Significance
Posttranslational modifications of the histone 3 (H3) tail in response to stress represent highly conserved epigenetic processes. However, the regulatory function of histone phosphorylation has hardly been studied in postmitotic cardiomyocytes. Here, we identified CaMKII as a catecholamine-sensitive kinase phosphorylating H3S28. We identified CaMKII-dependent H3S28p-regulated genes including hemoglobin, pointing to a previously unrecognized role of CaMKII for cardiac hemoglobin homeostasis. This study raises fundamental questions about the role of hemoglobin in nonhematopoietic cells such as cardiomyocytes and about the role of CaMKII for hematopoesis.
Keywords: histone phosphorylation, gene expression, heart
Abstract
Sympathetic activation of β-adrenoreceptors (β-AR) represents a hallmark in the development of heart failure (HF). However, little is known about the underlying mechanisms of gene regulation. In human ventricular myocardium from patients with end-stage HF, we found high levels of phosphorylated histone 3 at serine-28 (H3S28p). H3S28p was increased by inhibition of the catecholamine-sensitive protein phosphatase 1 and decreased by β-blocker pretreatment. By a series of in vitro and in vivo experiments, we show that the β-AR downstream protein kinase CaM kinase II (CaMKII) directly binds and phosphorylates H3S28. Whereas, in CaMKII-deficient myocytes, acute catecholaminergic stimulation resulted in some degree of H3S28p, sustained catecholaminergic stimulation almost entirely failed to induce H3S28p. Genome-wide analysis of CaMKII-mediated H3S28p in response to chronic β-AR stress by chromatin immunoprecipitation followed by massive genomic sequencing led to the identification of CaMKII-dependent H3S28p target genes. Forty percent of differentially H3S28p-enriched genomic regions were associated with differential, mostly increased expression of the nearest genes, pointing to CaMKII-dependent H3S28p as an activating histone mark. Remarkably, the adult hemoglobin genes showed an H3S28p enrichment close to their transcriptional start or end sites, which was associated with increased messenger RNA and protein expression. In summary, we demonstrate that chronic β-AR activation leads to CaMKII-mediated H3S28p in cardiomyocytes. Thus, H3S28p-dependent changes may play an unexpected role for cardiac hemoglobin regulation in the context of sympathetic activation. These data also imply that CaMKII may be a yet unrecognized stress-responsive regulator of hematopoesis.
Heart failure (HF) is a leading and increasing cause of morbidity and mortality worldwide (1). A characteristic feature of HF is the chronic activation of the sympathetic nervous system that leads to sustained stimulation of β-adrenoreceptors (β-ARs) and modulation of its downstream signaling pathways (2, 3). In the β-AR signaling pathway, the adenosine 3′,5′-cyclic monophosphate-dependent protein kinase A (PKA), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and Ser/Thr protein phosphatase 1 (PP1) are well-established modulators of intracellular protein phosphorylation and cardiac function (4, 5). A multitude of gene expression profiling studies in human and experimental HF models consistently show dysregulations of specific gene expression patterns, pointing to epigenetic mechanisms in this process (6–10). Critical epigenetic mechanisms involved in HF pathogenesis are histone modifications, whereas changes in DNA methylation seem to play a rather minor role in adult cardiomyocytes (11–13). Posttranslational modifications (PTMs) of histones consist of acetylation, methylation, ubiquitination, O-linked glycosylation, and phosphorylation, all modulating chromatin structure through alterations of histone interactions with DNA and other nuclear proteins (14–19). These modifications induce either activation or repression of transcription, depending on the type of modification and on the position of the modified amino acid residue. Histone methylation, for example, at H3K9 or H3K27 are considered as stable PTMs and are necessary for development, maintenance of cell identity, and heterochromatic silencing (20, 21), whereas other modifications such as histone acetylation and, in particular, phosphorylation appear to be transient and dynamic (22). Our previous work confirmed the importance of β-AR signaling for CaMKII-dependent nucleocytoplasmic shuttling of histone deacetylase 4 (HDAC4), which leads to the derepression of the transcription factor MEF2 and subsequent up-regulation of genes with maladaptive effects on cardiac remodeling (23, 24). Previous studies suggested so-called “methyl-phospho-acetyl” switches at distinct histone lysine residues, for example, H3K27, which derepress formerly silent chromatin states into transcriptionally active domains via the polycomb repressive complex (PRC) as well as HDACs and simultaneous attraction of histone acetylases (21, 25, 26). In this scenario, phosphorylation of the neighboring H3S28 residue (H3S28p) is an upstream event and induces the switch mechanism. Moreover, histone modifications, alone or in combination, may further alter chromatin structure and/or provide a “code” for the recruitment of specific proteins to chromatin, ultimately regulating gene transcription (27, 28). There is emerging evidence that increased H3S28p correlates with chronic stress-related disease states (e.g., atherosclerosis and stroke) in mammalian postmitotic cells (29, 30). Recently, we studied a Drosophila model with ectopic expression of constitutively activated H3S28A, which prevents PRC2 binding at H3S28 (31, 32). H3S28A mutated flies showed increased longevity, stress resistance, and cardiac function, pointing to a critical role of H3S28p in the heart (32).
In the present study, we investigated the mode of H3S28p regulation and started to identify the immediate target genes. We identified high levels of H3S28p in human HF and upon β-AR stimulation in experimental models. Catecholamine-induced elevation of H3S28p was partially CaMKII-dependent and sensitive to pharmacological β-AR blockade. Genome-wide analyses identified a subset of genes (including the adult hemoglobin genes), which we refer to as CaMKII-dependent H3S28p target genes. Strikingly, the majority of H3S28p-enriched genomic loci depended on CaMKII, highlighting its general role in the regulation of this histone modification.
