Significance
The currently used treatment regimen for the cancers of the adrenal cortex involves mitotane, a nonspecific derivative of the pesticide DDT (1,1-(dichlorobiphenyl)-2,2-dichloroethane), which has an unclear mechanism of action. Our studies in cell culture and genetic analysis of public databases demonstrate that human adrenocortical carcinomas (ACCs) are remarkably sensitive to a recently defined cell death pathway referred to as ferroptosis, indicating that induction of ferroptosis could be a promising treatment approach for ACCs. However, mitotane does not induce ferroptosis. Given the untoward side effect profile of mitotane, we suggest that ferroptosis-inducing agents may represent a more specific, more potent, and less toxic approach to treatment for patients with ACC than mitotane.
Keywords: adrenal, ferroptosis, regulated necrosis, endocrine tumors, adrenocortical carcinoma
Abstract
Adrenocortical carcinomas (ACCs) are rare and highly malignant cancers associated with poor survival of patients. Currently, mitotane, a nonspecific derivative of the pesticide DDT (1,1-(dichlorobiphenyl)-2,2-dichloroethane), is used as the standard treatment, but its mechanism of action in ACCs remains elusive. Here we demonstrate that the human ACC NCI-H295R cell line is remarkably sensitive to induction of ferroptosis, while mitotane does not induce this iron-dependent mode of regulated necrosis. Supplementation with insulin, transferrin, and selenium (ITS) is commonly used to keep NCI-H295R cells in cell culture. We show that this supplementation prevents spontaneous ferroptosis, especially when it contains polyunsaturated fatty acids (PUFAs), such as linoleic acid. Inhibitors of apoptosis (zVAD, emricasan) do not prevent the mitotane-induced cell death but morphologically prevent membrane blebbing. The expression of glutathione peroxidase 4 (GPX4) in H295R cells, however, is significantly higher when compared to HT1080 fibrosarcoma cells, suggesting a role for ferroptosis. Direct inhibition of GPX4 in H295R cells led to high necrotic populations compared to control, while cotreatment with ferrostatin-1 (Fer-1) completely reverted ferroptosis. Interestingly, the analysis of public databases revealed that several key players of the ferroptosis pathway are hypermethylated and/or mutated in human ACCs. Finally, we also detected that growth hormone-releasing hormone (GHRH) antagonists, such as MIA602, kill H295R cells in a nonapoptotic manner. In summary, we found elevated expression of GPX4 and higher sensitivity to ferroptosis in ACCs. We hypothesize that instead of treatment with mitotane, human adrenocortical carcinomas may be much more sensitive to induction of ferroptosis.
Adrenocortical carcinomas (ACCs) are rare cancers associated with limited options of treatment and poor survival of patients (1, 2). If ACCs can be surgically resected, they are currently treated with mitotane and a combination of chemotherapy (etoposide/doxorubicin/cisplatin). Unresectable ACCs are only treated with mitotane. In all cases, survival rates remain low, despite the completion of 2 clinical trials in 2007 and 2012 (3). More recently, comprehensive studies of genetic changes within these cancers (4, 5) have indicated clinical care guided by genomics (6), but molecular mechanisms of the evolution of ACCs remain elusive. Cell culture experiments on cell lines of ACC patients, such as NCI-H295R cells (herein referred to as H295R cells), have been problematic as this cell line is widely used for ACC research but kept under unphysiological conditions with special supplementation of the cell culture media requiring insulin, transferrin, selenium, and linoleic acid. Previous data based on morphology have pointed toward a central role of cell death in ACCs (7), and further analyses of positivity of TdT-mediated dUTP-biotin nick end labeling (TUNEL) in ACC cells (8) support this notion. However, today it is known that TUNEL positivity occurs in many regulated cell death pathways beyond classical apoptosis (9, 10). Regulated necrosis includes ferroptosis (11) and necroptosis (12), 2 pathways that were recently identified as important pathophysiological features of myocardial infarction (13, 14), stroke and neurodegeneration (15–17), acute kidney injury (18–20), sepsis (21), intoxications, and others (22). In contrast to apoptosis, which is immunologically uninvolved, all pathways of regulated necrosis release damage associated molecular patterns and are highly immunogenic (23, 24). The inflammation following regulated necrosis is referred to as necroinflammation and is thought to represent an important feature of the success of cancer immunotherapy (25, 26).
