Significance
The inextricable link between topsoil lead (Pb) and children’s blood lead (BPb) has not been widely accepted. Pb is associated with multiple health adversities. Urban residents are at risk from exposure to legacy Pb dust in topsoil resulting from smelting, industrial discharges, leaded gasoline emissions, leaded paint, and incineration. In New Orleans, topsoil median Pb decreased in ∼15 y from 99 mg/kg to 54 mg/kg, or ∼2.4 mg⋅kg⋅y−1. In ∼12 y, children’s median BPb declined from 3.6 μg/dL to 1.3 μg/dL, or ∼0.2 μg⋅dL⋅y−1. We argue that depletion of topsoil Pb is an important factor in the continuous decline of children’s BPb. Similar processes are expected in all US cities. Primary prevention requires curtailing Pb in all sources, including topsoil.
Keywords: urban soil, children’s blood lead, exposure map
Abstract
Lead (Pb) is extremely toxic and a major cause of chronic diseases worldwide. Pb is associated with health disparities, particularly within low-income populations. In biological systems, Pb mimics calcium and, among other effects, interrupts cell signaling. Furthermore, Pb exposure results in epigenetic changes that affect multigenerational gene expression. Exposure to Pb has decreased through primary prevention, including removal of Pb solder from canned food, regulating lead-based paint, and especially eliminating Pb additives in gasoline. While researchers observe a continuous decline in children’s blood lead (BPb), reservoirs of exposure persist in topsoil, which stores the legacy dust from leaded gasoline and other sources. Our surveys of metropolitan New Orleans reveal that median topsoil Pb in communities (n = 274) decreased 44% from 99 mg/kg to 54 mg/kg (P value of 2.09 × 10−08), with a median depletion rate of ∼2.4 mg⋅kg⋅y−1 over 15 y. From 2000 through 2005 to 2011 through 2016, children’s BPb declined from 3.6 μg/dL to 1.2 μg/dL or 64% (P value of 2.02 × 10−85), a decrease of ∼0.2 μg⋅dL⋅y−1 during a median of 12 y. Here, we explore the decline of children’s BPb by examining a metabolism of cities framework of inputs, transformations, storages, and outputs. Our findings indicate that decreasing Pb in topsoil is an important factor in the continuous decline of children’s BPb. Similar reductions are expected in other major US cities. The most contaminated urban communities, usually inhabited by vulnerable populations, require further reductions of topsoil Pb to fulfill primary prevention for the nation’s children.
Sometime in the near future it probably will be shown that the older urban areas of the United States have been rendered more or less uninhabitable by the millions of tons of poisonous industrial lead residues that have accumulated in cities during the past century.
Clair C. Patterson, NAS, 1980 (1)
Lead (Pb) poisoning has been known for at least 2,000 y, but its full extent was not recognized until the 1960s and 1970s. While commercial Pb production developed rapidly and dispersed massive quantities of Pb dust into the environment, appropriate analytical tools for measuring Pb evolved slowly (2). Pioneering research published in 1979 by Herbert Needleman (3) established that even small amounts of Pb affect children’s learning abilities. The research indicated that millions of children were being excessively exposed to Pb, resulting in long-term societal consequences. This understanding prompted the medical community’s involvement in decreasing children’s exposure to this toxic element.
Lead-based paint and tetraethyl lead (TEL), an additive to gasoline, were massive commercial sources of Pb and subsequent Pb exposure. Lead-based paint has been widely touted as the major source of childhood Pb exposure, particularly because of research conducted by employees of the Ethyl Corporation, the company responsible for TEL production (4). That report concluded: “. . . it is clear that nearly all of the Pb in dirt around these houses is due to paint from the houses. Lead antiknock additives are therefore not a significant contributor to the lead content of dirt around houses where children usually play” (4). Other researchers, however, have indicated that Pb contamination of soil from TEL is at least as important, if not more so, than lead-based paint as a source of children’s Pb exposure (5).
Primary prevention by reduction of Pb sources has markedly decreased exposure. For example, small quantities of Pb in fresh-caught tuna compared with large amounts of Pb in canned tuna prompted the elimination of Pb solder in the canning industry and investigation to remove Pb from all food products (6). The simultaneous decline of children’s blood Pb (BPb) and the decrease of TEL in gasoline spurred actions to further remove TEL from gasoline in the United States and abroad (7–9). After an initial rapid decline, children’s BPb continued to decline at a more gradual rate (10–12).
Secondary prevention was attempted using dimercaptosuccinic acid (or succimer) for intervention. A double-blind study included children who were considered (at the time) moderately exposed (∼15 μg/dL) (13). Although succimer decreased BPb in the treatment group, behavioral symptoms of the treated children became more pronounced. The study concluded: “. . . our inability to demonstrate effective treatment lends further impetus to efforts to protect children from exposure to Pb in the first place” (13). An editorial in the same journal issue suggested “the only solution” was primary prevention of Pb poisoning from lead-based paint and complete removal or replacement of lead-based paint before a child lives in a home (14). Other researchers do not support the paint-only approach because of its limited scope and the potential to increase Pb dust through the process of abatement (15).
The US lead-based paint and Pb hazard reduction programs continue to focus almost entirely on paint (16). Activities are directed at education and cleanup of dust from lead-based paint at “. . . an estimated 64 million (±7 million) homes.” These federal guidelines state that “83 (±9%) of the privately-owned housing units built before 1980 have lead-based paint somewhere in the building” (17). Assuming lead-based paint is the cause of elevated BPb, a study conducted after Hurricane Katrina predicted dire consequences for the future of New Orleans children because of unregulated, haphazard housing renovation and reconstruction throughout the city (18). As described in our study, the data from the city do not support this prediction.
To establish the need for intervention, the US childhood Pb exposure program tests children’s BPb using an arbitrary reference value to identify Pb hazards in the community. Categorically, the approach is not primary prevention because children’s BPb determines Pb cleanup actions (19). The Cochrane Collaboration, an international organization that evaluates the effectiveness of medical interventions, reviewed the US education and household intervention program for reducing children’s Pb exposure. The review concluded that the US program is ineffective for reducing children’s BPb (20).
The Cochrane Collaboration elaborated further by stating that the effects of soil remediation and/or a combination of actions on children’s exposure has not been reviewed because not enough data exist (20). In fact, the US guidelines for Pb dust cleanup preclude soil Pb (SPb) data by stating that “Soil sampling is necessary in a Pb hazard screen only if there are paint chips on the ground” (21). SPb evaluation and intervention are not included in the US program for Pb hazard mitigation. Primary prevention requires determining all sources and reducing or eliminating environmental Pb wherever it is found before, not after, children are exposed.