Results
H3S28p Is Elevated in End-Stage Human Failing Hearts.
First, we asked whether H3S28p is altered in myocardial tissues of HF patients. Therefore, we measured H3S28p in left ventricles of failing myocardium as well as myocardial tissue preparations from nonfailing hearts (NF). We found significantly higher H3S28p levels in end-stage HF compared to NF (see SI Appendix, Fig. S1 and Table S3 for patient characteristics).
H3S28p Is Catecholamine-Sensitive.
Using neonatal rat ventricular myocytes (NRVMs) we found a significant accumulation of H3S28p after preincubation with ocadaic acid (OA) in a concentration of more than 100 nM (SI Appendix, Fig. S2A). This pointed to a role of PP1 rather than PP2A, which would have been much more sensitive to OA (33). To test a potential role of PP1 for H3S28p in cardiomyocytes, we used its specific endogenous inhibitor (I-1; SI Appendix, Fig. S2B). Indeed, adenovirally overexpressed I-1 further increased H3S28p upon isoprenaline (Iso) stimulation in NRVMs (SI Appendix, Fig. S2B). Moreover, H3S28p was lower in cardiac extracts from I-1 knockout mice (KO), confirming the critical role of PP1 in dephosphorylating H3S28p in vivo (SI Appendix, Fig. S2C).
Because I-1 is a catecholamine-sensitive protein (34), these data pointed to a critical role of β-AR signaling in regulating H3S28p in the heart. Two protein kinases act typically downstream of β-ARs: PKA and CaMKII. Thus we determined in NRVMs the concentration of the β-AR agonist Iso to sufficiently induce H3S28p using a 3-min stimulation protocol. We found that 100 nM Iso was sufficient to induce H3S28p (SI Appendix, Fig. S2 D, Upper). Then we tested whether inhibition of PKA (by PKI) or CaMKII (by Autocamptide-2 Related Inhibitor Peptide [AIP]) would modulate Iso-induced H3S28p and found that 100 nM to 1 µM PKI (SI Appendix, Fig. S2 D, Middle) and 1 µM AIP (SI Appendix, Fig. S2 D, Lower) sufficiently inhibited Iso-induced H3S28p. Since PKA and CaMKII are known to be activated by β-AR in a different time course (35), we conducted kinetic analyses of H3S28p in NRVMs at different time points of Iso treatment (3 min to 24 h). We revealed a second Iso-induced increase in H3S28p starting after 2 h, which lasted up to 24 h (SI Appendix, Fig. S2 E, Upper). Both PKA inhibition by PKI and CaMKII inhibition by AIP prevented this increase in H3S28p. However, whereas the PKI effect occurred already after 2 h (SI Appendix, Fig. S2 E, Middle), the AIP effect occurred later, at 24 h (SI Appendix, Fig. S2 E, Lower). This points to 2 distinct mechanisms of H3S28 phosphorylation by 2 different downstream kinases of β-ARs, both similar in acute effects but CaMKII being apparently more specifically involved in the chronic H3S28p modulation after 24 h.
CaMKII Phosphorylates H3S28.
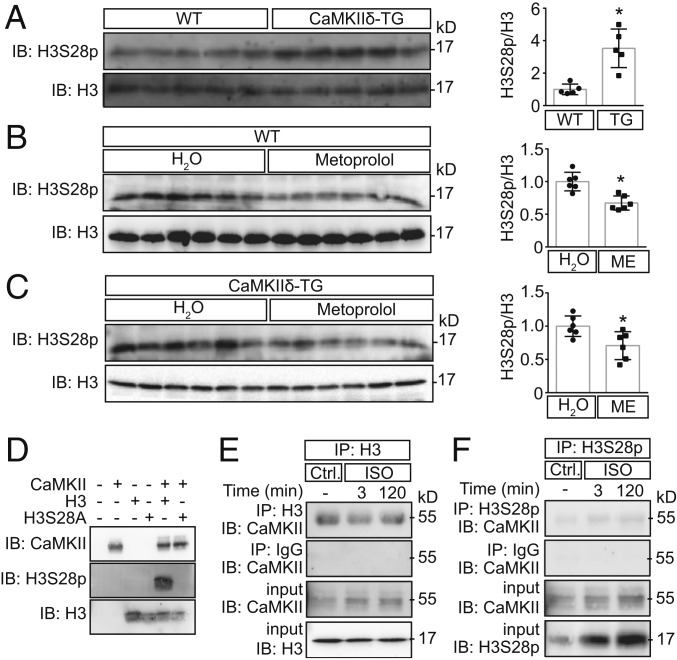
In the subsequent experiments, we focused on the specific role of CaMKII for H3S28p and used cardiac extracts from transgenic mice overexpressing CaMKIIδC (CaMKIIδ-TG) (36–38). CaMKIIδC is expressed in the cytosol and in the nucleus, although it lacks the nuclear localization signal (39). We found that H3S28p was significantly higher in CaMKIIδ-TG mice as compared to wild-type (WT) littermates (Fig. 1A). In addition, metoprolol (administered in the drinking water for 8 to 10 wk, 270 µg⋅g−1⋅d−1) lowered H3S28p in cardiac extracts from WT mice but also from mice that overexpress CaMKIIδ and thereby are characterized by a sensitized CaMKII pathway upon β-AR stimulation (Fig. 1 B and C).
Fig. 1.
