Significance
Human brains differ substantially from those of great apes, and equally important differences exist between their braincases. However, it remains unclear to which extent evolutionary changes in brain structure are related to changes in braincase structure. To study this question, we use combined computed tomography (CT) and MRI head data of humans and chimpanzees and quantify the spatial correlations between brain sulci and cranial sutures. We show that the human brain–braincase relationships are unique compared to chimpanzees and other great apes and that structural rearrangements in the brain and in the braincase emerged independently during human evolution. These data serve as an important frame of reference to identify and quantify evolutionary changes in brain and braincase structures in fossil hominin endocasts.
Keywords: brain reorganization, neurocranium, human evolution, morphological integration, biomedical imaging
Abstract
Throughout hominin evolution, the brain of our ancestors underwent a 3-fold increase in size and substantial structural reorganization. However, inferring brain reorganization from fossil hominin neurocrania (=braincases) remains a challenge, above all because comparative data relating brain to neurocranial structures in living humans and great apes are still scarce. Here we use MRI and same-subject spatially aligned computed tomography (CT) and MRI data of humans and chimpanzees to quantify the spatial relationships between these structures, both within and across species. Results indicate that evolutionary changes in brain and neurocranial structures are largely independent of each other. The brains of humans compared to chimpanzees exhibit a characteristic posterior shift of the inferior pre- and postcentral gyri, indicative of reorganization of the frontal opercular region. Changes in human neurocranial structure do not reflect cortical reorganization. Rather, they reflect constraints related to increased encephalization and obligate bipedalism, resulting in relative enlargement of the parietal bones and anterior displacement of the cerebellar fossa. This implies that the relative position and size of neurocranial bones, as well as overall endocranial shape (e.g., globularity), should not be used to make inferences about evolutionary changes in the relative size or reorganization of adjacent cortical regions of fossil hominins.
The human brain is approximately 3 times larger than that of any of our closest living relatives, the great apes. In various aspects, it can be understood as a scaled-up great ape brain (1–5). On the other hand, human brains differ from those of other living hominoids in several features, such as overall shape and structure (6), hemispheric asymmetries (7), degree of gyrification (8), sulcal patterns (9), cytoarchitecture (5), connectivity (10), function (11), and development (12, 13), implying that the hominin brain underwent substantial reorganization since the divergence from our last common ancestor with chimpanzees.
Macroscopically, 2 cortical regions stand out for being markedly distinct in humans and are commonly considered hallmarks of brain reorganization during hominin evolution. The first one is the frontal operculum—more commonly referred to as Broca’s area in our species. Humans, as compared to other living hominoids, exhibit an autapomorphic sulcal pattern in this region (14) which likely evolved in parallel or in concert with several neurological specializations—both in terms of cytoarchitecture and connectivity—that are related to human language capabilities (15). The second region is the one around the dorsolateral boundary of the parietal and occipital lobes. In nonhuman primates, this boundary is conspicuously demarcated by the lunate sulcus (lu) and essentially coincides with the anterolateral limit of the primary visual cortex (V1) (1, 16). In humans, on the other hand, the parietooccipital boundary is only vaguely defined dorsolaterally and does not coincide with the border of any major functional area (17). The anterior border of the V1 area is more caudally situated in humans than in great apes (18), and human lunate-like sulcal structures (which are always in a more caudal position than the lu of great apes) do not coincide with the border of V1 (17). Remarkably, the absolute size of the human V1 is similar to that of great apes, despite the 3-fold difference in brain size (19, 20). The smaller-than-expected size of the human V1 has been interpreted as evidence for relative enlargement of the parietotemporal association cortex in our species, possibly reflecting the evolution of new functional areas for the integration of visuospatial information (20, 21).
Based initially on ecto- (22) and later on endocranial (23) fossil data, neurocranial* globularity has also been hypothesized to represent a unique modern human feature not present in other members of the genus Homo. While adult modern humans do exhibit disproportionally large (24) and distinctly globular parietal bones, this has often been assumed to serve as morphological correlate of relative expansion of the underlying cortical parietal areas (21, 23, 25–28), both during evolution and early brain development. However, these assumptions not only have never been properly tested but also have been recently challenged on various grounds: first, it has been shown (27) that there is only weak spatial correlation between brain and endocranial features in the parietal region; second, endocranial parietal bulging is not associated with a local increase in surface area (28, 29); third, the globular shape of the human endocranial cavity is largely due to neurocranial–facial integration (29); and fourth, comparative fossil and modern data indicate that the pattern of early postnatal neurocranial development, which had been reported to lead to the uniquely globular form of the human neurocranium, is also present in the Neanderthals (30), as well as in gorillas and orangutans, while it is absent in our closest living relatives, the chimpanzees (29).
