Abstract
Siderophores, iron-scavenging small molecules, are fundamental to bacterial nutrient metal acquisition and enable pathogens to overcome challenges imposed by nutritional immunity. Multimodal imaging mass spectrometry allows visualization of host−pathogen iron competition, by mapping siderophores within infected tissue. We have observed heterogeneous distributions of Staphylococcus aureus siderophores across infectious foci, challenging the paradigm that the vertebrate host is a uniformly iron-depleted environment to invading microbes.
Keywords: siderophore, metallophore, mulitmodal molecular imaging, infectious disease, nutritional immunity
Metals are required by organisms to carry out metabolic processes (1). During infection, host metalloproteins sequester nutrient metals to prevent microbial colonization, a process termed nutritional immunity (2, 3). Bacteria have evolved sophisticated metal acquisition strategies, including the use of siderophores (4, 5). Siderophores are secondary metabolites (<1 kDa) characterized by a high binding affinity for iron (Fe) (dissociation constant [Kd] > 10−30 M) (5, 6). Staphylococcus aureus is an opportunistic pathogen that utilizes siderophores for Fe acquisition, and siderophore production is required for maximum virulence (5, 7).
One hallmark of S. aureus infection is the formation of tissue abscesses (8). Abscess architecture consists of staphylococcal abscess communities (SACs) segregated from host tissue by layers of necrotic and healthy innate immune cells (Fig. 1A) (9). In Fe-limiting environments, transcriptional repression of the S. aureus ferric uptake regulator (Fur) regulon is ceased, and bacteria increase expression of Fe acquisition machinery (5). Known mechanisms of staphylococcal Fe acquisition include heme uptake, inorganic Fe transport, and secretion of the siderophores staphyloferrin A (SA) and staphyloferrin B (SB) (10, 11). Emerging literature suggests that abscesses exhibit molecular heterogeneity, and, therefore, SACs elaborate differential gene expression, questioning the spatial and temporal importance of siderophores (12). However, the distribution of bacterial siderophores within vertebrate tissue has not been visualized. We sought to revisit the paradigm that bacteria are uniformly Fe-starved during vertebrate colonization, by mapping siderophore distributions in infected tissues using high-performance matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance imaging mass spectrometry (MALDI FT-ICR IMS) (2, 5, 12–19).
