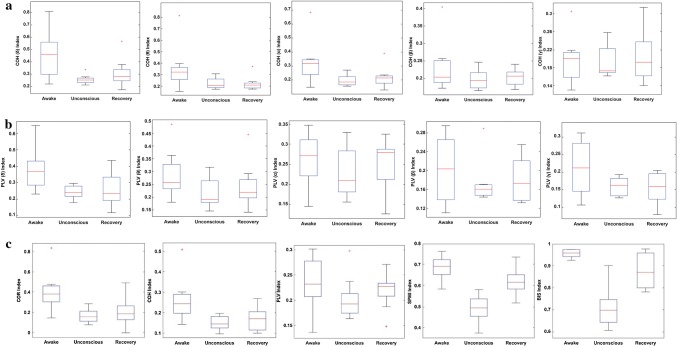
The ability of connectivity index to distinguish different anesthetic states is important for DoA monitoring. SPMI, COR, COH, and PLV index over 10-s epochs at three anesthetic states (awake, unconscious and recovery) are calculated for all subjects and the box plots of these indexes are shown in Fig. 4. This is done also for BIS index (Fig. 4). ANOVA test was used to evaluate the significant difference between awake and unconscious states and also between unconscious and recovery states for all indexes and P value of each index in different anesthesia states are calculated (Table 1). It can be seen from Fig. 4 and Table 1 that SPMI, could perfectly distinguish awake and unconscious state as well as unconscious and recovery state. However, other connectivity indexes cannot differentiate appropriately between awake and unconscious state and especially between unconscious and recovery state.
Fig. 4.
Box plots of EEG-derived functional connectivity indexes in different anesthetic states for all subjects. a, b COH and PLV in five frequency bands: Delta δ, Theta θ, Alpha α, Beta β and Gamma γ. c COR, COH (all frequency bands), PLV (all frequency bands), SPMI, and BIS index. The coefficient of variation (SD/mean) in SPMI index (0.122) is smaller than BIS index (0.131) at the unconscious state
Table 1.
P value of functional connectivity indexes (COR, COH, PLV, SPMI) and BIS index in awake and unconscious state, and also unconscious and recovery state
| Awake-unconscious | Unconscious-recovery | |
|---|---|---|
| COR | 0.003 | 0.753 |
| COH | 0.273 | 0.480 |
| PLV | 0.239 | 0.486 |
| COH(δ) | 0.016 | 0.294 |
| COH(θ) | 0.036 | 1 |
| COH(α) | 0.027 | 0.529 |
| COH(β) | 0.462 | 0.529 |
| COH(γ) | 0.834 | 1 |
| PLV(δ) | 0.009 | 1 |
| PLV(θ) | 0.141 | 0.462 |
| PLV(α) | 0.294 | 0.674 |
| PLV(β) | 0.462 | 0.916 |
| PLV(γ) | 0.294 | 0.834 |
| SPMI | 0.0007 | 0.0007 |
| BIS | 0.0009 | 0.0009 |
The P value of SPMI index is lower than BIS at awake and unconscious state as well as unconscious and recovery state which indicates that the proposed index is preferred than BIS. Moreover, the coefficient of variation of SPMI (0.122) index is considerably smaller in comparison with BIS (0.131) index at unconscious state. It should be noted that the stability of the index value during the unconscious sate is very important for the reliable DoA monitoring. These results illustrated that SPMI index directly reflects the neurophysiologic processes governing unconscious state and has a better robustness to noise than BIS during the propofol-induced anesthesia.
Discussion
Despite the widespread interest in DoA monitoring over the last decades, the neurophysiological mechanisms of anesthetic drugs are still unknown. In this study, a new nonlinear functional connectivity method named SPMI index between two-channel EEG from frontal and temporal is presented as a meaningful index for characterizing EEG dynamics during anesthesia. SPMI index showed a better performance than other indexes (COR, COH, and PLV) in evaluating EEG functional connectivity changes during anesthesia. It can follow the changes in propofol drug effect significantly (the highest PK values with Ceff (0.83)) and distinguish awake and unconscious states, and also unconscious and recovery states of the subject perfectly (Fig. 4, Table 1).