CaMKII phosphorylates H3S28. Representative immunoblots and cardiac H3S28p quantification normalized to total H3 from WT and CaMKIIδ-TG (A) under basal conditions (n = 5) and (B and C) after treatment with metoprolol (ME) or water (n = 6) (*P < 0.05). (D) In vitro kinase assay using recombinant H3 tail (WT) and H3S28A mutant and CaMKIIδ in the presence of ATP. No signal was detected by using H3S28A mutant, showing the specificity of the antibody to recognize the S28 residue. (E and F) Immunoblots for CaMKII after coimmunoprecipitation using H3 or H3S28p antibodies in cardiomyocytes upon Iso stimulation. IgG coimmunoprecipitation served as a negative control.
To investigate whether CaMKII phosphorylates H3 directly, we used recombinant H3 peptides with either the WT or the H3S28A mutant sequence. The phospho-antibody detected H3S28p upon addition of recombinant enzymatically active CaMKII in WT but not in mutant (Fig. 1D), indicating direct phosphorylation of H3S28 by CaMKII.
CaMKII usually shows physical interaction with its phosphorylation targets (23). To test whether it binds to H3, we performed coimmunoprecipitation experiments using NRVMs. As shown in Fig. 1 E and F, CaMKII coimmunoprecipitated with both H3 and H3S28p. CaMKII binding to H3 seems to be independent of its activity, because Iso did not increase CaMKII binding. These data suggest that CaMKII is permanently bound to the H3 tail, and, upon its activation, it phosphorylates H3S28.
CaMKII Is Required for H3S28p.
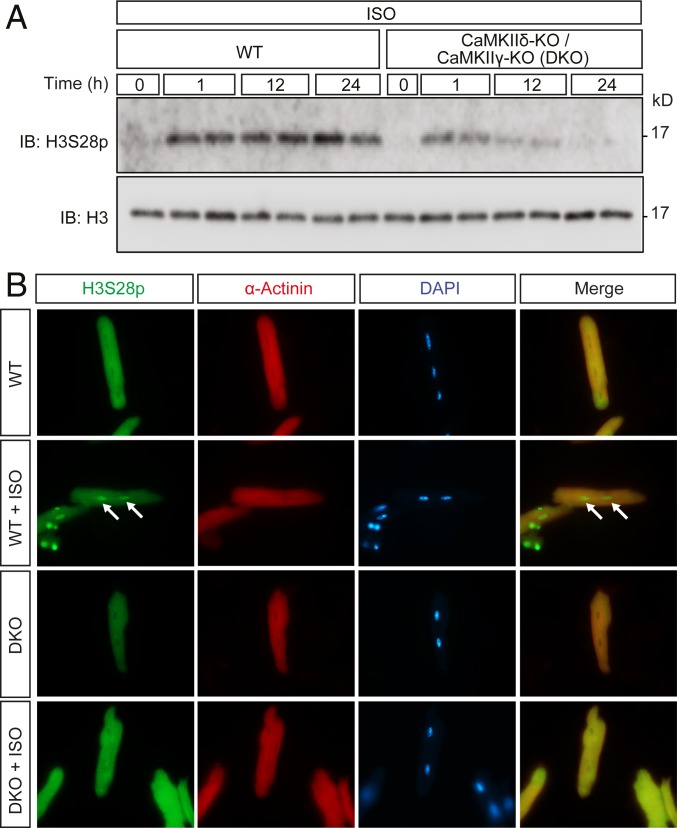
CaMKIIδ is the most abundant CaMKII gene in the myocardium (40). Immunoblot showed that H3S28p was lower in CaMKIIδ-KO as compared to WT littermates (SI Appendix, Fig. S3). We next used cardiomyocyte-specific double KO (DKO) lacking not only CaMKIIδ but also CaMKIIγ at the same time, which results in a complete loss of CaMKII activity in cardiomyocytes (40). We incubated adult mouse ventricular myocytes (AMVMs) with Iso and found higher H3S28p levels 12 h after Iso in WT, while H3S28 was still slightly phosphorylated early but not after 24 h of Iso treatment in DKO. This indicates that sustained catecholaminergic stimulation predominantly induces CaMKII-dependent H3S28p (Fig. 2A). These data confirmed the data in SI Appendix, Fig. S2E showing that CaMKII is required for H3S28p upon sustained β-AR activation. To confirm that CaMKII-dependent H3S28p occurs, indeed, in cardiomyocyte nuclei, we stained AMVMs after 24 h of Iso, with antibodies recognizing H3S28p, alpha-actinin, and DAPI (Fig. 2B). Only WT cardiomyocytes displayed a nuclear H3S28p signal upon Iso stimulation. These data prompted us to use this system for further genome-wide analyses.
Fig. 2.
CaMKII is required for H3S28p. (A) Immunoblot showing H3S28 phosphorylation in isolated AMVMs from WT or DKO mice upon Iso stimulation (100 nM) at different time intervals. (B) Immunostaining of cardiomyocytes using H3S28p (green), and α-actinin (red) antibodies and DAPI for nuclei (blue). Arrows indicate the nuclei of cardiomyocytes positive for H3S28p. (Original magnification, 63×.)
Identification of CaMKII-Dependent H3S28p Target Genes.