Fig. 1.
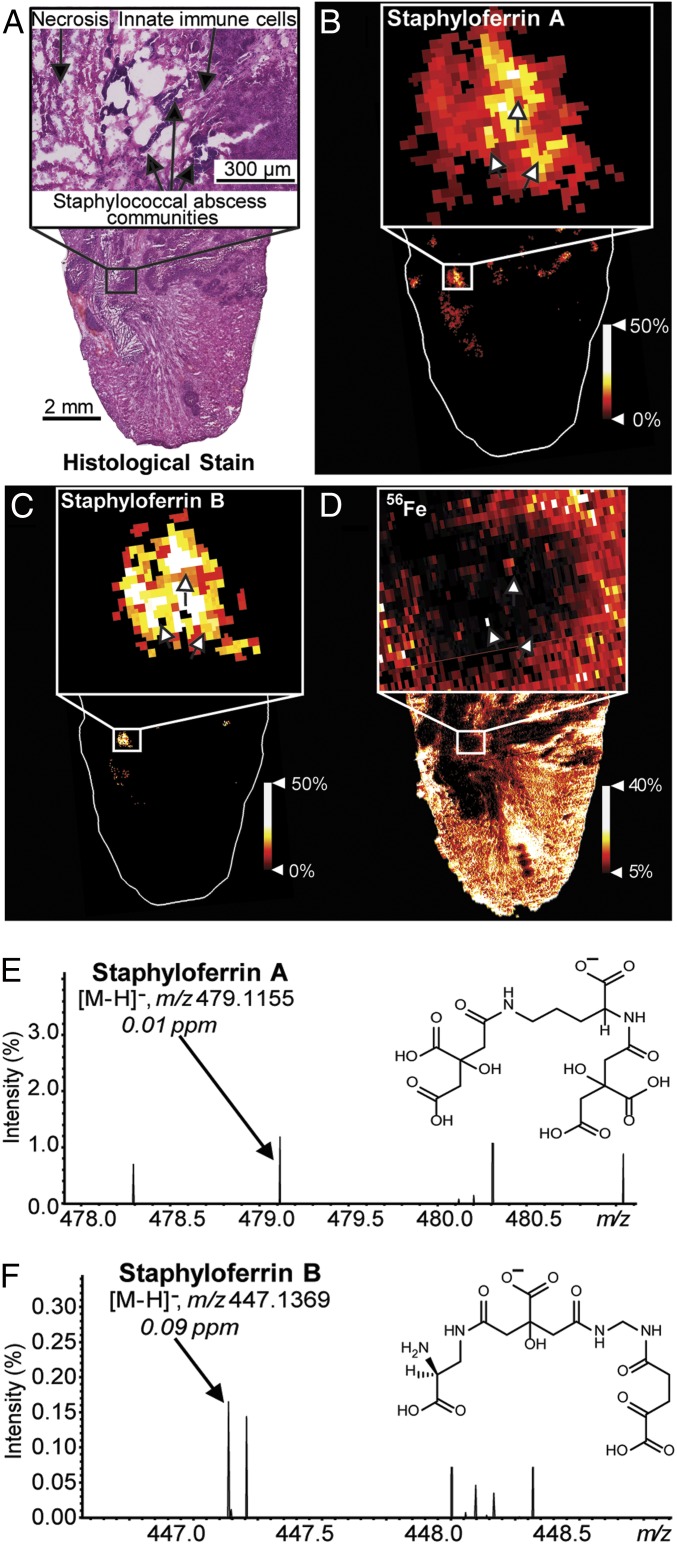
MALDI IMS reveals siderophores SA and SB within the infectious environment. (A) H&E stained abscesses. A zoom shows abscess morphology containing SACs (arrows). (B) MALDI FT-ICR IMS reveals SA colocalizing with infection sites. (C) SB is more closely localized to SACs than SA. (D) Fe distributions colocalize with select SACs (arrows). (E) A spectral zoom shows a signal corresponding to SA at m/z 479.1155, 0.01 ppm mass error, and the chemical structure of SA, [M-H]−. (F) A spectral zoom shows a signal corresponding to SB at m/z 447.1369, 0.09 ppm mass error, and the chemical structure of SB, [M-H]−.
Mice were infected with wild-type S. aureus, and tissue was harvested 7 d post infection (DPI) and frozen on dry ice (20). Tissues were sectioned serially for hematoxylin/eosin (H&E) staining, MALDI IMS, and 56Fe analysis using laser ablation—inductively coupled plasma (LA-ICP) IMS (Fig. 1 A–D). Using MALDI IMS, ions corresponding to S. aureus siderophores SA [M-H]− at m/z 479.1155 (mass accuracy: 0.01 parts per million [ppm] error) and SB [M-H]− at m/z 447.1369 (mass accuracy: 0.09 ppm error) were observed within tissue (Fig. 1 B and C). Tentative molecular identifications are based on accurate mass measurements. The SA molecular assignment was validated by MALDI IMS of mice infected with a S. aureus mutant genetically inactivated for SA production (∆sfa) (21). Methods, supplemental information, and raw data can be found at https://doi.org/10.6084/m9.figshare.9617633.v4.
Both siderophores localize to infection sites and expand beyond the perimeter of the SACs, highlighting the metabolic effort of S. aureus to acquire Fe (Fig. 1 A–C). Differences in siderophore production can be observed across abscesses. Comparing the 2 siderophore distributions, SA has increased prevalence at most infection sites. However, some foci show higher relative abundances of SB, suggesting differential Fe starvation at these sites. Notably, little to no siderophore is detected within some abscesses. Attempts to detect ferric SA and ferric SB were unsuccessful. It is possible that the complex is not stable due to the high basicity of the MALDI matrix or does not survive the MALDI process. Alternatively, but less likely, ferric SA and ferric SB are present but at low abundance and not detectable. Fe is largely excluded from infection sites (Fig. 1D). However, some pixels show colocalization of Fe to SACs (Fig. 1D, arrows), presumably highlighting successful acquisition of the metal.