Here we demonstrate that ACCs are highly sensitive to induction of ferroptosis and that mitotane induces a nonapoptotic, nonnecroptotic, nonferroptotic necrotic cell death. Implementation of this particular mitotane-induced cell death kills ACCs much less efficiently when compared to ferroptosis inducing agents (FINs). This is in line with mutations of human ACC that significantly affect ferroptosis regulating genes. We hypothesize that FINs will be very effective in the treatment of human ACCs once they enter clinical trials.
Results
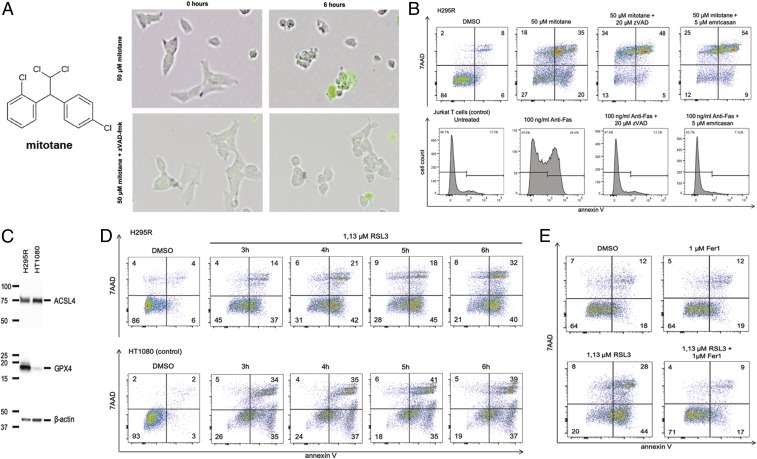
Healthy adrenocortical tissue exhibits a certain percentage of TUNEL positivity, whereas this feature is lost almost completely in ACCs (SI Appendix, Fig. S1A), indicating that ACCs generally are less sensitive to cell death when compared with normal adrenal tissue (10). Mitotane (Fig. 1A) is used as the standard treatment of ACCs with the purpose to kill the tumor cells. To understand the mechanism of mitotane-induced cell death, we investigated morphological changes in H295R cells in the presence of the necrosis marker SYTOX green in time lapse videos following exposure to 50 µM mitotane (Fig. 1A and Movie S1). Membrane blebbing and SYTOX positivity occurred within the first 6 h. The process of membrane blebbing was prevented by the pan-caspase inhibitor zVAD-fmk (herein referred to as zVAD). Unexpectedly, however, zVAD did not inhibit SYTOX positivity in these cells (Fig. 1A and Movie S2). When cell death was assessed by flow cytometry measuring the cell death marker 7AAD, indicating membrane rupture, simultaneously to annexin V, which represents a marker for phosphatidylserine exposure to the outer leaflet of the plasma membrane, neither zVAD nor the pan-caspase inhibitor emricasan prevented the H295R cells from 7AAD positivity (Fig. 1B). As a control of the function of caspase inhibitors, Jurkat T cells were treated with 100 ng/mL anti-Fas, a process that reliably induces apoptosis. In these controls, the caspase inhibitors used reversed annexin V positivity (Fig. 1B) and caspase-3 cleavage that was not observed in H295R cells treated with mitotane (SI Appendix, Fig. S1B). These experiments suggested that mitotane induces a necrotic cell death in H295R cells.
Fig. 1.
ACCs are sensitive to ferroptosis induction. (A) Structure of mitotane and time lapse video screen shots of H295R cells induced to undergo necrosis by mitotane. Membrane blebbing was sensitive to caspase inhibition by zVAD-fmk. (B) Caspase inhibition does not prevent mitotane-induced cellular necrosis after 6 h of incubation. Jurkat T cells serve as controls. (C) Expression of GPX4 in H295R cells in comparison with HT1080 cells, the standard cell line for ferroptosis research. (D) H295R cells are sensitive to the type 2 ferroptosis inducer RSL3 (HT1080 cells serve as controls). (E) RSL3-induced ferroptosis in H295R cells is prevented by addition of the small molecule ferrostatin-1 (Fer-1). All experiments were repeated at least 3 times; representative examples of primary data are shown.