Investigating when and how the gross neuroanatomical changes occurred during human evolution remains a challenge, notably because paleoneurology (the study of fossil neurocranial evidence) chiefly relies on preserved hard tissue structures—typically the endocranial cavity—to infer brain structures (31–33). Understanding the relationship between neurocranial and brain structures in extant humans and their closest living relatives is thus an important prerequisite for fossil inferences. While it is clear that endocranial imprints replicate sulcal/gyral morphology, the identification of the latter structures from fossil endocasts (endocranial casts) tends to have a strong observer bias. This issue has been addressed by comparative studies involving both cortical and neurocranial structures in humans and other primates (9, 34). The advent of biomedical imaging tools such as Computed Tomography (CT) and MRI has opened up new possibilities for quantitative studies examining the morphological and structural relationships between the brain and the neurocranium. At present, coregistration (=spatial alignment) of same-subject in vivo CT and MRI data (hereinafter referred to as CT/MRI) is set to become the method of choice, for these 2 modalities complement each other for the task at hand: while CT provides detail of neurocranial anatomy, MRI excels at imaging brain structures. CT/MRI data of living healthy subjects are rare, however, because ethical regulations limit the use of X-ray–based imaging such as CT to cases with a clear medical indication. Still, neurocranial structures can be recovered from MRI data at a level of detail that is sufficient for gross morphological analyses. For example, coregistration of T1- and T2-weighted MRI data offers a good alternative to CT-based imaging of the neurocranium in humans (35), and either T1- or T2-weighted MRI yields satisfying results in great apes. The MRI-based approach has already been used to study covariation between brain and endocranial landmarks in modern humans (27). These analyses were restricted to the midsagittal plane and thus focused on a specific set of neurocranial and brain structures. While acknowledging these limitations, the authors concluded that there exists “a limited degree of spatial integration between soft and hard tissues” and asked for “caution when making inferences about brain areas from the position of cranial sutures” (27).
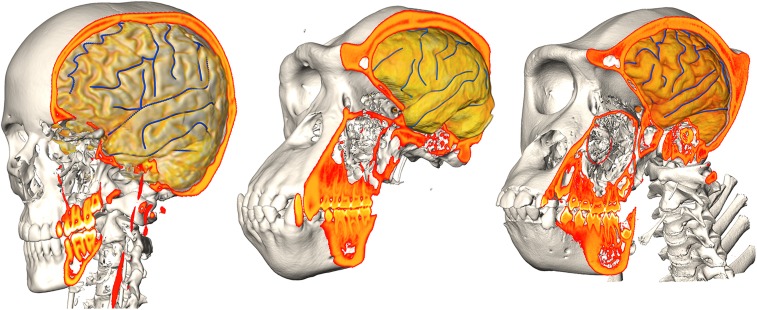
Here we expand the scope of earlier work on quantifying endocast–brain spatial relationships in the following way: first, we widen the taxonomic scope beyond humans, including chimpanzees; second, we quantify same-subject brain features (mostly cortical sulci) as well as internal and external neurocranial features (mostly sutures and foramina) in all 3 spatial dimensions; and third, we include same-individual coregistered CT/MRI scans (Fig. 1) to extract and quantify the location of endocranial features readily seen on CT but not on MRI.
Fig. 1.
Same-subject coregistered CT/MRI datasets of a human (Left), chimpanzee (Center), and gorilla (Right). Surface reconstructions of bony structures were derived from CT data, while volume renderings of brain segmentations were obtained from postprocessed MRI data. Delineations of some of the brain sulcal features used in the study are shown in blue.
We use these data to address one main question: to which extent is structural variation in neurocranial features correlated with structural variation in brain features—both within and between taxa? From the perspective of paleoneurology, quantitative answers to this question permit us to assess to which extent structural differences in neurocranial features of fossil hominins reflect structural differences in their long-gone brains. Furthermore, quantifying neurocranial–brain covariation in extant taxa permits testing the hypothesis of concerted evolution of the neurocranium and the brain.
In terms of methods, we use geometric morphometrics to quantify, in 3 dimensions, the external morphology of the brain, as well as the internal and external morphology of the neurocranium. In a first step, we analyze intra- and intertaxon variation of brain features and of neurocranial features. We then analyze the covariation between brain and neurocranial features, both within and between taxa, and assess to which extent the location of brain features can be inferred from the location of neurocranial features.
Results
Between-Taxon Variation.