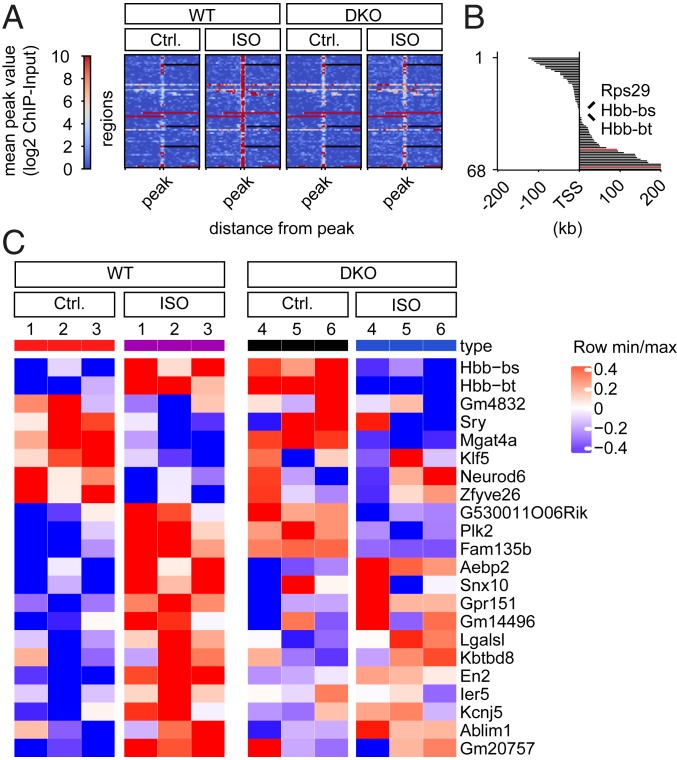
To identify direct CaMKII-dependent downstream genes, we conducted chromatin immunoprecipitation followed by massive parallel DNA sequencing (ChIP-seq). DKO and WT cells were subjected to long-term Iso treatment (24 h) to maximize CaMKII-dependent H3S28p. DiffBind Analysis (false discovery rate [FDR] 0.05) identified an enrichment of H3S28p at 68 genomic regions (Fig. 3A). Three enrichments occurred at promoter regions (Rsp29, Hbb-bs, and Hbb-bt; since Hbb-bs and Hbb-bt are duplicated genes with almost identical gene sequences, it remains unclear whether the enrichment occurs at the Hbb-bt and/or Hbb-bs locus). ChIP-PCR confirmed the enrichment at the genomic regions of Hbb-bt, Rps29, and En2 (SI Appendix, Fig. S4). Sixty-six enrichments (marked in black in Fig. 3B) did not occur in DKO (Fig. 3B and SI Appendix, Table S1), indicating that CaMKII is the predominant kinase that phosphorylates H3S28. Two enrichments (marked in red in Fig. 3B) were equally regulated in DKO, pointing to CaMKII-independently H3S28p-enriched genomic regions. Fig. 3B highlights the distance of the differential H3S28p enrichment from the transcriptional start site (TSS) of the nearest genes. SI Appendix, Table S1 lists the 68 peaks of the Iso-induced H3S28p-enriched or H3S28p-diminished genomic regions in WT and the nearest genes in WT. SI Appendix, Table S2 lists 23 peaks of Iso-induced H3S28p-enriched or H3S28p-diminished genomic regions in DKO and the nearest genes (SI Appendix). Two genomic regions overlap with regions listed in SI Appendix, Table S1 (Tmx3 and Ulbp1, red). Next, we performed a microarray analysis in an independent experiment to test whether messenger RNA (mRNA) expression correlates with H3S28p enrichment; 56 of the 66 nearest genes were covered by the Affymetrix gene chip (SI Appendix, Fig. S5), out of which 22 (39%) were differentially regulated upon Iso stimulation in WT (*P < 0.1, Fig. 3C). Moreover, 16 vs. 6 were associated with an increase in mRNA expression, indicating that H3S28p represents, rather, an activating mark. Hbb-bt/bs showed even a reciprocal behavior with up-regulation upon Iso in WT but down-regulation in DKO, raising our additional interest in globin genes, beside the fact that H3S28p enrichment occurred at the promoter region.
Fig. 3.
Identification of CaMKII-dependent H3S28p target genes. (A) Heatmap of H3S28p-enriched genomic regions in cardiomyocytes from WT (n = 68; correspond to regions with 66 different nearest genes), ordered (1 to 68) by the distance from the TSS in comparison to DKO. Heatmaps are centered to peaks (range: −2.0 kb to 2.0 kb; ±5 kb) showing the highest enrichment in WT/Iso. (B) Distances of peaks from TSS are depicted as columns next to the corresponding genomic region shown in the heatmap in A. Genomic regions corresponding to black columns are significantly H3S28p-enriched or H3S28p-diminished in WT but not in DKO; genomic regions corresponding to red columns were H3S28p-enriched or H3S28p-diminished in both WT and DKO upon Iso. (C) Gene expression analysis of the nearest genes shown in A. Only 56 (of 66) nearest genes were covered by the Affymetrix gene chip. Out of these, 22 (39%) were differentially regulated upon ISO stimulation in WT. These 22 gene expression data are shown as a hierarchically clustered heatmap. A false positive rate of a = 0.01 with FDR correction was used.
β-AR−dependent Expression of α- and β-Globin Genes.