The results show several benefits of SPMI index in comparison with the BIS index. First, the BIS value has a very complicated computation and takes more time to calculate than our method (Pilge et al. 2006; Shalbaf et al. 2013; Hagihira et al. 2001). Second, artifact removal in BIS is very difficult task (Pilge et al. 2006). Even without the complex artifact removal process, the SPMI index is more robust to artifacts than the BIS is. Third, the SPMI index reacts more quickly to changing drug concentrations, particularly about the change from awake to unconscious state (Fig. 2d). Fourth, the coefficient of variation in SPMI index is considerably smaller than BIS index in the unconscious state, and has less irregularity in the unconscious state during the propofol-induced anesthesia. Fifth, the SPMI index could distinguish different states better than BIS (Fig. 4, Table 1). Finally, through the PK statistics, it is revealed that the SPMI index correlated more closely with the Ceff than the BIS index.
The MI of two random variables evaluates the mutual dependence between them; which reflects how anesthesia alters the strength of information coupling between different cortical regions. The PMI method is an extension of MI methods but uses symbolic dynamic characters to represent the EEG patterns, rather than the raw EEG waveform. Previously, the use of symbolic dynamics to generate the permutation entropy has been shown to be a robust index of anesthetic effect (Li and Wen 2017). The PMI is based on the permutation entropy; thus the PMI is an integration index of the time series, which combines the symbolic dynamic theory, entropy theory, probability theory and information theory. However, PMI is unbounded because entropies can be arbitrarily large, so it difficult to interpret the strength of the association between two time series. Consequently, we have introduced the SPMI as a possible solution for this matter.
SPMI is a method of using the probability distribution of permutation patterns in evaluating functional connectivity changes in the EEG signal during anesthesia. Propofol causes a decrease of the available permutation patterns in the frontal–temporal EEG signals during the anesthetic period. Thus the frontal–temporal EEG signals become more regular when the subject is anesthetized. This is reflected in the decrease of the SPMI. Consequently, as seen in Fig. 2a, d, the SPMI is decreased when receiving intravenous anesthetics in the unconscious state and is increased upon recovery. These results illustrated that SPMI is able to characterize the EEG information coupling change that occurred with the changing drug concentration, and is a meaningful measure for characterizing EEG dynamics during anesthesia.
There is a significant increase in synchronization between separated channel pairs during anesthesia. Independent cortical subnetworks change to uniform activity patterns, more synchronous using anesthetics (Hasenstaub et al. 2005). Moreover, long-range connection input from other cortical areas is reduced under anesthesia (Ferrarelli et al. 2010; Mhuircheartaigh et al. 2010). The growth of gamma-amino-butyric-acid (GABA)-ergic currents move the synaptic interaction towards inhibition and weaken the long-range excitatory synaptic inputs, and finally lead to more synchronization between frontal and temporal regions (Drexler et al. 2005). Additionally, while using modest doses of propofol, the main pattern is the alpha frequency “sleep spindle”-like waveform (Sleigh et al. 2009). Since this is coordinated in the thalamus, and consequently is synchronous across widely spaced cortical areas, it is highly probable to be the most influential on the increase in EEG synchrony.
The results show that anesthetics impair brain function by inducing local synchronous patterns of activity, and so obstruct an exchange of independent data between cortical areas (Ferrarelli et al. 2010; Mhuircheartaigh et al. 2010; Schrouff et al. 2011). An experiment in which rats were received visual stimuli showed that volatile anesthetics agitate anterior–posterior synchronization of field responses (Imas et al. 2006). Another research on rats shows the reduction of the independent EEG signals recorded from various hemispheres (Hudetz 2002). In humans, anesthetics decouple gamma band activity between anterior and posterior cortical areas when the patients change from the awake stage to unconsciousness (John et al. 2001). It has been shown that feedforward and feedback connectivity is significantly reduced with symbolic transfer entropy (Ku et al. 2011) and a breakdown of cortical effective connectivity is revealed in the anesthetized state (Ferrarelli et al. 2010).
The following three points should be mentioned as areas to be further explored. First, although this paper has assessed EEG signals of just eight subjects anesthetized with propofol, significant results prove that the differences must be relatively robust. Second, the confusing effects of surgical stimulation are not included in the collected data which is based on the single deepening and lightening protocol. Extra interference may influence our index at surgical levels of anesthesia, because different arousal systems induce high frequency noise on EEG signal. However, results show that our index is robust to this type of high oscillation noise. Third, due to the EEG recording limitations in the operating room, only two EEG montages from frontal and temporal were recorded in this study. It would be better to use several EEG montages and choose the optimal one to properly assess depth of anesthesia in clinical application. However, it has been shown that anesthetic drugs mostly influence the frontal and temporal cortex of the brain.