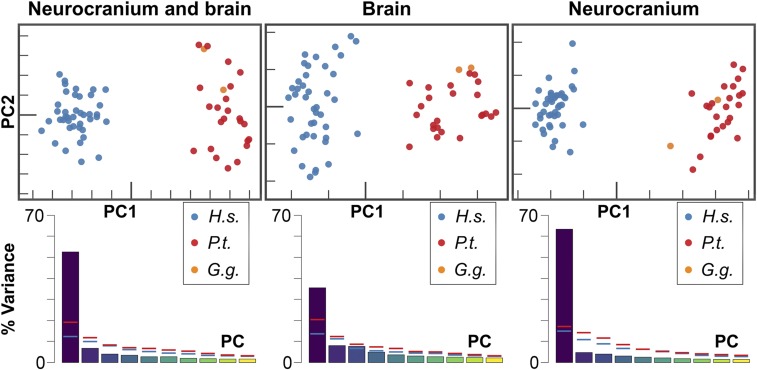
Principal component analysis (PCA) of shape of the combined neurocranial–brain (NB) data, as well as of brain (B) and neurocranial (N) data alone (B and N features are listed in SI Appendix, Table S1), reveals marked differences between human and chimpanzee configurations (Fig. 2). In all 3 analyses, a single dominant mode of variation (PC1) was found, accounting for 52.59, 35.44, and 63.43% of the total variance in NB, B, and N shape, respectively. Notably, the patterns of shape variation in physical space described by PC1 in the B and N shape analyses strongly resemble those of PC1 in the NB shape analysis (SI Appendix, Figs. S1–S3). Likewise, the spatial differences between the shapes associated with ±1 SD along PC1 in the NB, B, and N shape analyses essentially reflect differences between species mean shapes; these are visualized in Fig. 3, SI Appendix, Fig. S4, and Movie S1. In humans compared to chimpanzees, the precentral (pc), central (ce), and postcentral (pt) sulci are located more posteriorly, especially at their most inferior and lateral portions. Furthermore, the human inferior frontal sulcus (if) exhibits a more lateral position and follows a distinct course, reflecting a wider frontal operculum and frontal lobe. The posterior segments of the superior frontal sulcus (sf) and of the if exhibit little differences between taxa. Similarly, the lateral (Sylvian) sulcus (la), the superior temporal sulcus (st), and the intraparietal sulcus (ip) exhibit only moderate differences in relative location on the cortical surface across taxa. Finally, the parietal segment of the interhemispheric fissure is, in relative terms, only slightly longer and more rounded in humans than in chimpanzees, and the frontal and occipital segments exhibit almost identical proportions in both species.
Fig. 2.
Principal component analysis of neurocranium+brain, brain, and neurocranium shape variation in humans (blue) and chimpanzees (red). Data of 2 gorillas (orange) were projected into the human–chimpanzee shape space. H.s., Homo sapiens; P.t., Pan troglodytes; G.g., Gorilla gorilla. Within each graph, PC1 and PC2 axes are shown using the same scale. Scree plots show the percentage of variance explained by the first 10 PCs in the pooled sample of humans and chimpanzees (bars), as well as in human (blue horizontal lines) and chimpanzee (red horizontal lines) samples.
Fig. 3.
Comparison of human and chimpanzee mean configurations of brain and neurocranial features. (A–C) Human brain (A), neurocranium (C), and neurocranium+brain (B); (I–K) chimpanzee brain (I), neurocranium (K), and neurocranium+brain (J); (E–G) differences between human (blue) and chimpanzee (red) configurations for brain (E), neurocranium (G), and neurocranium+brain (F). (D, H, and L) Tensor maps visualizing the relative amount and direction of local variation of brain (green) and neurocranial (blue) features in humans (D), chimpanzees (L), and in the pooled sample of humans and chimpanzees (H); features only sampled in 1 species are rendered in yellow ellipsoids. Abbreviations as in SI Appendix, Table S1. The arrowhead in E indicates the position of the human po.
While between-taxon differences in relative size of the dorsolateral surface of the inferior and superior parietal lobules are moderate, differences in parietal bone shape and relative size are substantial. In humans, the parietal bones are relatively larger, and their midsagittal contour is more rounded than in chimpanzees. The apical portion of the human coronal suture (CO) is located more anteriorly, and the posterior portion of the parietotemporal suture (PT) more inferiorly. The human sigmoid sinus, transverse sinus, and internal occipital midsagittal contour exhibit a more anterior position compared to chimpanzees, in concert with the anterior shift of the foramen magnum. However, no substantial difference between taxa is present in the position of the lambdoid suture (LA), and the cerebellar tentorium is at a similar location in both species despite the substantially more anterior position of the human foramen magnum and posterior cranial fossa.