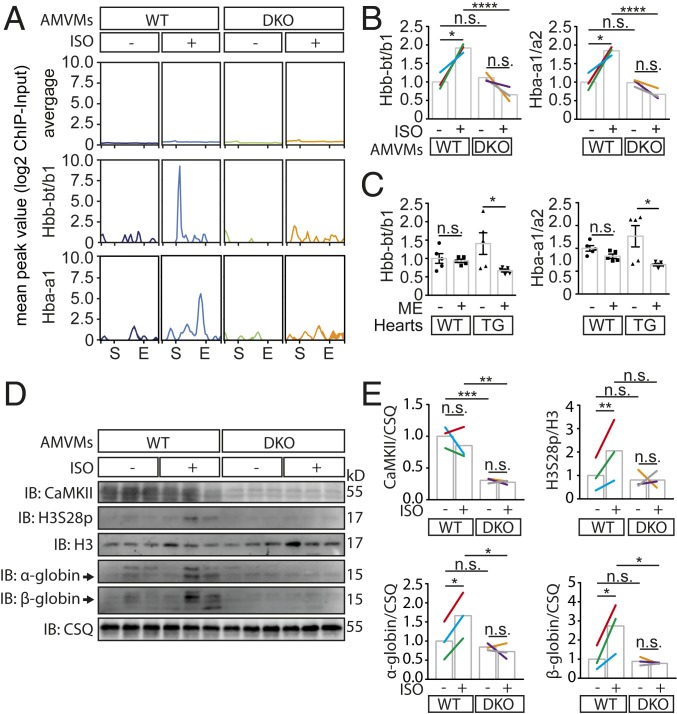
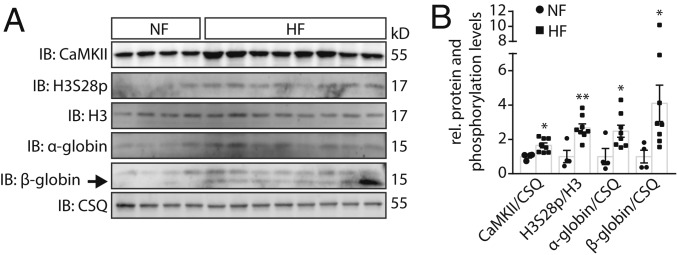
Because the globin genes are well known to be repressed by histone trimethylation of the adjunct amino acid H3K27 (H3K27me3) and to be regulated by cardiac stress (41), we further validated Hbb along with α-globins (Hba-a1/a2), which represent the adult isoform of hemoglobin. Intriguingly, H3S28p-binding occurred not only at the promoter region of Hbb-bt/bs but also at the transcriptional end site (E) of Hba-a1, which was not found by DiffBind Analysis (FDR 0.05) but by looking qualitatively at enriched H3S28p peaks (Fig. 4A). Next, we used qPCR to confirm mRNA up-regulation not only of Hbb-bt/b1 but also of Hba-a1/a2 (which was not part of the Affymetrix chip) after Iso stimulation in WT but not in DKO (Fig. 4B), indicating that the mode of regulation by H3S28p represents a group effect for the adult hemoglobin genes. To test whether H3S28p-dependent globin expression may have functional consequences in a more clinical-relevant setting, we measured cardiac gene expression upon β-blocker treatment of WT and CaMKII-TG mice. We found a trend toward higher mRNA expression in hearts of CaMKII-TG mice, which was significantly reduced upon β-AR blockade in CaMKII-TG (Fig. 4C). Next, we validated our findings at the protein level and show higher α- and β-globin abundance only in WT upon 24-h Iso incubation compared to untreated AMVMs. This again was abolished in DKO, which indicates that CaMKII is required for H3S28p upon sustained catecholaminergic stimulation. Importantly, we used isolated AMVM from the very same hearts in a paired fashion in the presence and absence of Iso, which proves that the up-regulation of α- and β-globin is a regulatory consequence of catecholaminergic stimulation and completely rules out confounding contaminations with blood cells (Fig. 4 D and E). Finally, we validated the enrichment of α- and β-globin along with elevated H3S28p and CaMKII levels in human myocardial tissues from HF patients and healthy donors (NF), showing higher CaMKII and higher H3S28p along with higher α- and β-globin protein abundance in HF in the very same samples (see Fig. 5 A and B and SI Appendix, Table S4 for patient characteristics).
Fig. 4.
β-AR-dependent expression of α- and β-globin genes. (A) H3S28p-enriched genomic regions within the Hbb-bt/bs and Hba-a1 locus in comparison with all differentially expressed genes in WT after Iso (average). These genes correspond to 1,188 genomic regions. The H3S28p enrichment peak locates to the TSS (S) of Hbb-bt/bs and to the transcriptional end site (E) of Hba-a1. (B) Relative mRNA expression of adult hemoglobin genes (Hbb-bt/bs, Hba-a1/a2) in AMVMs from WT or DKO under basal conditions and after Iso treatment (100 nM, 24 h). Colors indicate different hearts; n.s., not significant; *P < 0.05, ****P < 0.0001. (C) Relative expression levels of Hbb-bt/b1 and Hba-a1/a2 in heart tissue from WT and CaMKII-TG mice treated with metoprolol (ME) or H2O detected with qPCR; n.s., not significant; *P < 0.05. (D and E) Immunoblots and quantification showing CaMKII, H3S28p, H3, and α- and β-globin levels of Iso-treated (100 nM, 24 h) AMVMs from WT and DKO mice. Colors indicate different hearts; n.s. not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 5.
Increased protein expression of CaMKII-dependent H3S28p target genes in human HF. (A) Representative immunoblots and (B) quantification from protein lysates of left ventricular tissue of nonfailing hearts (NF, n = 4) and of patients with end-stage HF (n = 8). *P < 0.05, **P < 0.01 HF vs. NF.