Conclusion
Functional connectivity in neuronal networks has been increasingly recognized as a key feature for establishing the interaction and communication between different regions of the brain and reflects the functional relation between spatially separate cortical signals. This study reveals that a nonlinear functional connectivity measure, named the SPMI, between pairs of EEG signals at frontal and temporal can track the changes in propofol drug effect efficiently and estimate anesthetic state of patient better than other indexes. The SPMI index is also better than the commercially available BIS index, because of faster reaction, close correlation to the effect of drug concentration, low variability at the consciousness state, and finally better ability to distinguish the three anesthetic states. The SPMI index is a promising connectivity measure for quantifying EEG dynamics during anesthesia and is a significant index for designing a new two-channel EEG monitor system for DOA.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fahimeh Afshani, Email: fahimeh.afshani@gmail.com.
Ahmad Shalbaf, Phone: 00989122163201, Email: shalbaf@sbmu.ac.ir.
Reza Shalbaf, Email: shalbaf@iricss.org.
Jamie Sleigh, Email: Jamie.Sleigh@waikatodhb.health.nz.
References
- Abásolo D, Escudero J, Hornero R, Gómez C, Espino P. Approximate entropy and auto mutual information analysis of the electroencephalogram in Alzheimer’s disease patients. Med Biol Eng Comput. 2008;46(10):1019–1028. doi: 10.1007/s11517-008-0392-1. [DOI] [PubMed] [Google Scholar]
- Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, Purdon PL. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121(5):990–998. doi: 10.1097/ALN.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandt C, Pompe B. Permutation entropy: a natural complexity measure for time series. Phys Rev Lett. 2002;88(17):174102. doi: 10.1103/PhysRevLett.88.174102. [DOI] [PubMed] [Google Scholar]
- Breakspear M. Dynamic connectivity in neural systems. Neuroinformatics. 2004;2(2):205–224. doi: 10.1385/NI:2:2:205. [DOI] [PubMed] [Google Scholar]
- Drexler B, Roether CL, Jurd R, Rudolph U, Antkowiak B. Opposing actions of etomidate on cortical theta oscillations are mediated by different γ amino butyric acid type A receptor subtypes. Anesthesiology. 2005;102:346–352. doi: 10.1097/00000542-200502000-00017. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifani P, Rabiee HR, Hashemi MH, Zadeh MS, Taslimi P, Ghanbari M. Optimal fractal-scaling analysis of human EEG dynamic for depth of anesthesia quantification. J Frankl Inst. 2007;344:212–229. [Google Scholar]
- Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–428. doi: 10.1093/bja/87.3.421. [DOI] [PubMed] [Google Scholar]
- Hagihira S, Takashina M, Mori T, Mashimo T, Yoshiya I. Practical issues in bispectral analysis of electroencephalographic signals. Anesth Analg. 2001;93:966–970. doi: 10.1097/00000539-200110000-00032. [DOI] [PubMed] [Google Scholar]
- Hall CW, Jr, Sarkar A. Mutual information in natural position order of electroencephalogram is significantly increased at seizure onset. Med Biol Eng Comput. 2011;49(2):133–141. doi: 10.1007/s11517-010-0684-0. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, et al. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Mukai N, Sawa T. Simultaneous bicoherence analysis of occipital and frontal electroencephalograms in awake and anesthetized subjects. Clin Neurophysiol. 2014;125(1):194–201. doi: 10.1016/j.clinph.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Hejazi M, Nasrabadi AM. Prediction of epilepsy seizure from multi-channel electroencephalogram by effective connectivity analysis using Granger causality and directed transfer function methods. Cogn Neurodyn. 2019 doi: 10.1007/s11571-019-09534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Wang C, Chen Y, Wang W, Du J. Long-range temporal correlations of broadband EEG oscillations for depressed subjects following different hemispheric cerebral infarction. Cogn Neurodyn. 2017;11:529–538. doi: 10.1007/s11571-017-9451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG. Effect of volatile anesthetics on interhemispheric EEG crossapproximate entropy. Brain Res. 2002;954:123–131. doi: 10.1016/s0006-8993(02)03358-9. [DOI] [PubMed] [Google Scholar]