Despite decades of research on this topic, and in concert with the aforementioned paint-oriented focus for hazards and mitigation, the link between SPb and BPb has not been sufficiently evaluated. The goals of this study are to fill the data gap described by the Cochrane Collaboration and improve understanding about the role of SPb in children’s BPb exposure. To achieve these ends, we present data of SPb and children’s BPb and map them at the scale of a city for 2 distinct time intervals. Furthermore, we employ a metabolism of cities framework to characterize environmental signaling processes that contribute to the observed dynamics and pathways of exposure between SPb and children.
Methods
The location for this study is New Orleans, LA (30°N, 90°W). On August 29, 2005, Hurricane Katrina made landfall near metropolitan New Orleans and flooded 37% of the census tracts; 63% of the census tracts were not flooded. This study is a natural experiment of SPb and children’s BPb in residential communities. The study includes 274 (96%) of the census tracts of metropolitan New Orleans. The study was conducted by surveying each of the census tracts of the city after an interval of over a decade.
The approach is similar to methods used by John Snow, who, in 1854, mapped the occurrence of cholera in London and observed that people afflicted with cholera lived in the vicinity of the Broad Street pump for their daily water supply (22). Snow arranged to remove the pump handle, and the epidemic was arrested. The action verified that cholera is a waterborne disease (22). Snow’s methods demonstrate the need for collecting spatial data on environment and health and applying the observations to inform and advance health care in communities.
SPb Data.
SPb is invisible. Following Snow’s example, soil mapping of an entire metropolitan environment is the basis for verifying the spatial distribution of SPb. We used soil collection and analysis protocols that evolved over 3 decades, first in Baltimore, MD, and then in multiple cities in Minnesota and Louisiana, and were finally applied to more detailed study in metropolitan New Orleans (23–27). The refined protocol was applied to New Orleans collections conducted in 1999 to 2001 (i.e., the 2001 survey) and repeated in 2013 to 2017 (i.e., the 2017 survey) (27).
Topsoil samples 2 to 3 cm deep were collected for 285 census tracts of metropolitan New Orleans (27). The 1990 US census tract boundaries were used to establish the sampling areas for the 2 surveys (28). The collections consist of 5,431 topsoil samples for the 2001 survey and 5,434 topsoil samples for the 2017 survey. The 2 surveys were conducted at a median of 15 y apart. The refined protocol requires the collection of 19 topsoil samples per census tract. Sample locations were spaced to include residential sites throughout each census tract. The protocol requires collecting 4 different sample types in each census tract: 9 samples were collected within 1 m of the street (residential street-side soils); 4 samples were collected within 1 m of busy streets (busy street-side soils); 3 samples were collected within 1 m of house sides (foundation soils) and matched with street-side samples; and 3 samples were collected away from streets and foundations in yards, vacant land, or parks (open-space soils). Residential and busy street soil samples were collected in midblock locations away from intersections (27).
BPb Data.
Children’s BPb data were obtained from the Louisiana Healthy Homes and Childhood Lead Poisoning Prevention Program (LHHCLPPP) for children living in the same residential areas where SPb was surveyed (29). Individual children are unidentifiable per institutional review board requirements. In accordance with legislation, the LHHCLPPP requires case reporting of BPb of children 6 y and younger from health care providers (30). The datasets were geocoded and matched with the corresponding 1990 census tract boundaries. The criterion for inclusion in the database was at least 5 BPb samples per census tract. Of 285 census tracts, there were 274 census tracts (96.1%) that have matching SPb and BPb data over the 2 surveys. This study focuses only on results from residential areas located in 274 census tracts. The first BPb dataset was collected between 2000 and 2005 (pre-Katrina) and consists of 54,695 independent BPb results. The second BPb database was collected between 2011 and 2016 and consists of 27,249 BPb results from the same 274 census tracts. The 2 surveys for children’s BPb results were separated by a median interval of 12 y.
Geographic Information System Methods.
All data were integrated and mapped using the Environmental Systems Research Institute’s (ESRI’s) ArcGIS Desktop10 (31). The data were exported to kmz files for viewing in Google Earth or ESRI’s ArcGIS Earth.
Permutation Statistical Methods and Fisher’s Exact Test of Independence.
Statistical analysis of data often involves removal of outliers and data transformations to conform the data to the normal distribution to meet the assumptions of the statistical model. We use the multiresponse permutation procedure (MRPP) because it is a data-dependent model that uses all available, nontransformed data (32, 33). The MRPP calculates the exact moments of the underlying permutation distribution of all possible arrangements of the observed data and provides an approximate, but highly accurate, probability value without assuming normality or homogeneity of variance. The MRPP is part of a group of statistical tests developed by one of the authors (P.W.M.) and others. These procedures make statistical comparisons with distance-function based permutation tests (34).
Fisher’s exact test of independence evaluates the degree of association between 2 nominal variables. Assuming no difference, we test whether the proportions of one variable differ from another variable. In this case, we evaluate the proportions of elevated BPb in census tracts sorted, according to the median SPb, into high-Pb versus low-Pb groups. The results are expressed as a probability value of independence of the 2 variables (35).
Results
Geographic Information System View of SPb and BPb in New Orleans.
The spatial patterns of SPb and BPb in New Orleans can be visualized in Google Earth Pro or ArcGIS Earth as a kmz file. The URLs for the software and the kmz file for SPb data from 2001 and 2017 and BPb data from 2000 through 2005 and 2011 through 2016 for metropolitan New Orleans residential communities are available (SI Appendix, Fig. S1 and Dataset S1).
Declines of SPb and BPb in New Orleans.
For SPb in 274 census tracts of metropolitan New Orleans, the 2001 median was 99 mg/kg, whereas in the 2017 survey, the median was 54 mg/kg, a 44% decrease. Over 15 y, the decrease of SPb took place at a median rate, on average, of 2.4 mg⋅kg⋅y−1. The MRPP P value for the median SPb reduction between groups in the 2 surveys (n = 274) is 2.09 × 10−08.
The 2000 to 2005 children’s BPb survey result for the entire city was a median of 3.6 μg/dL, whereas the 2011 to 2016 children’s median BPb decreased 64%, to 1.3 μg/dL. The MRPP P value is 2.02 × 10−85 for the median group decrease in children’s BPb over ∼12 y for the data of the 2 surveys (n = 274). The median decline of children’s BPb was 0.2 μg⋅dL⋅y−1.