Regulated necrosis can be mediated by necroptosis and relies on the proteins receptor-interacting protein kinase 1 (RIPK1, RIPK3) and mixed lineage kinase domain-like protein (MLKL) (12). The expression of comparably low levels of RIPK1 was detected in H295R cells, whereas RIPK3 and MLKL could not be detected in significant amounts in this cell line (SI Appendix, Fig. S1C). In contrast, the key molecule required for the prevention of ferroptosis, glutathione peroxidase 4 (GPX4) (27–29), was expressed at much higher concentration in H295R cells as compared to the commonly used ferroptosis-sensitive cell line HT1080 (Fig. 1C). In contrast, another enzyme required for ferroptosis, acyl-CoA synthetase long-chain family member 4 (ACSL4) (30–32), was equally expressed in both cell lines (Fig. 1C). Inhibition of the enzymatic activity of GPX4 by the small molecule RSL3 (33) efficiently induced necrotic cell death in H295R and HT1080 cells within 6 h (Fig. 1D). As previously described for HT1080 cells (34), ferroptosis induced by RSL3 in H295R cells was efficiently prevented by coincubation with 1 µM ferrostatin-1 (Fer-1) (Fig. 1E). We concluded that H295R cells are exquisitely sensitive to induction of ferroptosis by RSL3.
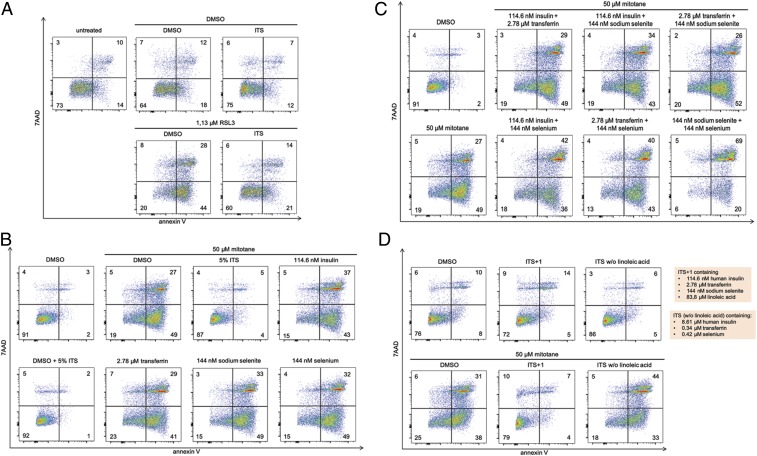
Previously published reports on H295R cells generally use an ITS supplement that contains insulin, transferrin, selenium, and linoleic acid (SI Appendix, Fig. S2A). We found that cells in culture without ITS supplementation exhibited a higher rate of spontaneous necrosis and lesser double negative staining in the FACS analysis of 7AAD and annexin V (Fig. 2A). Supplementation with ITS had a remarkable protective effect on the induction of RSL3-induced ferroptosis (Fig. 2A). We therefore decided to analyze the ferroptosis preventing factor in the ITS supplement.
Fig. 2.
ITS supplements prevent ferroptotic cell death in H295R cells. (A) H295R cells were treated with the type 2 ferroptosis inducer RSL3 for 6 h in the presence or absence of ITS (see also SI Appendix, Fig. S2, for details). Insulin, transferrin, selenium, and sodium selenite do not prevent mitotane-induced necrosis, either when applied (B) as single components or (C) in combination. (D) The absence of linoleic acid, however, resulted in failure of ITS to inhibit mitotane-induced necrosis. All experiments were repeated at least 3 times; representative examples of primary data are shown.