- Imas OA, Ropella KM, Wood JD, Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–221. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology. 2000;93:1336–1344. doi: 10.1097/00000542-200011000-00029. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS, Kox W, et al. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10:165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- Ku SW, Lee U, Noh GJ, Jun IG, Mashour GA. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS ONE. 2011;6:10. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann L, et al. Tracking electroencephalographic changes using distributions of linear models: application to propofol-based depth of anesthesia monitoring. IEEE Trans Biomed Eng. 2017;64:870–881. doi: 10.1109/TBME.2016.2562261. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8(4):194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, van Engeland H, Durston S. Changes in the developmental trajectories of striatum in autism. Biol Psychiatry. 2009;66(4):327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Lee U, Kim S, Noh G-J, Choi B-M, Hwang E, Mashour GA. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009;18(4):1069–1078. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lee U, Mashour GA, Kim S, Noh G-J, Choi B-M. Propofol induction reduces the capacity for neural information integration: implications for the mechanism of consciousness and general anesthesia. Conscious Cogn. 2009;18(1):56–64. doi: 10.1016/j.concog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Li T, Wen P. Depth of anaesthesia assessment using interval second-order difference plot and permutation entropy techniques. IET Signal Proc. 2017;11:221–227. [Google Scholar]
- Li D, Voss LJ, Sleigh JW, Li X. Effects of volatile anesthetic agents on cerebral cortical synchronization in sheep. Anesthesiology. 2013;119(1):81–88. doi: 10.1097/ALN.0b013e31828e894f. [DOI] [PubMed] [Google Scholar]
- Li D, Hambrechtwiedbusch VS, Mashour GA. Accelerated recovery of consciousness after general anesthesia is associated with increased functional brain connectivity in the high-gamma bandwidth. Front Syst Neurosci. 2017;11:16. doi: 10.3389/fnsys.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang F, Wu G. Monitoring depth of anesthesia using detrended fluctuation analysis based on EEG signals. J Med Biol Eng. 2017;37:171–180. [Google Scholar]
- Liang Z, Wang Y, Sun X, Li D, Voss LJ, Sleigh JW, Hagihira S, Li X. EEG entropy measures in anesthesia. Front Comput Neurosci. 2015;18:9–16. doi: 10.3389/fncom.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Huang C, Li Y, Hight DF, Voss LJ, Sleigh JW, Li X, Bai Y. Emergence EEG pattern classification in sevoflurane anesthesia. Physiol Meas. 2018;39(4):045006. doi: 10.1088/1361-6579/aab4d0. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen YF, Fan SZ, Abbod M, Shieh JS. Quasi-periodicities detection using phase-rectified signal averaging in EEG signals as a depth of anesthesia monitor. IEEE Trans Neural Syst Rehabil Eng. 2017;2:1–13. doi: 10.1109/TNSRE.2017.2690449. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen YF, Fan SZ, Abbod MF, Shieh JS. Improved spectrum analysis in EEG for measure of depth of anesthesia based on phase-rectified signal averaging. Physiol Meas. 2017;38:116–138. doi: 10.1088/1361-6579/38/2/116. [DOI] [PubMed] [Google Scholar]
- Mateos DM, Guevara Erra R, Wennberg R, Perez Velazquez JL. Measures of entropy and complexity in altered states of consciousness. Cogn Neurodyn. 2018;12:73–84. doi: 10.1007/s11571-017-9459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckay ID, Voss LJ, Sleigh JW, Barnard JP, Johannsen EK. Pharmacokinetic pharmacodynamic modeling the hypnotic effect of sevoflurane using the spectral entropy of the electroencephalogram. Anesth Analg. 2006;102:91–97. doi: 10.1213/01.ane.0000184825.65124.24. [DOI] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, et al. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpoory Z, Nasrolahzadeh M, Mahmoodian N, Sayyah M, Haddadnia J. Complex network based models of ECoG signals for detection of induced epileptic seizures in rats. Cogn Neurodyn. 2019;13:325–339. doi: 10.1007/s11571-019-09527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- Mumtaz W, Vuong PL, Xia L, Malik AS, Rashid RBA. An EEG-based machine learning method to screen alcohol use disorder. Cogn Neurodyn. 2017;11:161–171. doi: 10.1007/s11571-016-9416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallasamy N, Tsao DY. Functional connectivity in the brain: effects of anesthesia. Neuroscientist. 2011;17(1):94–106. doi: 10.1177/1073858410374126. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ky T, Wen P, Li Y. An improving detrended moving-average method for monitoring the depth of anaesthesia. IEEE Trans Biomed Eng. 2010;57:2369–2378. doi: 10.1109/TBME.2010.2053929. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ky T, Wen P, Li Y. Consciousness and depth of anesthesia assessment based on Bayesian analysis of EEG signals. IEEE Trans Biomed Eng. 2013;60:1488–1498. doi: 10.1109/TBME.2012.2236649. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ky T, Wen P, Li Y. Monitoring the depth of anaesthesia using Hurst exponent and Bayesian methods. IET Signal Process. 2014;8:907–917. [Google Scholar]