Discussion
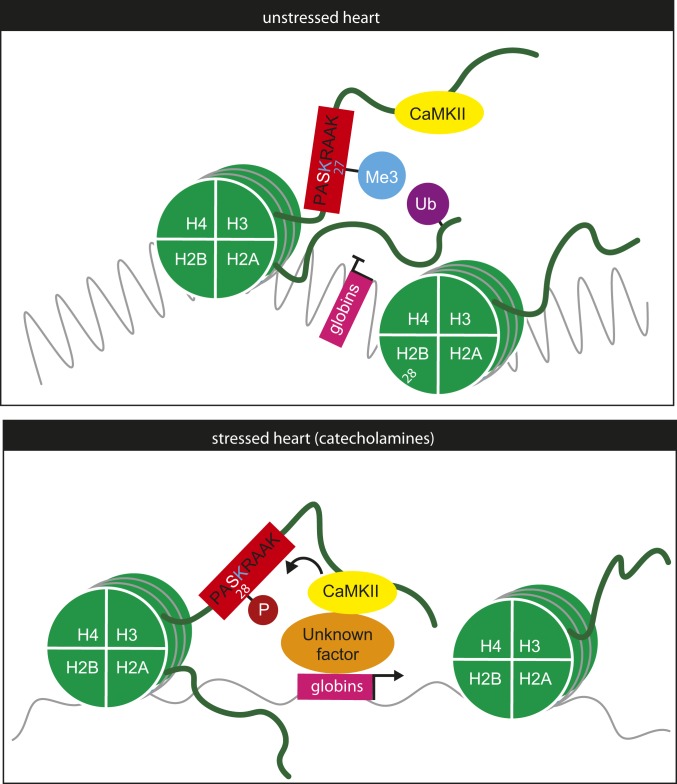
We found that, upon sympathetic stimulation, CaMKII directly phosphorylates H3S28 in cardiomyocytes. Thus, we propose to add CaMKII to the list of kinases involved in the phosphorylation of H3S28 including Msk1/2, Erk1/2, MAPK, and Aurora B (25, 26, 42). The studies that identified those kinases were done in mitotically active cells, in which H3 phosphorylation plays emerging roles for DNA stabilization during replication as well as transcription during and after mitosis (29, 30). However, we and others showed that H3S28p is also regulated in postmitotic cells, pointing to a functional role in stress responses in cardiac and neuronal cells, respectively (30, 32). Here, we demonstrate that CaMKII is sufficient and required for H3S28p upon sustained catecholaminergic stimulation. Thus we propose a molecular model in which CaMKII is bound to the H3 tail at baseline conditions and phosphorylates H3S28 upon β-AR stimulation (Fig. 6). This, in turn, induces CaMKII/H3S28-dependent target gene expression, including the 2 adult hemoglobin isoforms α- and β-globin.
Fig. 6.
Working model of CaMKII-dependent regulation of H3S28p. In the unstressed heart, CaMKII is bound to the H3 tail but does not phosphorylate H3S28, which is associated with a closed state at the genomic loci of α- and β-globins. In the stressed heart, CaMKII phosphorylates H3S28, leading to an open chromatin and gene expression of α- and β-globins that are typically repressed by H3K27me3 and H2AK119ub in nonhematopoietic cells.
Since catecholaminergic stimulation is a hallmark for the development of HF, we examined H3S28p levels in HF models and upon blockade of the β-AR system in an experimental model. An initial study from our laboratory showed high myocardial H3S28p in a canine model of pacing-induced HF (32). Here, we confirm these findings in humans and demonstrate high cardiac H3S28p levels in myocardial biopsies from patients with end-stage HF.
Numerous studies conducted gene expression analysis in response to catecholaminergic stimulation or upon pathophysiological stress leading to HF. However, it was difficult to conclude which (directly or indirectly) gene expression changes were caused by catecholamine-sensitive regulatory proteins. Here, we identified genes that are directly CaMKII/H3S28p-dependent. A certain number of these genes, like Kruppel-like factor 5 (Klf5), Polo-like kinase 2 (Plk2), or Actin Binding LIM Protein 1 (Ablim1), are regulated during heart development or cardiac differentiation processes. This might partially reflect the frequently observed “fetal response” of cardiomyocytes after chronic stress induction (43). Nevertheless, we also identified CaMKII-dependent H3S28p target genes encoding for adult hemoglobins (Hbb and Hba). Hemoglobins are well-established target genes of the polycomb complex that are typically repressed by H3K27 trimethylation (H3K27me3) and H2AK119 ubiquitination (H2AK119ub) but activated by H3K27 acetylation (H3K27ac) (44). Therefore, in future experiments, it needs to be investigated whether H3S28p cross talks to H3K27 and whether it may counteract H3K27me3 and H2AK119ub (see also Fig. 6). However, our data have further important implications that we briefly discuss in the following.
First, it is a remarkable finding of this study that hemoglobins are expressed in cardiomyocytes. We used isolated cardiomyocytes, excluding artificial contaminations with hematopoietic cells. There is one report showing that hemoglobins are expressed in the heart at the protein level (45). Our data add that detectable levels of α-globin in isolated cardiomyocytes depend on CaMKII. Hemoglobin mRNAs were recently also shown to be regulated in an experimental HF mouse model (41). Notably, GeneAtlas databases show high gene expression levels of Hbb-bt mRNA (http://biogps.org/#goto=genereport&id=101488143) and Hba-a1 mRNA (http://biogps.org/#goto=genereport&id=15122) in the heart. These databases actually show expression levels close to those in bone marrow but much higher levels than, for example, in the liver. Collectively, these data suggest that cardiac hemoglobin expression has been, so far, underestimated. Notably, cardiomyopathy is a well-known lifespan limiting complication of β-thalassemia major (caused by genetic deletion of β-globins) and is traditionally thought to be mainly mediated by iron overload (46). However, despite a decrease in mortality due to the treatment with iron chelators, the phenotype persists (both in the human disease and in mouse models) (47). From this point of view, it would be important to clinically monitor cardiac function in the context of new “curative” approaches in “ex-thalassemic” patients by hematopoietic stem cell transplantation or gene therapy (48). In these patients, only the genetic defect in hematopoietic cells would be corrected, but not in the heart. In contrast, neuronal hemoglobin was suggested to protect from hypoxia in the brain (49, 50), maybe providing a hint for a potential cardiac function. To investigate the role of cardiac hemoglobin, cell type-specific gain and loss of function studies are needed. Of note is that the existing hemoglobin mouse models consist, to the best of our knowledge, only of global gene deletions (47), and conditional cell-type specific studies have, so far, not be conducted.