GPX4 is a selenoprotein, and selenium is a component of the ITS supplement. Selenium supplementation can be performed by providing selenium or sodium selenite to the cell culture (SI Appendix, Fig. S2B). After confirming that 50 µM mitotane exhibits a maximal cell death inducing effect on H295R cells (SI Appendix, Fig. S2C), we investigated the effect of equimolar concentrations of insulin, transferrin, sodium selenite, and selenium compared to ITS supplement and found that none of these components altered mitotane-induced cell death (Fig. 2B). We also confirmed that such combinations did not induce cell death on their own, without mitotane (SI Appendix, Fig. S2 D and E). Also, combinations of any of these factors did not prevent mitotane-induced necrosis (Fig. 2C and SI Appendix, Fig. S2D). We additionally confirmed that the presence or absence of 5% ITS did not affect the efficacy of Fer-1 to inhibit RSL3-induced ferroptosis (SI Appendix, Fig. S2G). To assess whether selenium has an impact on mitotane-induced cell death, we gradually increased the concentration up to 720 nM selenium, but no difference was detected (SI Appendix, Fig. S2F). In contrast, in ITS media that did not contain the polyunsaturated fatty acid (PUFA), linoleic acid failed to prevent mitotane-induced necrosis (Fig. 2D). These data indicate that the ITS supplementation prevents spontaneous necrosis and mitotane-induced necrosis by providing PUFAs as ROS scavenger (SI Appendix, Fig. S2H).
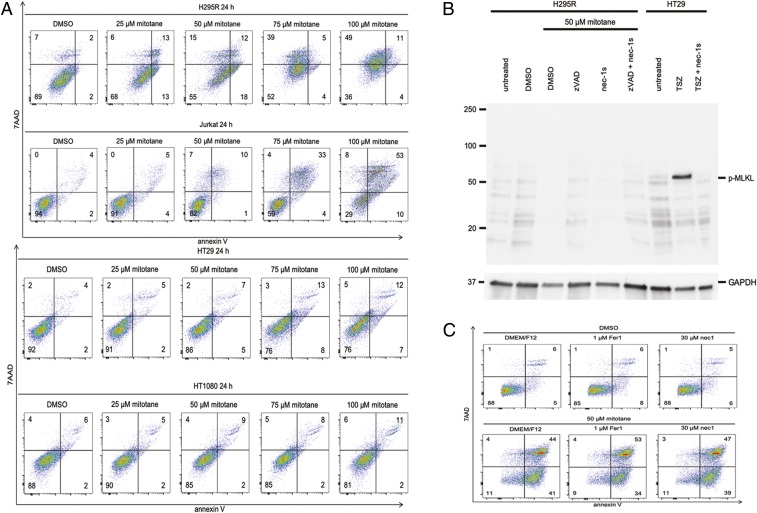
Next, we decided to continue work on the mechanism of mitotane-induced cell death in the light of the currently known pathways of regulated necrosis. Unlike H295R cells, the prototype cell lines of apoptosis (Jurkat T cells), necroptosis (HT29 cells), and ferroptosis (HT1080 cells) were not sensitive to mitotane-induced cell death at concentrations of 50 µM (Fig. 3A). Some toxicity, however, was noticed in Jurkat T cells with 100 µM mitotane, which we consider unphysiologically high (SI Appendix, Fig. S3A). Similarly, no effect of mitotane was detected on other cell lines commonly used in cell death research, such as L929, HEK, and NIH 3T3 cells (SI Appendix, Fig. S3B).
Fig. 3.
Mitotane induces a specific form of cell death. (A) H295R cells, Jurkat T cells, HT29 cells, and HT1080 cells were treated with different doses of mitotane for 24 h. At 50 µM, the number of annexin V/7AAD double negative cells significantly decreased in the H295R cells, but all other cell lines were resistant. (B) Western blot for human phosphorylated MLKL, the downstream mediator of necroptosis. Mitotane does not induce necroptosis, as controlled by HT29 cells that were treated with TNFα, smac mimetics, and a pan-caspase inhibitor zVAD (together indicated as TSZ). GAPDH serves as a loading control. (C) Mitotane does not induce ferroptosis, as indirectly demonstrated by the absence of a protective effect of Fer-1 or Nec-1. All experiments were repeated at least 3 times; representative examples of primary data are shown.