Within-Taxon Variation.
In contrast to the pattern of variation between species, in which a single dominant component (PC1) was found, the within-taxon variation is more evenly distributed across principal components in chimpanzees and even more so in humans (Fig. 2). Moreover, the major patterns of within-taxon shape variation in both species did not show a clear principal direction of variation in physical space and were dissimilar in both species.
Our analysis of variation per semilandmark—measured as the deviation from the taxon-specific means obtained from separate taxon-specific Procrustes registrations—reveals the regions with highest local variation (Fig. 3).
In the human brain (Fig. 3D), these regions are the posterior ends of the st and la, the inferior portion of the pt, and the anterior portion of the if. Other regions with higher-than-average sulcal variation include the anterior portion of the sf and the entire horizontal and ascending rami of the la (hr and ar, respectively). In the chimpanzee brain (Fig. 3L), the anterior portions of the sf and of the if, as well as the inferior portion of the pc exhibit remarkably high local variation. Finally, the lu and the posterior portion of the chimpanzee st also exhibit high variability, although the latter not as much as in humans.
Location of Nonhomologous Cortical Sulci.
As mentioned, 2 brain regions are of special interest in terms of evolutionary differentiation between chimpanzees and humans—the opercular region and the region of the lu. As shown in Fig. 3E and SI Appendix, Fig. S4D, the lu of chimpanzees originates medially at the same relative position as the human parietooccipital sulcus (po). It then runs laterally, reaching the same position of the posterior ends of the human and chimpanzee ip, and ends bending caudally at its most lateral and inferior segment. The second sulcus of interest is the frontoorbital sulcus (fo) of chimpanzees, which is located largely in the same brain region as the human sulci delimiting pars triangularis. Inferiorly, it originates at the la and runs superiorly approaching the if, but never reaching it. In humans, the if runs more laterally than the fo of chimpanzees, implying that the human brain is relatively wider at the frontal opercular region. Compared to the fo of chimpanzees, the human hr and ar are more posteriorly and laterally located. Interestingly, the ar is on average more posteriorly located than the inferior portion of the chimpanzee pc.
Taxon-Specific Topographical Relationships.
Overall, humans and chimpanzees exhibit distinct patterns of topographical relationships between neurocranial and brain features. Most conspicuously, the human pc is consistently situated posteriorly to the ectocranial CO, while the chimpanzee pc crosses it. Our CT/MRI datasets of humans show that endocranial CO, which is difficult to determine on MRI, tends to be slightly more posterior than ectocranial CO while still being substantially anterior to pc (SI Appendix, Fig. S5). In contrast, the endocranial COs in our adult chimpanzee CT/MRI dataset are located more posteriorly than their ectocranial counterparts, so that they cross the inferior portion of the ce. This same pattern—the ce crossing the endocranial CO—is also seen in both hemispheres of 1 of the 2 gorilla CT/MRI datasets (8-y-old female) of our outgroup sample (see sample structure in Materials and Methods).
Another topographical relationship with relevance for paleoneurology is the position of LA with respect to the lu in chimpanzees and the dorsolateral segment of the po in humans. Our results show that endocranial lambda is, on average, slightly more posteriorly located in humans than in chimpanzees. Moreover, endocranial lambda is, also on average, posterior to each species’ po and even to lu in chimpanzees.
Importantly, the 2 gorillas in our outgroup sample exhibit topographical relationships between brain and neurocranial features that strongly resemble those observed in chimpanzees. Additionally, the NB, B, and N configurations of both gorillas appear within or very near the chimpanzee cluster in shape space (Fig. 2).
Covariation Between Neurocranial and Brain Features.
Given the substantial between-species difference in relative location of neurocranial and brain features, we ask here whether these differences can be explained by a common pattern of size-related (allometric) variation and/or by a common pattern of covariation between neurocranial and brain features. This was tested by regressing neurocranial and brain shape on size (log centroid size) and by evaluating multivariate multiple regression (MMR), partial least squares (PLS), and covariance ratios (CR) between neurocranial and brain features, both within and between species.
No within-species size-related effects on neurocranial and brain shape variation were found in humans and chimpanzees (SI Appendix, Table S2). As per MMR, PLS, and CR, respectively, lower correlation, lower degree of integration, and higher degree of modularity (sensu Adams [36]) between the full set of neurocranial features and the full set of brain features were found in humans compared to chimpanzees (Table 1). MMR, PLS, and CR were also computed between the subset of 5 neurocranial features that comprise the parietal bone and a subset of 6 neighboring brain features that lie underneath (SI Appendix, Table S1). Like in the previous analysis, higher morphological independence between these submodules was found in humans than in chimpanzees (Table 1). However, these subsets of features were correlated less and showed noticeably lower integration and higher modularity compared to the whole brain and neurocranium analysis. Using a reduced set of sparse semilandmarks per feature or only the 2 extreme landmarks per feature did not alter our findings (SI Appendix, Table S3). Likewise, computing MMR, PLS, and CR on both brain and neurocranial hemispheres or on each hemisphere independently yielded similar results (SI Appendix, Table S3).