Second, CaMKII is ubiquitously expressed, but most studies focused on the role of CaMKII in the brain or heart (51). Therefore, studies are needed to investigate whether CaMKII may regulate hematopoesis in a stress-responsive manner, for example, upon sympathetic stimulation. Because the 2 ubiquitously expressed CaMKII genes are CaMKIIδ and CaMKIIγ, double conditional knockouts (DKO) deficient for these 2 genes (as used here in a cardiomyocyte-specific manner) in hematopoietic cells would be needed (since global DKO are not viable) (40). Intriguingly, β-blocker treatment of HF patients has been associated with anemia (52), but, surprisingly, no follow-up studies investigated the relevance or the mode of action. Interestingly, iron therapy became a treatment option for HF patients and is currently investigated in phase III studies to explore effects on prognosis (53). Thus, our data should stimulate efforts to study anemia as a side effect of β-blocker therapy.
In summary, we identified CaMKII as a stress-responsive kinase phosphorylating H3S28 after sustained catecholaminergic stress, regulating CaMKII-dependent H3S28p target genes, including adult hemoglobins. We propose to add CaMKII to the group of stress-responsive chromatin-modifying enzymes that directly connects environmental changes to the genome. Moreover, important implications with regard to the role of hemoglobin in cardiomyocytes and the role of CaMKII for hematopoiesis arise from this study.
Materials and Methods
The study conforms to the Declaration of Helsinki and was approved by the ethics committee of the University Medical Center Göttingen (Aktenzeichen 31/9/00). Samples were obtained from healthy donor hearts that could not be transplanted for technical reasons or from explanted hearts of patients with HF. Informed written consent was obtained from all donors and patients. A detailed list of patient characteristics is shown in SI Appendix, Table S3. The animal studies were performed according to the European Community guiding principles in the care and use of animals (2010/63/UE, 22 September 2010) and were authorized by the “Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit” (Germany).
Detailed description is provided in SI Appendix, including Affymetrix gene expression (GSE131796) and ChIP.
Statistical Analysis.
Data are presented as means ± SEM. Statistical analysis was performed by unpaired Student’s t test (Fig. 1 A–C and SI Appendix, Figs. S1, S2C, and S3), paired Student’s t test (Fig. 4 B and E), or 1-way ANOVA with Bonferroni’s multiple-comparison post hoc test (SI Appendix, Fig. S2 A, B, D, and E and Fig. 4 B, C, and E) using the Graph-Pad Prism Software (V5.0, GraphPadInc). Differences were considered to be statistically significant if P < 0.05 or P < 0.1 (Fig. 3C). Statistics underlying Fig. 4A are as follows: All genes were up-regulated in WT vs. WT + Iso (P < 0.05; unpaired Student’s t test) but not regulated in KO vs. KO + Iso (P > 0.1; unpaired Student’s t test). From 650 genes, 500 were annotated in the mouse genome (mm9) and annotated to 1,188 genomic regions.
Supplementary Material
Acknowledgments
We thank Ulrike Oehl, Jutta Krebs-Haupenthal, Qiang Sun, and Joshua Hartmann for technical help. L.H.L. is a recipient of the clinician scientist program of the German Society of Cardiology. S.K. was supported by the “Habilitationsförderung für Frauen” at Technische Universität Dresden. J.B. was supported by the Sonderforschungsbereich (SFB) 1118 (Deutsche Forschungsgemeinschaft [DFG]), by the German Centre for Cardiovascular Research, and by the Grant of the “Bundesministeriums für Bildung und Forschung” (BMBF) and the Grant of the Ministerium für Wissenschaft, Forschung und Kunst (MWK) Baden Württemberg (Inflamyokard). A.E.-A. was supported by the DFG Transregio-SFB CRC/TRR 205, DFG International Research Training Group (IRTG) 2251: “Immunological and Cellular Strategies in Metabolic Disease” (ICSMD), and DFG EL-270/7-1.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816521116/-/DCSupplemental.