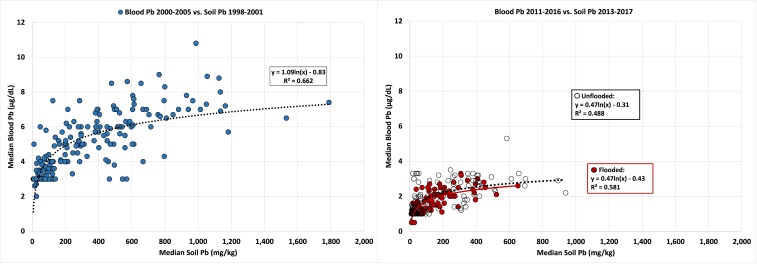
Fig. 1 plots the corresponding SPb and BPb for the 2 surveys. The interval for the SPb surveys is a median of 15 y (range: 13 to 19 y) and a median of 12 y (range: 9 to 15 y) for the 2 BPb surveys. The 2001 SPb survey was sampled from 1998 to 2001 and was matched with BPb data obtained from 2000 to 2005. The 2017 SPb survey was sampled from 2013 to 2017 and matched with BPb data obtained from 2011 to 2016.
Fig. 1.
SPb and BPb data for the 2 surveys (n = 274). (Left) Association of SPb and BPb observed in the 2001 survey. (Right) Unflooded and flooded communities distinguished to observe the effect of flooding by Hurricane Katrina on SPb and BPb. The regressions of flooded and unflooded communities are similar, showing that the reduction in SPb and BPb is occurring in all communities.
Fisher’s Exact Test of Independence.
In Table 1, the matched data for SPb and BPb are arranged in 2-by-2 contingency tables to calculate the probabilities between SPb and BPb for the 2 surveys (35). The null hypothesis is that SPb has no influence on BPb. In the 2001 survey, the median SPb is 99 mg/kg and the median BPb is 3.6 µg/dL In the 2017 survey, the median SPb is 54 mg/kg and the median BPb is 1.3 μg/dL. Fisher’s exact test P values are 2.1 × 10−17 and 7.3 × 10−11 for 2001 and 2017, respectively. These results indicate that we can reject the null hypothesis that the decline in SPb has no influence on the decline in BPb.
Table 1.
Association between BPb and SPb for 2 New Orleans surveys completed in 2001 and 2017
| Soil lead (SPb) | Census tracts (N) | Blood lead (BPb) | Census tracts (N) | Fisher’s exact test P value (ref. 35) |
| 2001 Mdn SPb 99 mg/kg | 2001 Mdn BPb 3.6 µg/dL | |||
| SPb ≥ 99 mg/kg | 137 | BPb ≥ 3.6 µg/dL | 124 | |
| SPb < 99 mg/kg | 137 | BPb < 3.6 µg/dL | 13 | 2.1 × 10−17 |
| 2017 Mdn SPb 54 mg/kg | 2017 Mdn BPb 1.3 µg/dL | |||
| SPb ≥ 54 mg/kg | 137 | BPb ≥ 1.3 µg/dL | 113 | |
| SPb < 54 mg/kg | 137 | BPb < 1.3 µg/dL | 24 | 7.3 × 10−11 |
The median (Mdn) SPb decreased from 99 mg/kg to 54 mg/kg between 2001 and 2017. The Mdn BPb declined from 3.6 μg/dL to 1.3 μg/dL in the interval between the 2 SPb surveys. The small P values indicate that the decline in BPb is not unrelated to the decline in SPb.
Discussion
For the 2001 survey in New Orleans, strong spatial associations were identified between SPb and children’s BPb (36). Thus, communities where SPb results were highest also had the highest BPb results, and, vice versa, the lowest SPb communities had the lowest children’s BPb results. The associations between SPb and children’s BPb are linked to community location because this variable corresponds to traffic volumes within individual communities, and hence differences in community Pb aerosol exposure (25, 36, 37). Our contribution is a temporal dimension from 2 surveys conducted at an interval of 12 to 15 y. The temporal dimension assists with identifying urban ecosystem processes that contribute to the continuous national decline of children’s BPb.
The Metabolism of Cities Framework and Pb Contamination at Individual Properties.
The results of 2 surveys, summarized graphically in Fig. 1, show that both SPb and children’s BPb have undergone substantial reductions during the given time interval. While these 2 phenomena may potentially be independent, the results (P < 10−11) presented in Table 1 indicate that they are not. In order to understand the intricacies of environmental dynamics and human health that pertain to decreasing SPb and declining children’s BPb, we employ a “metabolism of cities” framework (38). At the spatial scale of cities, there are attributes similar to those found in cell metabolism. The metabolism of cities includes inputs, transformations, storages, and outputs that result in variable dynamics of environmental signals affecting children’s Pb exposure (39). The result of such processes within a city are shown in the SI Appendix, Fig. S2 by the kriged SPb maps for the 2 surveys.
Individual urban properties exist within the context of the spatial distribution of SPb. In a previous study, New Orleans was divided into high-Pb (median census tract soil ≥ 100 mg/kg) and low-Pb (median census tract soil < 100 mg/kg) areas (40). Within the high-SPb communities toward the interior of the city, the sample medians for busy streets, residential streets, house side foundations, and open-space locations were 367, 313, 1,228, and 103 mg/kg, respectively. For the same series of busy streets, residential streets, house sides, and open-space locations in low-SPb areas in outlying communities of the city, the sample medians were 64, 46, 32, and 28 mg/kg, respectively. For children, the outlying communities were safer by factors ranging from 3 to 38 contingent on the specific location of the soil samples (40).
Depending on the community location of individual properties, environmental signals mirror urban patterns of Pb aerosol inputs, Pb dust deposition on topsoil, seasonal Pb dust resuspension, and aerosol transport (41, 42). The outdoor environmental signaling also contributes to indoor environmental Pb contamination via aerosol transfers and footwear track-in to home interiors (43, 44). Children are exposed to environmental Pb signals from aerosols (inhalation) or settled dusts (hand-to-mouth behavior), and children’s behavioral responses are compounded by multiple sources of contact and feedback mechanisms at play.
Air Pb Inputs, Decreases, and Legacy Pb.
Atmospheric Pb drives the metabolism of the city and, in this context, is the major input source, which is largely derived from Pb additives in gasoline. TEL is a xenobiotic additive in gasoline that was introduced into commerce in the mid-1920s (45) and became almost universally used by 1950. The Pb tonnages emitted from TEL grew exponentially through the 1950s into the 1970s and peaked in 1975 (46). TEL usage declined steadily after the 1975 catalytic converter requirement, an air pollution control device that necessitated the removal of TEL additives to prevent damaging the catalyst (47). Urban Pb dust inputs gradually declined in accord with sales of vehicles (equipped with catalytic converters) after 1975 and then decreased quickly after the January 1, 1986 rapid phase-down of TEL additives in gasoline (48). Total Pb emitted from TEL in the United States was about 6 million tons, and, in New Orleans, ∼9,100 tons of Pb were emitted as exhaust particles (49). In addition to direct deposition onto the soil, Pb aerosol particles accumulate on building surfaces and wash down into soils along foundations (50).