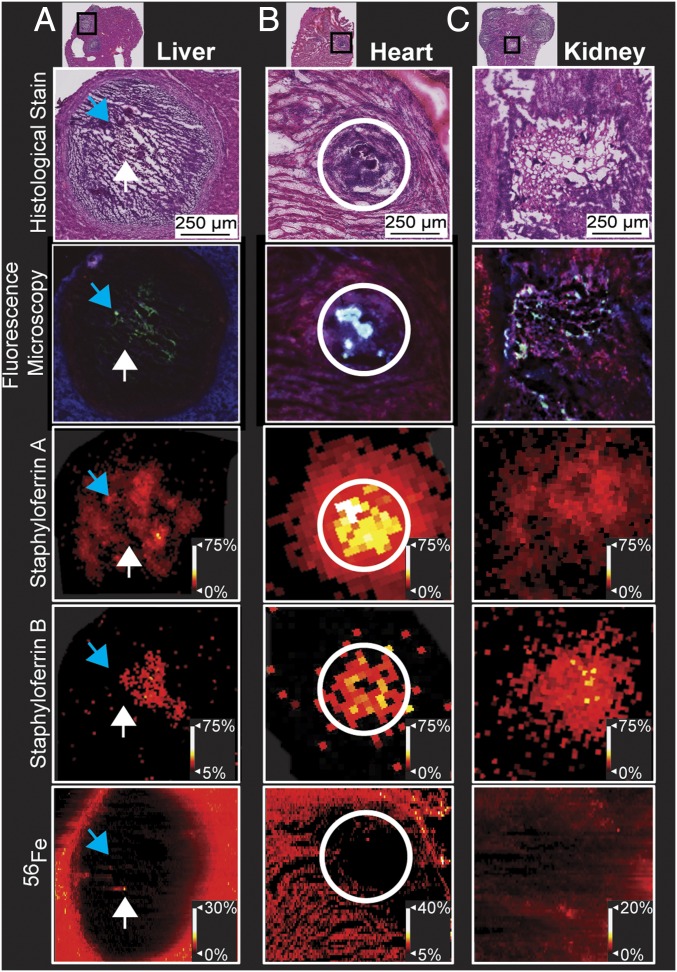
To further investigate host−pathogen Fe competition, heart, liver, and kidney lesions were compared using a multimodal approach integrating IMS, H&E staining, and fluorescence microscopy. Mice were infected with S. aureus PisdAgfp, where green fluorescent protein (GFP) expression is driven by the Fur-regulated isdA promoter (12). After 10 DPI, tissues presented with abscesses (Fig. 2). Fluorescence micrographs of GFP expression allow for a MALDI IMS-compatible technique to visualize SAC responses to host Fe sequestration (Fig. 2). Fig. 2 shows siderophores colocalizing with GFP expression. Comparing H&E stains to Fe distributions, some SACs colocalize with Fe, while others do not. This observation supports nutritional heterogeneity of SACs and suggests differential molecular responses. Siderophores are produced by S. aureus across necrotic abscesses in all tissues examined. In the heart abscess, SA extends beyond the abscess, highlighting SA diffusion (Fig. 2B). Siderophore distributions localize to GFP expression. Fe distributions within the liver and kidney lesions (Fig. 2 A and C) localize to GFP absence and lessened siderophore signals. These data highlight differential siderophore production across abscesses from liver, heart, and kidney tissues.
Fig. 2.
Multimodal imaging of 10-DPI S. aureus PisdAgfp infection characterizes utilization of SA and SB across tissue types. (A) Siderophore distributions localize to regions of staphylococcal Fe starvation (blue arrows). Fe distributions colocalize to areas that lack GFP signal in the fluorescent micrograph (white arrows). (B) Siderophore distributions in the heart expand outside of the abscess. (C) Heterogeneity in siderophore and Fe distributions as well as Fe starvation can be observed from zooms of a single kidney abscess, similar to distributions within the liver.
These results provide insight into staphylococcal metal acquisition during infection and emphasize the capabilities of IMS to investigate host−microbe interactions. While it is accepted that siderophores play a role in pathogenesis, it is less clear why bacteria produce multiple distinct siderophores. In addition to Fe, glucose represses SA production, and heme affects SB production (7, 22, 23). Furthermore, SA and SB differentially impact infection outcomes in murine models (5, 7, 21, 22). These results suggest a niche-specific role for each siderophore, rather than functional redundancy. Differential distributions of these siderophores may be explained by molecular heterogeneity within the abscess. Use of spatial molecular technologies such as MALDI IMS reveals siderophore distributions in tissue, and, when combined with multimodality integration, enables an unprecedented view of the struggle for metal between host and pathogen. The ability to image bacterial metabolites within tissue has the potential to be broadly applicable to infection biology, microbiome studies, and clinical microbiology.