Table 1.
Multivariate multiple regression, partial least squares, and covariance ratios between the spatial position of brain and neurocranial modules
| Pooled | Human | Chimpanzee | |
| MMR (r2) | |||
| All features | 0.628 (0.001) | 0.463 (0.001) | 0.678 (0.001) |
| Parietal region | 0.485 (0.001) | 0.362 (0.003) | 0.498 (0.122) |
| PLS (RV) | |||
| All features | 0.959 (0.001) | 0.836 (0.002) | 0.851 (0.006) |
| Parietal region | 0.937 (0.001) | 0.680 (0.073) | 0.833 (0.020) |
| CR (CR) | |||
| All features | 0.936 (0.001) | 0.718 (0.001) | 0.778 (0.001) |
| Parietal region | 0.869 (0.001) | 0.564 (0.001) | 0.626 (0.001) |
P values (inside parenthesis) represent the proportion of instances with higher r2 and lower RV and CR coefficients (ref. 36) observed in 1,000 random models. The sample sizes for the pooled, human, and chimpanzee samples are 65, 41, and 24 individuals, respectively. The RV coefficient ranges from 0 to 1, with higher values indicating higher covariation between modules. The CR coefficient ranges from 0 to positive values, with higher values indicating higher proportion of covariation between modules relative to the total amount of covariation within modules. The subset of 6 brain and 5 neurocranial features that comprised the modules used in the analyses of the parietal region is listed in SI Appendix, Table S1.
Discussion
During hominin evolution, 2 processes had a substantial impact on the spatial arrangement of neurocranial relative to viscerocranial structures of the head: a shift from quadrupedal to bipedal locomotion and an increase in brain size (29, 37). For example, human–great ape differences in endocranial shape are largely due to differences in the size and orientation of the face relative to the brain (29). The question that arises is: to which extent do the human–chimpanzee differences in sulcal patterns and/or in neurocranial structures reported here reflect the effects of bipedality, brain expansion, and/or brain reorganization?
Our results show substantial rearrangement of homologous sulcal structures in humans compared to chimpanzees. In humans, the inferior regions of the pc, ce, and (to a smaller extent) pt are at a more posterior position compared to chimpanzees, while the superior portions of these sulci are at similar relative positions in both species (Fig. 3 and Movie S1). This pattern reflects a conspicuous reorganization of the human opercular region—evidenced by the relative enlargement of the frontal operculum (9, 16) and by the reported caudal shift of the inferior aspects of the pre- and postcentral gyri—that is likely related to the reorganization of the Broca’s area and the evolution of language. However, the extent to which the presence of an imprint of a caudally positioned inferior pc in fossil endocasts can serve as evidence for human-like language capacities is a matter that cannot be resolved here.
Our results also point to limitations of interpreting sulcal imprints on fossil hominin endocasts as “great ape-like” versus “human-like”. Fig. 3 shows that the ar of humans and the inferior pc of chimpanzees exhibit similar position and orientation. Accordingly, endocranial imprints of a great ape-like sulcal pattern of pc in Broca’s area cannot readily be discriminated from a human-like pattern of ar (38). An MRI-based analysis of sulcal patterns of chimpanzee brains also showed that interindividual variation in the frontal opercular region is greater than documented previously, which further complicates identification (16).
The hypothesis (9, 25, 39) that the hominin parietal lobe increased in relative size after the divergence from our last common ancestor with chimpanzees can be evaluated, at least partially, in light of the position of the dorsolateral segment of the human po (Fig. 3E and SI Appendix, Fig. S4D). This structure is slightly more posteriorly situated than its chimpanzee homolog, thus leading to a slight increase in the relative length of the superior border of the precuneus in humans. Although this confirms previous research (25), this increase is moderate at most, as is the between-species difference in relative size of the dorsolateral surfaces of the inferior and superior parietal lobules (Fig. 3E and SI Appendix, Fig. S4D).