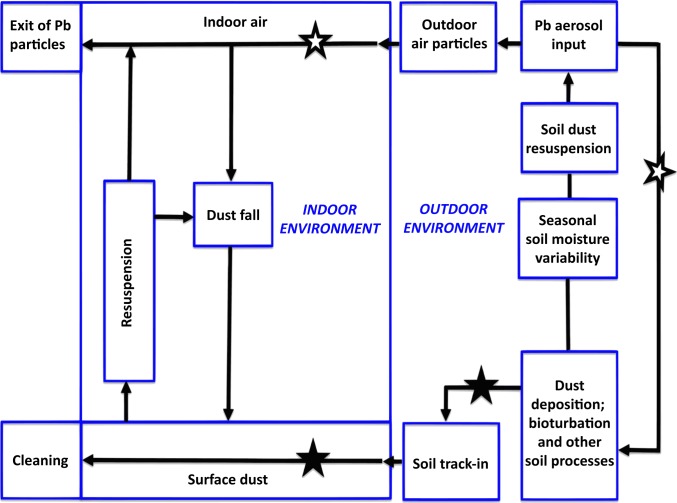
With the restriction of TEL additives, most exhaust Pb aerosols ceased and Pb loading of topsoil abruptly declined. However, the Pb stored in the topsoil remained as a legacy Pb reservoir of ongoing exposure (51). Pb species emitted from automobile exhaust are generally particulate Pb halides and double salts with ammonium halides (e.g., PbBrCl, NH4Cl). The formation of more stable PbSO4 compounds can occur quickly by reactions with neutral and acid sulfate droplets and coagulation in the atmosphere (52). Photochemical decomposition can produce Pb oxides, and the range of aged exhaust particles is most prominently found as carbonates, oxycarbonates, sulfates, and oxysulfates in soil (53). The stability of Pb in these forms, along with their strong adsorption to soil particles, is the mechanism for the relatively long residence time of Pb in soils. This process is indicated in Fig. 2, Right. On a seasonal basis, especially during late summer and early fall, soil moisture is diminished, and soil particles are resuspended into the air, resulting in seasonal increases in children’s BPb through inhalation or hand-to-mouth pathways of exposure (41, 42, 54, 55).
Fig. 2.
Environmental signaling within the context of individual properties of the city (after refs. 38, 56). Key components are inputs of air Pb, SPb deposition, and Pb particle resuspension that serve as environmental signals in children’s inhalation (open stars) and hand-to-mouth (closed stars) routes or pathways of exposure.
Environmental Signaling and Combined Issues of Children’s Pb Exposure Disparities.
The combination of traffic fueled with gasoline containing TEL plus urban traffic congestion became an international public health disaster (57, 58). Pb is a xenobiotic environmental signal, and associated long-term health damage occurs through at least 2 mechanisms. Pb mimics calcium (Ca) and is readily absorbed in its place. Ca is required for signaling across neuron synapses. If Pb is in the synapse instead of Ca, nerve transmission signals are blocked, and the neurons become weakened and die (59, 60). Multigenerational epigenetic inheritance in humans has also been shown. Genetic methylation deviation in the developing brain is associated with maternal exposure to Pb and can be inherited by grandchildren (61).
Societal consequences of Pb exposure result from multiple deleterious health impacts of Pb. Exposure is often irreversible, particularly for children, and includes behavioral or learning problems, decreased intelligence quotient, hyperactivity, delayed growth, hearing problems, anemia, kidney disease, and cancer, and, in rare cases, it can lead to seizures, coma, or death (3, 62–64). In metropolitan New Orleans, children living in communities with elevated SPb and BPb have the lowest school performance scores and, vice versa, children living in communities with lowest SPb have the highest school performance scores (65, 66). In a national study on the federal Interstate Highway System legacy SPb and preschool cognitive ability, a strong association was observed between legacy SPb and the ability of young boys (not young girls) to perform learning, memory, and decision-making activities (67). In New Orleans, the outcomes of temporal increases and decreases of TEL have been related to latent trends of aggravated assault rates that rose about 20 y later than the upswing TEL and decreased about 20 y after TEL use subsided (68). Additional societal consequences of childhood Pb exposure extend into adulthood and are characterized by psychopathology traits and mental well-being (69). Furthermore, Pb was recently cited as a “leading risk factor for premature death in the USA, particularly from cardiovascular disease,” and is responsible for 412,000 premature deaths each year (70).
While the physiological mechanisms for Pb dose–response may be similar for all children, exposure pathways are, in fact, mediated and compounded by socioeconomic factors, racial inequalities, and a host of health and nutritional factors (71, 72). Racial segregation and poverty have long been acknowledged as contributing to inequitable health outcomes for vulnerable populations in general (73) and Pb exposure in particular (74). Non-Hispanic black children were shown to be nearly 3-fold more likely than white children to have BPb > 10 μg/dL on the National Health and Nutrition Examination Survey III from 1999 to 2004 (75).
Structural factors that may explain racial disparities include socioeconomic status and education, type and age of housing, and proximity to major roads and industry (76). While the metabolism of the city could theoretically affect all residents equally, in reality, social formations produce inequitable outcomes in which vulnerable populations tend to bear greater burdens of contaminant exposure. This is, by definition, an environmental justice issue, which highlights the significance of the findings presented here.
Previous research from the 2001 survey documented similar patterns in the demographic and geographic associations between socioeconomic characteristics and SPb in New Orleans. The absolute population was found to decline as SPb levels increased, except, importantly, at concentrations ranging from 200 to 400 mg/kg to 400 to 1,000 mg/kg, where population density was found to increase. African Americans were found to comprise a disproportionate share of the population exposed to high SPb. Elevated SPb was found to occur in the inner city, which is home to the largest populations of African Americans in New Orleans (77).
Recognizing the associations of BPb and socioeconomic indicators, could the demographic changes observed between the 2 surveys be responsible for the reductions in New Orleans? The demographic characteristics of New Orleans have shifted. From 2000 to 2018, the black population declined from 67 to 59% and households with children decreased from 30 to 18%. While educational attainment is increasing at a comparable rate to the US average, poverty rates decreased only slightly, from 28 to 26% (78). With respect to socioeconomic factors, the BPb and SPb decreases may not be occurring equitably for all groups, and this topic requires further investigation (79).
Temporal Decreases of Topsoil Pb and Processes of Attenuation.