Acknowledgments
We thank N. H. Patterson for assistance in elemental image generation and J. Yang for assistance in MALDI matrix application methods. Work was funded by grants from the NIH: National Institute of General Medical Sciences (2P41 GM103391-07 [to R.M.C.]) and National Institute of Allergy and Infectious Diseases (NIAID) (R01AI138581 [to E.P.S. and J.M.S.], R01AI069233 [to E.P.S.], and R01AI073843 [to E.P.S.]). The 15T FT-ICR MS and LA-ICP-MS in the Mass Spectrometry Research Center at Vanderbilt University were acquired through the NIH Shared Instrumentation Grant Programs 1S10OD012359 and 1S10RR026742-01A1, respectively. J.E.C. is supported by NIH: NIAID (1R01AI132560 and 1R01AI132560) and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. D.E.H. is supported by an operating grant from the Canadian Institutes of Health Research.
Footnotes
The authors declare no competing interest.
Data deposition: Methods, supplemental information, and raw data can be found at https://doi.org/10.6084/m9.figshare.9617633.v4.
References
- 1.Andreini C., Bertini I., Cavallaro G., Holliday G. L., Thornton J. M., Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 13, 1205–1218 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Weinberg E. D., Iron and susceptibility to infectious disease. Science 184, 952–956 (1974). [DOI] [PubMed] [Google Scholar]
- 3.Aisen P., Leibman A., Zweier J., Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 253, 1930–1937 (1978). [PubMed] [Google Scholar]
- 4.Neilands J. B., A crystalline organo-iron pigment from a rust fungus (Ustilago sphaerogena). J. Am. Chem. Soc. 74, 4846–4847 (1952). [Google Scholar]
- 5.Sheldon J. R., Heinrichs D. E., Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 39, 592–630 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Ahmed E., Holmström S. J. M., Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 7, 196–208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beasley F. C., Marolda C. L., Cheung J., Buac S., Heinrichs D. E., Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect. Immun. 79, 2345–2355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall A., Pirofski L. A., Host-pathogen interactions: Basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 68, 6511–6518 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A. G., DeDent A. C., Schneewind O., Missiakas D., A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 19, 225–232 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konetschny-Rapp S., Jung G., Meiwes J., Zähner H., Staphyloferrin A., Staphyloferrin A: A structurally new siderophore from staphylococci. Eur. J. Biochem. 191, 65–74 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Cheung J., Beasley F. C., Liu S., Lajoie G. A., Heinrichs D. E., Molecular characterization of staphyloferrin B biosynthesis in Staphylococcus aureus. Mol. Microbiol. 74, 594–608 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Cassat J. E., et al. , Integrated molecular imaging reveals tissue heterogeneity driving host-pathogen interactions. Sci. Transl. Med. 10, eaan6361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nriagu J. O., Skaar E. P., Trace Metals and Infectious Diseases Lupp J., Ed. (The MIT Press, Cambridge, MA, 2015). [PubMed] [Google Scholar]
- 14.Caprioli R. M., Farmer T. B., Gile J., Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69, 4751–4760 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Moore J. L., Caprioli R. M., Skaar E. P., Advanced mass spectrometry technologies for the study of microbial pathogenesis. Curr. Opin. Microbiol. 19, 45–51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juttukonda L. J., et al. , Dietary manganese promotes staphylococcal infection of the heart. Cell Host Microbe 22, 531–542.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehl-Fie T. E., et al. , MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect. Immun. 81, 3395–3405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spraggins J. M., et al. , MALDI FTICR IMS of intact proteins: Using mass accuracy to link protein images with proteomics data. J. Am. Soc. Mass Spectrom. 26, 974–985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakeman C. A., et al. , The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 7, 11951 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrum B., et al. , A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 7, 233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beasley F. C., et al. , Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol. Microbiol. 72, 947–963 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Sheldon J. R., Marolda C. L., Heinrichs D. E., TCA cycle activity in Staphylococcus aureus is essential for iron-regulated synthesis of staphyloferrin A, but not staphyloferrin B: The benefit of a second citrate synthase. Mol. Microbiol. 92, 824–839 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Laakso H. A., Marolda C. L., Pinter T. B., Stillman M. J., Heinrichs D. E., A heme-responsive regulator controls synthesis of staphyloferrin B in Staphylococcus aureus. J. Biol. Chem. 291, 29–40 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]