One candidate pathway of regulated necrosis is necroptosis. We found that addition of the highly potent and specific necroptosis inhibitor necrostatin-1s (Nec-1s, 7-O-Cl-Nec-1) prevented necroptosis induced by TNFα, the smac mimetic birinapant, and zVAD-fmk (referred to as TSZ treatment) in HT29 cells but had no effect on mitotane-induced necrosis (SI Appendix, Fig. S2C). In line with these observations, TSZ treatment allowed detection of the necroptosis mediator phospho-MLKL (pMLKL) in HT29 cells, but as expected, no phosphorylation of MLKL was observed in H295R cells upon treatment with mitotane (Fig. 3B). As observed for necroptosis, the ferroptosis inhibitors Fer-1 and Nec-1 did not exhibit any influence on mitotane-induced cell death (Fig. 3C). The presented data indicate that a nonnecroptotic, nonferroptotic, nonapoptotic cell death pathway is induced by mitotane. Importantly, however, the ACCs appeared to be much more sensitive to ferroptosis inducers (e.g., RSL3). We therefore hypothesized that alteration of ferroptosis controlling proteins might be a means of malignant transformation of ACC.
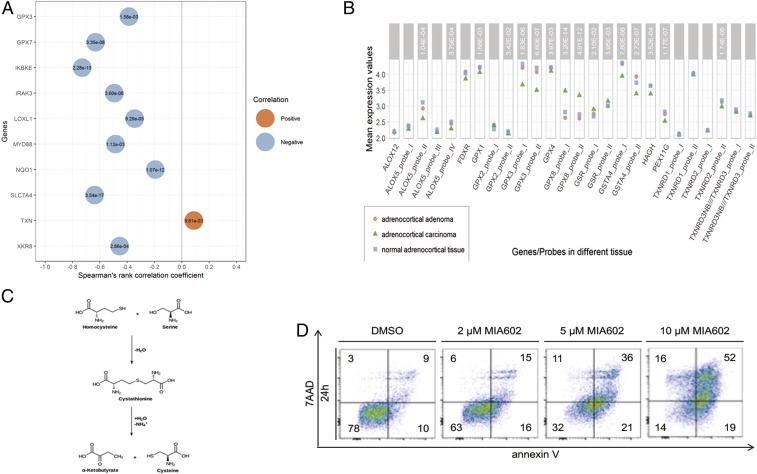
Mitochondria represent an important source of oxygen radicals that have been demonstrated to contribute to ferroptosis sensitivity (35). In keeping with this finding, mitochondria from adrenocortical tissues exhibit a unique ultrastructure (SI Appendix, Fig. S4A) that has been described morphologically (36), but a potential connection to ferroptosis has not been considered. To investigate if human ACCs mutate ferroptosis proteins, we took advantage of 2 recently published public databases. A comprehensive pangenomic database of ACC patients (5) exhibited several ferroptosis-associated genes that are mutated and/or hypermethylated (Fig. 4A and SI Appendix, Fig. S4B). Importantly, genes associated with glutathione metabolism were altered in a highly significant manner. Examples of these proteins are glutathione peroxidases 3 and 5 (GPX3, GPX5), lipoxygenase L1, the phospholipid scramblase cofactor XKR8, and others (Fig. 4A). As expected, expression analysis was further confirmed by Giordano et al. (4), the examination of which confirmed the GPX3 and GPX5 findings and further pointed to a key role of GPX4, which is highly expressed in the adrenal in general, not restricted to ACCs (Fig. 4B). In addition to these findings, the Giordano et al. dataset exhibited highly significant changes in cystathionine gamma lyase (CTH, cystathionase [SI Appendix, Fig. S4C]), an enzyme that is critically involved in the generation of α-ketoglutarate and cysteine from cystathionine (Fig. 4C) (4, 5). In summary, the cell culture and the human data clearly point to a high ferroptosis sensitivity of ACCs.
Fig. 4.