Our results indicate that the human parietal bone is relatively larger than that of chimpanzees. Fig. 3 shows that differences in parietal shape are localized: the human parietal bone extends more anteriorly and inferolaterally, resulting in a relatively longer parietal midsagittal arc length and a deep parietal notch. This pattern can tentatively be interpreted in terms of taxon-specific differences in parietal bone growth. In humans compared to chimpanzees, parietal bone growth is likely more intense along the apical region of the CO and along the parietomastoid suture (PM). As an effect, the superior portion of the human CO appears shifted anteriorly compared to chimpanzees, while the inferior regions of the CO in both human and chimpanzees (landmark “pterion”) are at similar positions on the neurocranial surface.
In chimpanzees (9), the pc crosses the CO in its middle region such that its inferior part is anterior to that suture; in humans, on the other hand, the entire pc is located posterior to the CO. Furthermore, the human PM tends to be inferior to the transverse sinus, while the posterior PT is well above this sinus in chimpanzees. The taxon-specific differences in topographical relationships between neurocranial and brain structures likely reflect evolutionary shifts. Since it is often difficult to disambiguate great ape-like from human-like morphologies on fossil endocasts, the topographical relationships between taxon-specific brain and neurocranial features described above are not only informative about the evolution of both modules and the interaction between them, but also of great interest for the examination of paleoneurological evidence. In this context, the similarities in physical and shape space between the 2 gorillas of our outgroup sample and the chimpanzees from the main sample suggest that the latter, unlike humans, largely retained their ancestral brain–neurocranium topographical relationships after the split between the hominin–panin lineages.
The human neurocranium (and, by inference, the brain) has been characterized as being globular in comparison to that of chimpanzees (22, 26, 29, 40). Fig. 3 and Movie S1 illustrate these differences for the midsagittal plane of the neurocranium. Interestingly, they reveal a previously unnoticed disparity between endocranial and brain shape in humans: while the human endocranial outline is clearly more rounded than in chimpanzees, this is less so in the corresponding region of the brain (Fig. 3F). This disparity results in a larger interstitial space between brain and endocast in humans compared to chimpanzees, likely reflecting a more voluminous sagittal venous sinus.
Apart from these differences, several neurocranium–brain relationships appear to be conserved. Most conspicuously, the cerebellar tentorium is positioned similarly in both species despite substantial differences in the antero–posterior location of the foramen magnum and the entire cerebellar fossa (Fig. 3). This suggests that changes in relative size and position of the cerebellum—likely related to the acquisition of obligate bipedalism—may have had little correlation with changes in relative size and position of the cerebrum during hominin evolution.
The results presented in Table 1 indicate that there is little covariation between neurocranial and brain structures, both within humans and within chimpanzees. Within species, structural variation in the brain is thus largely independent of structural variation in the neurocranium. From an evolutionary developmental perspective, this suggests that brain and cranial ontogenies are not strictly correlated and that evolutionary changes in brain structure did not entail changes in neurocranial structure.
An earlier study on endocranial integration (29) showed that variation in overall endocranial (and thus brain) shape largely reflects variation in size and orientation of the brain relative to the face and cranial base. Positive evidence for integration of neuroviscerocranial shape (29) and negative evidence for integration of neurocranial and brain structures (this study) only seemingly contradict each other: while brain shape as a whole is tightly integrated with overall skull shape, structural reorganization of the brain in terms of sulcal patterns is largely independent of structural reorganization of the neurocranium. Correlated changes in neurocranial and brain structures in the human–chimpanzee sample as a whole (Table 1) are thus unlikely to reflect concerted evolutionary change. It is more likely that they reflect the combined result of 2 independent evolutionary processes. On the one hand, the acquisition of obligate bipedalism led to anterior–inferior displacement of the foramen magnum and cerebellar fossa; on the other, human-specific brain reorganization led to rearrangement of the cortical sulcal pattern.
Our analyses further show that humans, as compared to chimpanzees, exhibit less morphological integration and more modularity between N and B features and also between features of the parietal bone and a subset of 6 neighboring brain sulci. Further research will be needed to confirm and establish the biological significance of these findings, especially at a finer scale—for instance, at the level of individual N and B features. For now, we hypothesize that our results might reflect higher sulcal variability and lower spatial covariation within cortical structures in humans, in line with previous work showing significantly less genetic heritability of cerebral cortical anatomy in our species than chimpanzees (41) while sharing with them modular brains (42).