An especially intriguing outcome of the results from the interval between the 2 surveys is the observed change in SPb. Nonurban studies indicate that the amount of time required to decrease half of the Pb in the top 16- to 20-cm horizons of nonurban soil is 700 y, with less than 0.1% of the mobile Pb pool migrating out of the topsoil each year (80, 81). While the results presented here for metropolitan New Orleans refer only to the top 2 to 3 cm of the urban topsoil, we found that the 2001 median was 99 mg/kg, whereas the 2017 median was 54 mg/kg, a 44% decrease over about 15 y, or an overall median depletion rate of ∼2.4 mg⋅kg⋅y−1. The 2- to 3-cm deep topsoil of New Orleans decreased by nearly half within 2 decades, and not centuries as indicated for the 16- to 20-cm soil horizon.
Given our suggestion that SPb decreases are contributing to BPb reductions, it is important to consider mechanisms that might be causing the observed SPb declines. One potential mechanism for Pb reduction may be soil erosion. Hurricanes Katrina and Rita were responsible for depositing more than 131 × 106 metric tons of sediments on coastal lands (82). While deposition rates were highest near the coastline and decreased inland, the reduction of SPb in flooded areas is likely due more to sediment emplacement than to erosion. While this hypothesis was initially the most reasonable explanation for SPb reductions in flooded areas (83), as shown in Fig. 1, substantial SPb reductions also occur in unflooded areas of the city. Floodwater samples taken after the storms did show higher concentrations of Pb than were generally expected in stormwater runoff, with average values of 906 μg/L and 1,088 μg/L in a drainage canal in the center of the city (84). It is plausible that some of the Pb was removed during this process.
Given that unflooded areas show similar SPb declines as flooded areas, Pb mobility and translocation within soil profiles may be another explanation for the observed declines across the city. Pb mobility is dependent upon a wide range of processes governing transformation between solution and solid phases. Solid phase components, including organic matter (OM) and iron oxides, have high capacities to adsorb Pb (85). Changes in pH also affect Pb solubility, which is generally immobilized in slightly acidic to alkaline conditions (86). Soil OM decomposition also impacts Pb speciation and partitioning. For example, Pb initially adsorbed to OM surfaces can be redistributed to iron oxides and other pedogenic minerals during decomposition (87).
While Pb mobility and leaching have been documented in nonurban soils, it is important to note that processes in urban soils may differ substantially. The alluvial soils of New Orleans are generally young (classified as Entisols), contain high OM (classified as Histosols), retain moisture, drain poorly (with aquic regimes), and contain high quantities of expanding clays (smectites) (88). It is difficult to generalize about urban soils, because processes interact at various scales, but as a result of concrete and other additions, pH values in urban areas are often alkaline (89). Infiltrating rainfall may nonetheless be transporting Pb particles into deeper horizons in New Orleans. Given the limited sampling depth of the data presented here, further research is necessary to verify the mobility process.
Studies on long-term patterns of Pb accumulation often assumed that inputs of Pb are irreversibly retained. In several studies, however, Pb mobility within forested ecosystems has been shown to be greater than previously expected. For example, ∼30% of the atmospheric Pb inputs at the Hubbard Brook Experimental Forest between 1926 and 1987 was removed from soils, with potential to pollute drainage water (90). In 3 decades of research in the Vienna Woods, the declining atmospheric deposition of metals is reflected by decreases of metals in soil and foliage (91). A study of roadside SPb conducted in Israel indicated a Pb depletion rate of about 1 to 7 mg⋅kg⋅y−1 (92).
Biological activity and bioturbation in the topsoil may explain the reduction of New Orleans topsoil Pb over a short time scale. The abundance of lifeforms in topsoil ecosystems is fully recognized (93, 94). The biochemical impact of subsurface soil microorganisms on metal content in the above-ground plant growth provides evidence of processes that diminish topsoil metals. An investigation of the impact of Bacillus subtilis on fractionation, translocation, and accumulation in the subsurface rhizosphere soil of maize (Zea mays L.) found that B. subtilis tended to immobilize Pb, Sb, Ni, Zn, and Cu in the rhizosphere soil, thereby preventing the transport of these metals to plants above the soil surface (95). The low Pb organic matter transported by plants to the soil surface would attenuate the amount of Pb accumulating in topsoil. While microbial activities that regulate metal partitioning in urban contaminated soils may decrease in some cases (96), bacterial diversity and abundance may also be high in anthropogenically altered urban soils (97). The soil biological communities and activities were not assessed here. The influences of biological, physical, and chemical processes on attenuating soil metals are fundamental to understanding resilience, regeneration, and sustainability of urban ecosystems, and require ongoing investigation in urban contexts (98).
Conclusions.
Clair Patterson’s supposition that Pb in urban environments will render cities uninhabitable is supported by the legacy of Pb in soil identified in New Orleans. While SPb and BPb have declined, the levels are still higher than naturally occurring soil concentrations, and there is no known safe level of Pb in a human system. The effects of inequitable Pb exposure across class and race, along and with the lifelong health effects resulting from children’s Pb exposure, result in dire societal consequences. Because household interventions have been deemed ineffective, and environmental factors have contributed to the contamination process in the first place, we suggest that the declines in BPb in metropolitan New Orleans over 12 to 15 y can be explained by dynamic metabolism of the city processes. We contend that the various declines in inputs, transformations, storage, and outputs of Pb in the topsoil play a substantial role in reducing children’s Pb exposure.
The removal of TEL from gasoline curtailed most of the Pb dust inputs in urban topsoil. Declining air Pb had the immediate effect of reducing inhalation of Pb particles and decreasing children’s BPb. After the initial decrease, a gradual decline in children’s BPb continued. In New Orleans, topsoil Pb decreased substantially (MRPP P value of 2.4 × 10−08), and this change transformed children’s response to Pb dust with an even more marked decline (MRPP P value of 2.02 × 10−85). Decreases in resuspended Pb dust aerosols resulted in a reduction of children’s Pb inhalation and hand-to-mouth pathways of exposure in outdoor and interior environments of individual properties. The overall outcome is declining BPb in association with decreasing SPb. Given the robust statistical power of SPb and BPb declines observed between 2 surveys in New Orleans and the national restrictions of Pb aerosol inputs, along with combined erosion, leaching, and soil microbial processes, it is likely that decreases in SPb and BPb are occurring in all US urban communities.
Our findings show that SPb and BPb in New Orleans noticeably improved during an interval of 12 to 15 y. However, the current topsoil data also show that many inner-city communities remain too Pb-contaminated to appropriately support children’s health and welfare trajectory into adulthood. Along with other measures for reducing exposure and upholding the principles of environmental justice, primary prevention requires interventions that decrease SPb in excessively contaminated communities.