Analyses of public databases on human ACCs suggest a role for glutathione peroxidases and ferroptosis. (A) Gene expression and DNA methylation of epigenetically silenced genes in human ACCs, according to Zheng et al. in ref. 5. (B) Selected differentially expressed genes in normal adrenocortical tissue, adrenocortical adenomas and ACCs indicate a role for ferroptosis sensitivity in ACCs. (C) Cystathionase (CTH cystathionine gamma-lyase) catalyzed reaction and generation of cysteine, initially identified in ref. 4. (D) Five µM of MIA602 are sufficient to kill more than 50% of H295R cells in cell culture.
Several endocrine tumors, such as pheochromocytoma, have been demonstrated to be sensitive to induction of cell death by growth hormone-releasing hormone (GHRH) antagonists (37). MIA602 is a GHRH antagonist, and it has been shown to induce cell death in other endocrine cells (38, 39). The expression of GHRH receptor in ACCs (4) prompted us to investigate the effects of MIA602 on H295R cells. As demonstrated in Fig. 4D, whereas 5 µM MIA602 killed more than 50% of H295R cells within 24 h, 10 µM MIA602 was sufficient to kill more than 85% of H295R cells, even in the presence of ITS supplement. MIA602 at 20 µM concentration induced necrosis in H295R cells, inhibition of apoptosis (zVAD), necroptosis (Nec-1s), and the combination of apoptosis and necroptosis, and the inhibition of ferroptosis (Fer-1) did not prevent MIA620-induced cell demise. In summary, our results suggest that MIA602 or an inducer of ferroptosis might be useful to kill ACCs.
Discussion
Our work demonstrates the sensitivity of ACCs to ferroptosis induction. Given the current inadequate treatment options for ACCs, such as mitotane, our findings point toward a better targeted therapy by inducing ferroptosis. To the best of our knowledge, in comparison with all other tumor cells investigated so far, no other cell line exhibits a higher GPX4 expression than H295R cells. The exquisite sensitivity of ferroptosis is further supported by the analysis of the 2 public databases containing solid expression profiles of ACCs (Fig. 4).
The protein p53 has been reported to be frequently mutated in ACCs (6, 40). It is worth mentioning that p53, besides its plethora of functions in cancers (41), was attributed important implications in regulation of ferroptosis (42, 43). Specifically, this might point to ferroptosis in ACCs induced by metabolic stress (44).
Glutathione peroxidases (GPX3, GPX4, and GPX7) are highly redundant proteins (45). It is therefore not surprising to see cancer mutation in ACCs in particularly this class of proteins, all of which might add to the protection against ferroptosis in a redundant manner. The most likely benefit for the tumor is the alteration of ferroptosis sensitivity as the absence or the inhibition of GPX4 is sufficient to kill kidney tubular cells (46) or adrenal cells, respectively. In addition, the cystathionine gamma lyase (cystathionase) regulates sensitivity to ferroptosis indirectly and represents yet another clinical target for the control of the ferroptosis set point.
Finally, treating ACCs with mitotane has significant limitations of treatment, and it is well recognized that novel treatment options, such as anti-PD-1-treatment employing nivolumab (47), are urgently awaited. However, anti–PD-1–based approaches may rely on the necroinflammatory capacity of necrosis (26), e.g., as driven by ferroptosis, as demonstrated in models of myocardial necrosis (48, 49). Induction of ferroptosis, as recently demonstrated in mice with IKE (imidazole ketone erastin) (50), might represent a future treatment option for ACC patients. However, compounds such as the GHRH antagonist MIA602 are also capable of effectively killing ACCs, but the mechanism of this cell death remains obscure because inhibition of apoptosis, necroptosis, and ferroptosis does not affect the activity of MIA602. Importantly, we suggest that the current standard therapy with mitotane be revisited, given the side effect profile of this drug. In this sense, preclinical data on both MIA602 and ferroptosis inducers such as IKE are urgently awaited.
Materials and Methods
Cell Lines and Cell Culture Conditions.