The advent of geometric morphometrics to analyze virtual endocasts has revolutionized the field of paleoneurology. Among other things, it has enabled the identification of subtle differences in ecto- and endocranial shape across extinct and extant species (22, 23) and the quantification of shared and species-specific patterns of endocranial variation and brain growth during development (26, 28–30, 37, 40). At the same time, morphometric analyses of endocranial shape and/or neurocranial structure (i.e., relative position of cranial bones) of fossil hominins are often assumed to be informative for making inferences about the external morphology of the brain. This last assumption, however, has not been substantiated. A first consideration is that neurocranial sutures do not coincide at all with structural or functional boundaries on the external cortical surface (43). For instance, the inner table of the human parietal bones extends well beyond the dorsolateral parietal cortex, reaching portions of the frontal, temporal, and occipital lobes (SI Appendix, Fig. S5). In evolutionary terms, neurocranial globularity might not necessarily reflect relative enlargement of the underlying cerebral cortex; it might well reflect a relative expansion of white matter and/or other deep subcortical structures, and/or of the interstitial space between the brain and the endocranial surface. Moreover, neurocranial globularity does not seem to increase local surface area (28), and nonnegligible position shifts of the major external districts of the brain cannot be ruled out in analyses of endocranial shape alone. Finally, while differences in the relative sizes of specific brain regions across extant and extinct species are important on their own, a thorough examination must also include an estimation of their absolute size and amount of within-group variation.
In conclusion, our results indicate that structural changes in the brain and the neurocranium reflect largely independent evolutionary developmental processes. This implies that inferences about brain structure cannot and should not be carried out from endocranial shape or from neurocranial structure of fossil hominins unless they are accompanied by clear sulcal imprints. The reported changes in the structure of the human neurocranium—the relative expansion of the parietal bone, its characteristic globular shape, and the anterior shift of the foramen magnum and posterior cranial fossa—most likely reflect increased encephalization and/or neurocranial-to-facial reorientation during the acquisition of bipedalism. Changes in the external morphology of the human brain, on the other hand, most likely reflect simple rules of allometric scaling and, in some areas, neurological specializations acquired during hominin evolution.
Materials and Methods
Sample Structure.
The sample consists of 41 humans, 24 chimpanzees, and 2 gorillas. It can be divided into 2 subsets: 1) individuals represented by combined CT/MRI data (anonymized clinical data from 9 nonsymptomatic human patients, 1 postmortem chimpanzee from the Digital Morphology Museum of the Kyoto University Primate Research Institute (KUPRI), and 2 postmortem gorillas from Swiss zoos); 2) individuals represented by MRI data alone (clinical datasets of 32 human volunteers and of 23 live chimpanzees from the Yerkes Primate Center). Details of the sample structure are provided in SI Appendix, Table S4. Details of CT and MRI data acquisition protocols and of image-processing procedures are provided in SI Appendix, Materials and Methods. This study makes retrospective use of CT/MRI datasets. The study design was reviewed and deemed exempt by the institutional review boards of the University Children’s Hospital Zurich, the University Hospital Zurich, and the Catholic University Leuven.
Feature Extraction.
The neurocranial features considered here comprise foramina and internal and external suture lines, as well as vascular imprints that are consistently observable on the endocasts of all specimens in the sample (SI Appendix, Table S1). Brain features comprise major sulci that exhibit intra- and/or intertaxon homology (SI Appendix, Table S1). The morphology of all features was quantified by means of anatomical landmarks (indicating fixed anatomical points of reference) and semilandmarks (indicating points along curves). Landmark data of the brain features were sampled on the volume renderings of the MRI data. The landmarks of the neurocranial features were sampled on surface reconstructions derived from CT data and/or on volume renderings of postprocessed MRI data (procedures for MRI-based segmentation of neurocranial structures are described in SI Appendix, Materials and Methods).
Statistical Analysis.
Generalized Procrustes Analysis (GPA) was used for size normalization and optimal alignment of individual landmark configurations. For combined neurocranium and brain (NB) landmark configurations, GPA was performed using a subset of features that are homologous across taxa, that are located at the surface of the brain or in contact to it, and that do not exhibit exceedingly high variability within and between taxa (SI Appendix, Table S1). PCA of shape was used to explore patterns of within- and between-taxon variation in multidimensional shape space. These patterns were also rendered in 3D physical space. Two-block PLS analysis (2B-PLS) (44, 45) was used to quantify covariation between the neurocranial and brain landmark sets. These data can be used as a measure of morphological integration between sets. To assess the relative independence (modularity) of brain and neurocranial features, the covariance ratio (CR) was evaluated (36), which expresses the amount of covariation between modules as a fraction of the total amount of covariation within modules. CR is a global measure of covariance proportions and, therefore, does not provide information on the exact nature of modularity. To test specific hypotheses about the covariation between neurocranial and brain features, we used MMR to assess how well a given set of brain features can be predicted by a given set of neurocranial features. All 3 analyses—PLS, CR, and MMR—were computed using a reduced set of semilandmarks per feature and also using only the 2 extreme landmarks (fixed landmarks). In addition, these analyses were performed on both brain and neurocranial hemispheres and also on each hemisphere independently (SI Appendix, Table S3).