- Nicolaou N, Georgiou J. Spatial analytic phase difference of EEG activity during anesthetic-induced unconsciousness. Clin Neurophysiol. 2014;125(10):2122–2131. doi: 10.1016/j.clinph.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Pal D, Silverstein BH, Sharba L, Li D, Hambrecht-Wiedbusch VS, Hudetz AG, Mashour GA. Propofol, sevoflurane, and ketamine induce a reversible increase in delta-gamma and theta-gamma phase-amplitude coupling in frontal cortex of rat. Front Syst Neurosci. 2017;14:41. doi: 10.3389/fnsys.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palus M. Coarse-grained entropy rates for characterization of complex time series. Physica D. 1996;93:64–77. [Google Scholar]
- Pereda E, Quiroga RQ, Bhattacharya J. Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol. 2005;77(1):1–37. doi: 10.1016/j.pneurobio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Pilge S, Zanner R, Schneider G, Blum J, Kreuzer M, Kochs EF. Time delay of index calculation: analysis of cerebral state, bispectral, and narcotrend indices. Anesthesiology. 2006;104:488–494. doi: 10.1097/00000542-200603000-00016. [DOI] [PubMed] [Google Scholar]
- Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- Saadeh W, Khan F, Altaf MAB. Design and implementation of a machine learning based EEG processor for accurate estimation of depth of anesthesia. IEEE Trans Biomed Circuits Syst. 2019;13:658–669. doi: 10.1109/TBCAS.2019.2921875. [DOI] [PubMed] [Google Scholar]
- Schrouff J, Perlbarg V, Boly M, et al. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE, Gan TJ, Domino KB. The incidence of awareness during anesthesia: a multicenter United States study. Anesth Analg. 2004;99:833–839. doi: 10.1213/01.ANE.0000130261.90896.6C. [DOI] [PubMed] [Google Scholar]
- Shalbaf R, Behnam H, Sleigh JW, Voss LJ. Using the Hilbert–Huang transform to measure the electroencephalographic effect of propofol. Physiol Meas. 2012;33:271–285. doi: 10.1088/0967-3334/33/2/271. [DOI] [PubMed] [Google Scholar]
- Shalbaf R, Behnam H, Sleigh JW, Voss LJ. Measuring the effects of sevoflurane on electroencephalogram using sample entropy. Acta Anaesthesiol Scand. 2012;56:880–889. doi: 10.1111/j.1399-6576.2012.02676.x. [DOI] [PubMed] [Google Scholar]
- Shalbaf R, Behnam H, Sleigh JW, Steyn-Ross A, Voss LJ. Monitoring the depth of anesthesia using entropy features and an artificial neural network. J Neurosci Methods. 2013;218:17–24. doi: 10.1016/j.jneumeth.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Shalbaf A, Saffar M, Sleigh JW, Shalbaf R. Monitoring the depth of anesthesia using a new adaptive neuro-fuzzy system. IEEE J Biomed Health Inform. 2018;22:671–677. doi: 10.1109/JBHI.2017.2709841. [DOI] [PubMed] [Google Scholar]
- Shalbaf A, Shalbaf R, Saffar M, et al. Monitoring the level of hypnosis using a hierarchical SVM system. J Clin Monit Comput. 2019 doi: 10.1007/s10877-019-00311-1. [DOI] [PubMed] [Google Scholar]
- Sleigh JW, Vizuete JA, Voss L, Steyn-Ross A, Steyn-Ross M, et al. The electrocortical effects of enflurane: experiment and theory. Anesth Analg. 2009;109:1253–1262. doi: 10.1213/ANE.0b013e3181add06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WD, Dutton RC, Smith NT. Measuring the performance of anesthetic depth indicators. Anesthesiology. 1996;84(1):38–51. doi: 10.1097/00000542-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005;116(10):2266–2301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Talebi N, Nasrabadi AM, Mohammad-Rezazadeh I. Estimation of effective connectivity using multi-layer perceptron artificial neural network. Cogn Neurodyn. 2018;12:21–42. doi: 10.1007/s11571-017-9453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Sleigh JW. Auditory recall and response to command during recovery from Propofol anaesthesia. Anaesth Intensive Care. 1999;27:265–268. doi: 10.1177/0310057X9902700307. [DOI] [PubMed] [Google Scholar]