Supplementary Material
Acknowledgments
Trina Williams and Ngoc Huynh, LHHCLPPP, provided the blood lead data for metropolitan New Orleans. Survey 2001 was funded by Agency for Toxic Substances and Disease Registry (ATSDR) and US Department of Housing and Urban Development (HUD). Survey 2017 was funded by The Ling and Ronald Cheng Fund, Al French, Mary An Godshall, Allen and Laura Carmen, P.W.M. and Roberta R. Mielke, Thomas Beller, Jack Eichenbaum, Gabriel Filippelli, Community Church Unitarian Universalist members, and the Department of Pharmacology.
Footnotes
Competing interest statement: H.W.M. is the unremunerated president of Lead Lab, Inc., which obtained private funding to conduct the 2017 survey of New Orleans.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906092116/-/DCSupplemental.
References
- 1.Patterson C. C., “An alternative perspective-lead pollution in the human environment: Origin, extent, and significance” in Lead in the Human Environment, Committee on Lead in the Human Environment, Ed. (National Academy of Sciences, Washington, DC, 1980), pp. 265–349. [Google Scholar]
- 2.Parsons P. J., McIntosh K. G., Human exposure to lead and new evidence of adverse health effects: Implications for analytical measurements. Int. Cent. Diffr. Data 25, 283–301 (2010). [Google Scholar]
- 3.Needleman H. L., et al. , Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 300, 689–695 (1979). [DOI] [PubMed] [Google Scholar]
- 4.Haar G. T., Aronow R., New information on lead in dirt and dust as related to the childhood lead problem. Environ. Health Perspect. 7, 83–89 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mielke H. W., Reagan P. L., Soil is an important pathway of human lead exposure. Environ. Health Perspect. 106 (suppl. 1), 217–229 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Settle D. M., Patterson C. C., Lead in albacore: Guide to lead pollution in Americans. Science 207, 1167–1176 (1980). [DOI] [PubMed] [Google Scholar]
- 7.Annest J. L., et al. , Chronological trend in blood lead levels between 1976 and 1980. N. Engl. J. Med. 308, 1373–1377 (1983). [DOI] [PubMed] [Google Scholar]
- 8.Pirkle J. L., et al. , The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 272, 284–291 (1994). [PubMed] [Google Scholar]
- 9.Landrigan P. J., et al. , The Lancet Commission on pollution and health. Lancet 391, 462–512 (2018). [DOI] [PubMed] [Google Scholar]
- 10.McClure L. F., Niles J. K., Kaufman H. W., Blood lead levels in young children: US, 2009-2015. J. Pediatr. 175, 173–181 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Pantic I., et al. , Children’s blood lead concentrations from 1988 to 2015 in Mexico City: The contribution of lead in air and traditional lead-glazed ceramics. Int. J. Environ. Res. Public Health 15, 2153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsoi M.-F., Cheung C.-L., Cheung T. T., Cheung B. M. Y., Continual decrease in blood lead level in Americans: United States National Health Nutrition and Examination Survey 1999-2014. Am. J. Med. 129, 1213–1218 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Rogan W. J., et al. ; Treatment of Lead-Exposed Children Trial Group , The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N. Engl. J. Med. 344, 1421–1426 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Rosen J. F., Mushak P., Primary prevention of childhood lead poisoning–The only solution. N. Engl. J. Med. 344, 1470–1471 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Ryan D., Calls for removing all lead paint from US housing are misguided. Am. J. Public Health 103, e5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Housing and Urban Development Guidelines for the Evaluation and Control of Lead-Based Paint Hazards in Housing. Office of Healthy Homes and Lead Hazard Control 2nd ed., July 2012. https://www.hud.gov/sites/documents/SECOND_EDITION_2012.PDF. Accessed 23 September 2019.
- 17.US EPA , Report on the National Survey of Lead-Based Paint in Housing. https://www.epa.gov/sites/production/files/documents/r95-003.pdf (1995). Accessed 23 September 2019.
- 18.Rabito F. A., Iqbal S., Perry S., Arroyave W., Rice J. C., Environmental lead after Hurricane Katrina: Implications for future populations. Environ. Health Perspect. 120, 180–184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulson J. A., Brown M. J., The CDC blood lead reference value for children: Time for a change. Environ. Health 18, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nussbaumer-Streit B., et al. , Household interventions for preventing domestic lead exposure in children. Cochrane Database Syst. Rev. 10, CD006047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Office of Healthy Homes and Lead Hazard Control , Guidelines for the Evaluation and Control of Lead-Based Paint Hazards in Housing (US Department of Housing and Urban Development, 2012), ed. 2, chap. 5, section I-7, pp. 5–61. https://www.hud.gov/sites/documents/SECOND_EDITION_2012.PDF. Accessed 20 September 2019.
- 22.Johnson J., The Ghost Map: The Story of London’s Most Terrifying Epidemic—and How It Changed Science, Cities, and the Modern World (Penguin Books, NY, 2007). [Google Scholar]
- 23.Mielke H. W., et al. , Lead concentrations in inner-city soils as a factor in the child lead problem. Am. J. Public Health 73, 1366–1369 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielke H. W., Blake B., Burroughs S., Hassinger N., Urban lead levels in Minneapolis: The case of the Hmong children. Environ. Res. 34, 64–76 (1984). [DOI] [PubMed] [Google Scholar]
- 25.Mielke H. W., Adams J. L., Reagan P. L., Mielke P. W. Jr, Soil-dust lead and childhood lead exposure as a function of city size and community traffic flow: The case for lead abatement in Minnesota. Environ. Geochem. Health 9, 253–271 (1989). [Google Scholar]
- 26.Mielke H. W., Lead dust contaminated USA communities: Comparison of Louisiana and Minnesota. Appl. Geochem. 8 (suppl. 2), 257–261 (1993). [Google Scholar]
- 27.Mielke H. W., Gonzales C., Powell E., Mielke P. W. Jr, Changes of multiple metal accumulation (MMA) in New Orleans soil: Preliminary evaluation of differences between survey I (1992) and survey II (2000). Int. J. Environ. Res. Public Health 2, 308–313 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Census Bureau , 1990 Census - Census Tract Outline Maps. https://www.census.gov/geographies/reference-maps/1990/geo/1990-census-tract-maps.html. Accessed 20 September 2019. [Google Scholar]
- 29.Louisiana Healthy Homes and Childhood Lead Poisoning Prevention Program Health Health Care Provider and Parent Toolkit 2013–2015. http://ldh.la.gov/assets/oph/Center-PHCH/Center-PH/genetic/LEAD/LAHHCLPP_Toolkit-Final2013-2015.pdf. Accessed 20 September 2019.
- 30.Louisiana Department of Health and Hospitals, Bureau of Family Health , Welcome to the Louisiana Healthy Homes and Childhood Lead Poisoning Prevention Program (2015). http://ldh.la.gov/index.cfm/page/466. Accessed 23 September 2019.