All of the cell lines used were grown in a humidified 5%CO2/95%O2 atmosphere at 37 °C. NCI-H295R (gender: female) were cultured in DMEM/F12 (Modified) medium (Gibco; 11330032) supplemented with 2.5% Nu-Serum (Corning Nu-serum Culture Supplement; 355500) and 1% penicillin-streptomycin (Gibco; 15140122). Jurkat T cells were cultured in RPMI (Modified) (Gibco; 21875091) supplemented with 10% FBS (Gibco; 10270106) and 1% penicillin-streptomycin. HT29, HT1080, L929, NIH 3T3, and HEK cells were cultured in DMEM (Modified) medium (Gibco; 41966029) supplemented with 10% FBS and 1% penicillin-streptomycin.
Nu-Serum and FBS were heat inactivated in 56 °C water bath for 20 min and filtered afterward before any use. The GHRH antagonist MIA602 was synthetized in the laboratory of A.V.S.
Plating and Treatment of Cells.
For detaching the H295R cells from the flasks Accutase (Thermo Fisher; 00455556) was used, while for HT29, HT1080, L929, NIH/3T3, and HEK cells, Trypsin-EDTA (Gibco; 25200056) was used. Afterward, cells were washed with their normal medium twice, and Ficoll Paque Plus (Sigma–Aldrich; GE17-1440-02) gradient was performed before every experimental procedure. Cells were seeded in 6-well plates (Sarstedt; 83.3920) (5 × 105 cells per well for H295R and 8 × 105 cells per well for all of the other cell lines) in their respective medium. The next day, the medium was removed, and cells were washed with 1 mL PBS. New medium with the respective reagents was added in a total volume of 1 mL.
Please refer to SI Appendix, Supplementary Material and Methods, section for further details.
Supplementary Material
Acknowledgments
Work in the A.L. laboratory is funded by the Medical Clinic 3, University Hospital Carl Gustav Carus Dresden, Germany, and supported by the Sonderforschungsbereich/Transregio (SFB-TRR) 205, SFB-TRR 127, and the international research training group 2251. This work was supported by the German Research Foundation (Heisenberg Professorship to A.L. [project no. 324141047] and KR3363/3-1 to N.P.K.). We received further funding for this project by the transCampus initiative to S.R.B. and the Heuberg Foundation to C. Hantel. We cordially thank Romy Opitz for expert technical assistance.
Footnotes
Competing interest statement: S.R.B. and A.V.S. hold patents on MIA602. A.V.S. patents are assigned to the University of Miami and Veterans Affairs. Reviewer W.K. was a coauthor with A.L. on this manuscript on a 2018 paper that is a committee report. Apart from this, all authors declare no competing interest regarding any of the presented data in this manuscript.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912700116/-/DCSupplemental.
References
- 1.Fassnacht M., Libé R., Kroiss M., Allolio B., Adrenocortical carcinoma: A clinician’s update. Nat. Rev. Endocrinol. 7, 323–335 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Megerle F., Kroiss M., Hahner S., Fassnacht M., Advanced adrenocortical carcinoma—What to do when first-line therapy fails? Exp. Clin. Endocrinol. Diabetes 127:109–116 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Tierney J. F., et al. , National treatment practice for adrenocortical carcinoma: Have they changed and have we made any progress? J. Clin. Endocrinol. Metab., jc.2019-00915 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Giordano T. J., et al. , Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 15, 668–676 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng S., et al. ; Cancer Genome Atlas Research Network , Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 30, 363 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Crona J., Beuschlein F., Adrenocortical carcinoma—Towards genomics guided clinical care. Nat. Rev. Endocrinol. 15, 548–560 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Wolkersdörfer G. W., Marx C., Brown J. W., Scherbaum W. A., Bornstein S. R., Evaluation of apoptotic parameters in normal and neoplastic human adrenal. Endocr. Res. 22, 411–419 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Kanczkowski W., et al. , Abrogation of TLR4 and CD14 expression and signaling in human adrenocortical tumors. J. Clin. Endocrinol. Metab. 95, E421–E429 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L., Kepp O., Krautwald S., Kroemer G., Linkermann A., Molecular mechanisms of regulated necrosis. Semin. Cell Dev. Biol. 35, 24–32 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Galluzzi L., et al. , Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockwell B. R., et al. , Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linkermann A., Green D. R., Necroptosis. N. Engl. J. Med. 370, 455–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luedde M., et al. , RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc. Res. 103, 206–216 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Newton K., et al. , RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23, 1565–1576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D., et al. , TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell 174, 1477–1491.