References
- 1.Ambrosy A. P., et al. , The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 63, 1123–1133 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Cohn J. N., et al. , Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984). [DOI] [PubMed] [Google Scholar]
- 3.Koitabashi N., Kass D. A., Reverse remodeling in heart failure–Mechanisms and therapeutic opportunities. Nat. Rev. Cardiol. 9, 147–157 (2011). [DOI] [PubMed] [Google Scholar]
- 4.El-Armouche A., Eschenhagen T., Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail. Rev. 14, 225–241 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Kreusser M. M., Backs J., Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front. Pharmacol. 5, 36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baccarelli A., Ghosh S., Environmental exposures, epigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 15, 323–329 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C. P., Bruneau B. G., Epigenetics and cardiovascular development. Annu. Rev. Physiol. 74, 41–68 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Papait R., Greco C., Kunderfranco P., Latronico M. V., Condorelli G., Epigenetics: A new mechanism of regulation of heart failure? Basic Res. Cardiol. 108, 361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirodkar A. V., Marsden P. A., Epigenetics in cardiovascular disease. Curr. Opin. Cardiol. 26, 209–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papait R., et al. , Genome-wide analysis of histone marks identifying an epigenetic signature of promoters and enhancers underlying cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 110, 20164–20169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backs J., Olson E. N., Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 98, 15–24 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Nührenberg T., Gilsbach R., Preissl S., Schnick T., Hein L., Epigenetics in cardiac development, function, and disease. Cell Tissue Res. 356, 585–600 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Gilsbach R., et al. , Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat. Commun. 9, 391 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee T., Chakravarti D., A peek into the complex realm of histone phosphorylation. Mol. Cell. Biol. 31, 4858–4873 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger S. L., The complex language of chromatin regulation during transcription. Nature 447, 407–412 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Kouzarides T., Chromatin modifications and their function. Cell 128, 693–705 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Taverna S. D., Li H., Ruthenburg A. J., Allis C. D., Patel D. J., How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat. Struct. Mol. Biol. 14, 1025–1040 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zentner G. E., Henikoff S., Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 20, 259–266 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Baek S. H., When signaling kinases meet histones and histone modifiers in the nucleus. Mol. Cell 42, 274–284 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Smith E., Shilatifard A., The chromatin signaling pathway: Diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Mol. Cell 40, 689–701 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawicka A., Seiser C., Sensing core histone phosphorylation–A matter of perfect timing. Biochim. Biophys. Acta 1839, 711–718 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth T. K., Imhof A., Fast signals and slow marks: The dynamics of histone modifications. Trends Biochem. Sci. 35, 618–626 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Backs J., Song K., Bezprozvannaya S., Chang S., Olson E. N., CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Invest. 116, 1853–1864 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann L. H., et al. , A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat. Med. 24, 62–72 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Lau P. N., Cheung P., Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes polycomb silencing. Proc. Natl. Acad. Sci. U.S.A. 108, 2801–2806 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gehani S. S., et al. , Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol. Cell 39, 886–900 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Lee J. S., Smith E., Shilatifard A., The language of histone crosstalk. Cell 142, 682–685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohl M., et al. , HDAC4 controls histone methylation in response to elevated cardiac load. J. Clin. Invest. 123, 1359–1370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawicka A., et al. , H3S28 phosphorylation is a hallmark of the transcriptional response to cellular stress. Genome Res. 24, 1808–1820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K., et al. , Neuronal necrosis is regulated by a conserved chromatin-modifying cascade. Proc. Natl. Acad. Sci. U.S.A. 111, 13960–13965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yung P. Y., Stuetzer A., Fischle W., Martinez A. M., Cavalli G., Histone H3 serine 28 is essential for efficient polycomb-mediated gene repression in Drosophila. Cell Rep. 11, 1437–1445 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Joos J. P., et al. , Ectopic expression of S28A-mutated Histone H3 modulates longevity, stress resistance and cardiac function in Drosophila. Sci. Rep. 8, 2940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber S., et al. , PDE2 at the crossway between cAMP and cGMP signalling in the heart. Cell. Signal. 38, 76–84 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Weber S., Meyer-Roxlau S., El-Armouche A., Role of protein phosphatase inhibitor-1 in cardiac beta adrenergic pathway. J. Mol. Cell. Cardiol. 101, 116–126 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Backs J., et al. , Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J. Cell Biol. 195, 403–415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewenter M., et al. , Calcium/calmodulin-dependent protein kinase II activity persists during chronic β-adrenoceptor blockade in experimental and human heart failure. Circ Heart Fail 10, e003840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T., et al. , The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ. Res. 92, 912–919 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Maier L. S., et al. , Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 92, 904–911 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Mishra S., Gray C. B., Miyamoto S., Bers D. M., Brown J. H., Location matters: Clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ. Res. 109, 1354–1362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreusser M. M., et al. , Cardiac CaM Kinase II genes δ and γ contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation 130, 1262–1273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobin S. W., et al. , Heart failure and MEF2 transcriptome dynamics in response to β-blockers. Sci. Rep. 7, 4476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giet R., Glover D. M., Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dirkx E., da Costa Martins P. A., De Windt L. J., Regulation of fetal gene expression in heart failure. Biochim. Biophys. Acta 1832, 2414–2424 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Kim Y. W., Kim A., Characterization of histone H3K27 modifications in the β-globin locus. Biochem. Biophys. Res. Commun. 405, 210–215 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Hsu P. L., et al. , Activation of mitochondrial function and Hb expression in non-haematopoietic cells by an EPO inducer ameliorates ischaemic diseases in mice. Br. J. Pharmacol. 169, 1461–1476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kremastinos D. T., et al. , Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ Heart Fail 3, 451–458 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Kumfu S., Fucharoen S., Chattipakorn S. C., Chattipakorn N., Cardiac complications in beta-thalassemia: From mice to men. Exp. Biol. Med. (Maywood) 242, 1126–1135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter J., Beyond transfusion therapy: New therapies in thalassemia including drugs, alternate donor transplant, and gene therapy. Hematology Am. Soc. Hematol. Educ. Program 2018, 361–370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terraneo L., et al. , Brain adaptation to hypoxia and hyperoxia in mice. Redox Biol. 11, 12–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Y., et al. , Effects of cerebral ischemia on neuronal hemoglobin. J. Cereb. Blood Flow Metab. 29, 596–605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckendorf J., van den Hoogenhof M. M. G., Backs J., Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 113, 29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komajda M., et al. , The impact of new onset anaemia on morbidity and mortality in chronic heart failure: Results from COMET. Eur. Heart J. 27, 1440–1446 (2006). [DOI] [PubMed] [Google Scholar]
- 53.von Haehling S., Ebner N., Evertz R., Ponikowski P., Anker S. D., Iron deficiency in heart failure: An overview. JACC Heart Fail. 7, 36–46 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.