- 31.ESRI , ArcGIS Desktop10 (Environmental Systems Research Institute, Redlands, CA, 2011).
- 32.Berry K. J., Johnston J. E., Mielke P. W. Jr, A Chronicle of Permutation Statistical Methods; 1920–2000, and Beyond (Springer, New York, 2014). [Google Scholar]
- 33.Cade M. K., Richards B. S., User Manual for Blossom Statistical Package (Open-File Report 2005-1353, US Geological Survey, Fort Collins Science Center, 2005). https://pdfs.semanticscholar.org/863f/fe3eb6de056481e85f5d70343cdcdcf81d00.pdf. Accessed 20 September 2019.
- 34.Mielke P. W. Jr, Berry K. J., Permutation Methods: A Distance Function Approach (Springer, NY, ed. 2, 2007). [Google Scholar]
- 35.McDonald J. H., “Fisher’s exact test of independence” Handbook of Biological Statistics (Sparky House Publishing, Baltimore, MD, 2015), pp. 77–85. http://www.biostathandbook.com/fishers.html. Accessed 20 September 2019. [Google Scholar]
- 36.Mielke H. W., Gonzales C. R., Powell E., Jartun M., Mielke P. W. Jr, Nonlinear association between soil lead and blood lead of children in metropolitan New Orleans, Louisiana: 2000-2005. Sci. Total Environ. 388, 43–53 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Mielke H. W., Laidlaw M. A. S., Gonzales C. R., Estimation of leaded (Pb) gasoline’s continuing material and health impacts on 90 US urbanized areas. Environ. Int. 37, 248–257 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Wolman A., The metabolism of cities. Sci. Am. 213, 179–190 (1965). [PubMed] [Google Scholar]
- 39.Senesi G. S., Baldassarre G., Senesi N., Radina B., Trace element inputs into soils by anthropogenic activities and implications for human health. Chemosphere 39, 343–377 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Mielke H. W., Gonzales C. R., Powell E. T., Mielke P. W., Environmental and health disparities in residential communities of New Orleans: The need for soil lead intervention to advance primary prevention. Environ. Int. 51, 73–81 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Zahran S., Laidlaw M. A. S., McElmurry S. P., Filippelli G. M., Taylor M., Linking source and effect: Resuspended soil lead, air lead, and children’s blood lead levels in Detroit, Michigan. Environ. Sci. Technol. 47, 2839–2845 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Laidlaw M. A. S., Zahran S., Mielke H. W., Taylor M. P., Filippelli G. M., Re-suspension of lead contaminated urban soil as a dominant source of atmospheric lead in Birmingham, Chicago, Detroit and Pittsburgh, USA. Atmos. Environ. 49, 302–310 (2012). [Google Scholar]
- 43.Hunt A., Johnson D. L., Suspension and resuspension of dry soil indoors following track-in on footwear. Environ. Geochem. Health 34, 355–363 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Hunt A., Johnson D. L., Griffith D. A., Mass transfer of soil indoors by track-in on footwear. Sci. Total Environ. 370, 360–371 (2006). [DOI] [PubMed] [Google Scholar]
- 45.US Public Health Service , Treasury Department and United States Public Health Service Proceedings of a Conference to Determine Whether or Not There Is a Public Health Question in the Manufacture, Distribution, or Use of Tetraethyl Lead Gasoline (Public Health Bulletin No. 158, Government Printing Office, Washington, DC, 1925). [Google Scholar]
- 46.Krachler M., Zheng J., Fisher D., Shotyk W., Direct determination of lead isotopes (206Pb, 207Pb, 208Pb) in arctic ice samples at picogram per gram levels using inductively coupled plasma-sector field MS coupled with a high-efficiency sample introduction system. Anal. Chem. 76, 5510–5517 (2004). [DOI] [PubMed] [Google Scholar]
- 47.O’Connor J. T., The automobile controversy-federal control of vehicular emissions. Ecol. Law Q. 4, 661–691 (1975). [Google Scholar]
- 48.US Senate , “Hearings before the committee on environment and public works” in Proceedings of the S2609–A Bill to Amend the Clean Air Act with Regard to Mobile Source Emission Control (Government Printing Office, Washington, DC, 1984), 98th Congress, 2nd Session.
- 49.Mielke H. W., Gonzales C. R., Mielke P. W. Jr, The continuing impact of lead dust on children’s blood lead: Comparison of public and private properties in new Orleans. Environ. Res. 111, 1164–1172 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Mielke H. W., Lead in the inner-cities. Am. Sci. 87, 62–73 (1999). [Google Scholar]
- 51.Laidlaw M. A. S., Filippelli G. M., Resuspension of urban soils as a persistent source of lead poisoning in children: A review and new directions. Appl. Geochem. 23, 2021–2039 (2008). [Google Scholar]
- 52.Biggins P. D. E., Harrison R. M., Atmospheric chemistry of automotive lead. Environ. Sci. Technol. 13, 558–565 (1979). [Google Scholar]
- 53.Olson K. W., Skogerboe R. K., Identification of soil lead compounds from automotive sources. Environ. Sci. Technol. 9, 227–230 (1975). [Google Scholar]
- 54.Laidlaw M. A. S., Mielke H. W., Filippelli G. M., Johnson D. L., Gonzales C. R., Seasonality and children’s blood lead levels: Developing a predictive model using climatic variables and blood lead data from Indianapolis, Indiana, Syracuse, New York, and New Orleans, Louisiana (USA). Environ. Health Perspect. 113, 793–800 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue J., et al. , A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 27, 411–420 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Layton D. W., Beamer P. I., Migration of contaminated soil and airborne particulates to indoor dust. Environ. Sci. Technol. 43, 8199–8205 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovarik W., Ethyl-leaded gasoline: How a classic occupational disease became an international public health disaster. Int. J. Occup. Environ. Health 11, 384–397 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Huang S., et al. , Road proximity influences indoor exposures to ambient fine particle mass and components. Environ. Pollut. 243, 978–987 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Cecil K. M., et al. , Decreased brain volume in adults with childhood lead exposure. PLoS Med. 5, e112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lidsky T. I., Schneider J. S., Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 126, 5–19 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Sen A., et al. , Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 5, 14466 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellinger D. C., The protean toxicities of lead: New chapters in a familiar story. Int. J. Environ. Res. Public Health 8, 2593–2628 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellinger D. C., Bellinger A. M., Childhood lead poisoning: The torturous path from science to policy. J. Clin. Invest. 