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J., Lipinski M., Degterev A., Diversity in the mechanisms of neuronal cell death. Neuron 40, 401–413 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Zhou W., Yuan J., Necroptosis in health and diseases. Semin. Cell Dev. Biol. 35, 14–23 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Kers J., Leemans J. C., Linkermann A., An overview of pathways of regulated necrosis in acute kidney injury. Semin. Nephrol. 36, 139–152 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Linkermann A., Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int. 89, 46–57 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Garg J. P., Vucic D., Targeting cell death pathways for therapeutic intervention in kidney diseases. Semin. Nephrol. 36, 153–161 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Blériot C., Lecuit M., The interplay between regulated necrosis and bacterial infection. Cell. Mol. Life Sci. 73, 2369–2378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonnus W., Linkermann A., The in vivo evidence for regulated necrosis. Immunol. Rev. 277, 128–149 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Land W. G., Agostinis P., Gasser S., Garg A. D., Linkermann A., DAMP-induced allograft and tumor rejection: The circle is closing. Am. J. Transplant. 16, 3322–3337 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Land W. G., Agostinis P., Gasser S., Garg A. D., Linkermann A., Transplantation and damage-associated molecular patterns (DAMPs). Am. J. Transplant. 16, 3338–3361 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Mulay S. R., Linkermann A., Anders H. J., Necroinflammation in kidney disease. J. Am. Soc. Nephrol. 27, 27–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarhan M., Land W. G., Tonnus W., Hugo C. P., Linkermann A., Origin and consequences of necroinflammation. Physiol. Rev. 98, 727–780 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Friedmann Angeli J. P., et al. , Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingold I., et al. , Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422.e21 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Yang W. S., et al. , Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon S. J., et al. , Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doll S., et al. , ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagan V. E., et al. , Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W. S., Stockwell B. R., Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon S. J., et al. , Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaschler M. M., et al. , Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem. Biol. 13, 1013–1020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bornstein S. R., et al. , Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary-adrenal function, body weight, and metabolic regulation. Endocrinology 139, 1546–1555 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Ziegler C. G., et al. , Anti-tumor effects of peptide analogs targeting neuropeptide hormone receptors on mouse pheochromocytoma cells. Mol. Cell. Endocrinol. 371, 189–194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellyei S., et al. , GHRH antagonists reduce the invasive and metastatic potential of human cancer cell lines in vitro. Cancer Lett. 293, 31–40 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Wang H., et al. , Inhibition of experimental small-cell and non-small-cell lung cancers by novel antagonists of growth hormone-releasing hormone. Int. J. Cancer 142, 2394–2404 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Ohgaki H., Kleihues P., Heitz P. U., p53 mutations in sporadic adrenocortical tumors. Int. J. Cancer 54, 408–410 (1993). [DOI] [PubMed] [Google Scholar]
- 41.Moon S. H., et al. , p53 represses the mevalonate pathway to mediate tumor suppression. Cell 176, 564–580.e19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D., et al. , NRF2 is a major target of ARF in p53-independent tumor suppression. Mol. Cell 68, 224–232.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang L., et al. , Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarangelo A., et al. , p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 22, 569–575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y. I., Wei P. C., Hsu J. L., Su F. Y., Lee W. H., NPGPx (GPx7): A novel oxidative stress sensor/transmitter with multiple roles in redox homeostasis. Am. J. Transl. Res. 8, 1626–1640 (2016). [PMC free article] [PubMed] [Google Scholar]
- 46.Linkermann A., et al. , Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 111, 16836–16841 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carneiro B. A., et al. , Nivolumab in metastatic adrenocortical carcinoma: Results of a phase II trial. J. Clin. Endocrinol. Metab., jc.2019-00600 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Fang X., et al. , Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 116, 2672–2680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W., et al. , Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Invest. 129, 2293–2304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., et al. , Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem. Biol. 26, 623–633.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.