116, 853–857 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanphear B. P., et al. , Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 113, 894–899 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mielke H. W., Berry K. J., Mielke P. W., Powell E. T., Gonzales C. R., Multiple metal accumulation as a factor in learning achievement within various New Orleans elementary school communities. Environ. Res. 97, 67–75 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Zahran S., et al. , Associations between standardized school performance tests and mixtures of Pb, Zn, Cd, Ni, Mn, Cu, Cr, Co, and V in community soils of New Orleans. Environ. Pollut. 169, 128–135 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Clay K., Portnykh M., Severnini E., The legacy lead deposition in soils and its impact on cognitive function in preschool-aged children in the United States. Econ. Hum. Biol. 33, 181–192 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Mielke H. W., Zahran S., The urban rise and fall of air lead (Pb) and the latent surge and retreat of societal violence. Environ. Int. 43, 48–55 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Reuben A., et al. , Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry 76, 418–425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanphear B. P., Rauch S., Auinger P., Allen R. W., Hornung R. W., Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 3, e177–e184 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Bellinger D. C., Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotoxicology 29, 828–832 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manton W. I., Angle C. R., Stanek K. L., Reese Y. R., Kuehnemann T. J., Acquisition and retention of lead by young children. Environ. Res. 82, 60–80 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Massey D. S., Segregation and stratification: A biosocial perspective. Du Bois Rev. 1, 7–25 (2004). [Google Scholar]
- 74.Lanphear B. P., Weitzman M., Eberly S., Racial differences in Urban children’s environmental exposures to lead. Am. J. Public Health 86, 1460–1463 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones R. L., et al. , Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988-2004. Pediatrics 123, e376–e385 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Sampson R. J., Winter A. S., The racial ecology of lead poisoning: Toxic inequality in Chicago neighborhoods, 1995-2013. Du Bois Rev. 13, 261–283 (2016). [Google Scholar]
- 77.Campanella R., Mielke H. W., Human geography of New Orleans’ high-lead geochemical setting. Environ. Geochem. Health 30, 531–540 (2008). [DOI] [PubMed] [Google Scholar]
- 78.The Data Center New Orleans , Who lives in New Orleans and Metro Parishes Now? (2019). https://www.datacenterresearch.org/data-resources/who-lives-in-new-orleans-now/. Accessed 15 August 2019.
- 79.Bullard R. D., Wright B., Race, Place, and Environmental Justice after Hurricane Katrina: Struggles to Reclaim, Rebuild, and Revitalize New Orleans and the Gulf Coast (Routledge, Boulder, 2009). [Google Scholar]
- 80.Bacon J. R., Jones K. C., McGrath S. P., Johnston A. E., Isotopic character of lead deposited from the atmosphere at a grassland site in the United Kingdom since 1860. Environ. Sci. Technol. 30, 2511–2518 (1996). [Google Scholar]
- 81.Semlali R. M., et al. , Modeling lead input and output in soils using lead isotopic geochemistry. Environ. Sci. Technol. 38, 1513–1521 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Turner R. E., Baustian J. J., Swenson E. M., Spicer J. S., Wetland sedimentation from hurricanes Katrina and Rita. Science 314, 449–452 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Mielke H. W., Gonzales C. R., Powell E. T., Soil lead and children’s blood lead disparities in pre- and post-Hurricane Katrina New Orleans (USA). Int. J. Environ. Res. Public Health 14, E407 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pardue J. H., et al. , Chemical and microbiological parameters in New Orleans floodwater following Hurricane Katrina. Environ. Sci. Technol. 39, 8591–8599 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Bargar J. R., Brown G. E., Parks G. A., Surface complexation of Pb(II) at oxide-water interfaces: II. XAFS and bond-valence determination of mononuclear Pb(II) sorption products and surface functional groups on iron oxides. Geochim. Cosmochim. Acta 61, 2639–2652 (1997). [Google Scholar]
- 86.Sauvé S., Martínez C. E., McBride M., Hendershot W., Adsorption of free lead (Pb 2+) by pedogenic oxides, Ferrihydrite, and leaf compost. Soil Sci. Soc. Am. J. 64, 595–599 (2000). [Google Scholar]
- 87.Schroth A. W., Bostick B. C., Kaste J. M., Friedland A. J., Lead sequestration and species redistribution during soil organic matter decomposition. Environ. Sci. Technol. 42, 3627–3633 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Weindorf D. C., An Update of the Field Guide to Louisiana Soil Classification (LSU Agcenter Research Bulletin 889, Baton Rouge, LA, 2008). [Google Scholar]
- 89.Herrmann D. L., Schifman L. A., Shuster W. D., Widespread loss of intermediate soil horizons in urban landscapes. Proc. Natl. Acad. Sci. U.S.A. 115, 6751–6755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson C. E., Siccama T. G., Driscoll C. T., Likens G. E., Moeller R. E., Changes in lead biogeochemistry in response to decreasing atmospheric inputs. Ecol. Appl. 5, 813–822 (1995). [Google Scholar]
- 91.Türtscher S., Berger P., Lindebner L., Berger T. W., Declining atmospheric deposition of heavy metals over the last three decades is reflected in soil and foliage of 97 beech (Fagus sylvatica) stands in the Vienna Woods. Environ. Pollut. 230, 561–573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pariente S., Zhevelev H., Sachs E., Fragin G. A., Zilbershtein M., Roadside effect on lead content in sandy soil. Catena 107, 301–307 (2019). [Google Scholar]
- 93.Bahram M., et al. , Structure and function of the global topsoil microbiome. Nature 560, 233–237 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Wilkinson M. T., Richards P. J., Humphreys G. S., Breaking ground: Pedological, geological, and ecological implications of soil bioturbation. . Earth Sci. Rev. 97, 257–272 (2009). [Google Scholar]
- 95.Li X., et al. , Occurrence, fate, and transport of potentially toxic metals (PTMs) in an alkaline rhizosphere soil-plant (Maize, Zea mays L.) system: The role of Bacillus subtilis. Environ. Sci. Pollut. Res. Int. 26, 5564–5576 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Huot H., et al. , Characterizing urban soils in New York City: Profile properties and bacterial communities. J. Soils Sediments 17, 393–407 (2017). [Google Scholar]
- 97.Scharenbroch B. C., Lloyd J. E., Johnson-Maynard J. L., Distinguishing urban soils with physical, chemical, and biological properties. Pedobiologia (Jena) 49, 283–296 (2005). [Google Scholar]
- 98.Pouyat R. V., Szlavecz K., Yesilonis I. D., Groffman P. M., Schwarz K., “Chemical, physical, and biological characteristics of urban soils” in Urban Ecosystem Ecology, Aitkenhead-Peterson J., Volder A., Eds. (Agronomy Monograph 55, Soil Science Society of America, Madison, WI, 2010), pp. 119–152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.