Data and Materials Availability.
There are restrictions on accessing data discussed in the paper. Access to the clinical CT/MRI datasets requires the approval of the respective institutional review boards. All other relevant code and data supporting the findings of this study are available at the Zurich Open Repository Archive (ZORA), doi.org/10.5167/uzh-174973.
Supplementary Material
Acknowledgments
We thank W. Coudyzer, W. Develter, C. Kellenberger, S. Kollias, and L. Michels for access to clinical data, as well as the reviewers for providing constructive comments that helped improve the manuscript. This research was funded by the Swiss National Science Foundation Grant #31003A_135470 (to C.P.E.Z.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
*Following the conventions of the anthropological literature, we use neurocranium and braincase as synonyms.
Data deposition: All relevant code and data supporting the findings of this study are available at the Zurich Open Repository Archive (ZORA), doi.org/10.5167/uzh-174973.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905071116/-/DCSupplemental.
References
- 1.Schoenemann P. T., Evolution of the size and functional areas of the human brain. Annu. Rev. Anthropol. 35, 379–406 (2006). [Google Scholar]
- 2.Herculano-Houzel S., The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. U.S.A. 109, 10661–10668 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semendeferi K., Damasio H., The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J. Hum. Evol. 38, 317–332 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Semendeferi K., Lu A., Schenker N., Damasio H., Humans and great apes share a large frontal cortex. Nat. Neurosci. 5, 272–276 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Semendeferi K., et al. , Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb. Cortex 21, 1485–1497 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Aldridge K., Patterns of differences in brain morphology in humans as compared to extant apes. J. Hum. Evol. 60, 94–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Robles A., Hopkins W. D., Sherwood C. C., Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc. R. Soc. B Biol. Sci. 280, 20130575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rilling J. K., Insel T. R., The primate neocortex in comparative perspective using magnetic resonance imaging. J. Hum. Evol. 37, 191–223 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Connolly C. J., External Morphology of the Primate Brain (CC Thomas, Springfield, IL, 1950). [Google Scholar]
- 10.Rilling J. K., et al. , The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 11, 426–428 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Rilling J. K., “Searching for human brain specializations with structural and functional neuroimaging” in The Human Brain Evolving: Paleoneurological Studies in Honor of Ralph L. Holloway, Broadfield D., Yuan M., Schick K., Toth N., Eds. (Stone Age Institute Press, Gosport, IN, 2010), pp. 157–170. [Google Scholar]
- 12.Sakai T., et al. , Differential prefrontal white matter development in chimpanzees and humans. Curr. Biol. 21, 1397–1402 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Sakai T., et al. , Fetal brain development in chimpanzees versus humans. Curr. Biol. 22, R791–R792 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Keller S. S., Deppe M., Herbin M., Gilissen E., Variability and asymmetry of the sulcal contours defining Broca’s area homologue in the chimpanzee brain. J. Comp. Neurol. 520, 1165–1180 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Rilling J. K., Comparative primate neurobiology and the evolution of brain language systems. Curr. Opin. Neurobiol. 28, 10–14 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Falk D., et al. , Identification of in vivo sulci on the external surface of eight adult chimpanzee brains: Implications for interpreting early hominin endocasts. Brain Behav. Evol. 91, 45–58 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Allen J. S., Bruss J., Damasio H., Looking for the lunate sulcus: A magnetic resonance imaging study in modern humans. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 867–876 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Holloway R. L., Broadfield D. C., Yuan M. S., Morphology and histology of chimpanzee primary visual striate cortex indicate that brain reorganization predated brain expansion in early hominid evolution. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 273, 594–602 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Stephan H., Frahm H., Baron G., New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. (Basel) 35, 1–29 (1981). [DOI] [PubMed] [Google Scholar]
- 20.Gilissen E., Zilles K., The relative volume of the primary visual cortex and its intersubject variability among humans: A new morphometric study. C. R. Acad. Sci. Paris Ser. II 320, 897–902 (1995). [Google Scholar]
- 21.Bruner E., Iriki A., Extending mind, visuospatial integration, and the evolution of the parietal lobes in the human genus. Quat. Int. 405, 98–110 (2016). [Google Scholar]
- 22.Lieberman D. E., McBratney B. M., Krovitz G., The evolution and development of cranial form in Homo sapiens. Proc. Natl. Acad. Sci. U.S.A. 99, 1134–1139 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruner E., Manzi G., Arsuaga J. L., Encephalization and allometric trajectories in the genus Homo: Evidence from the Neandertal and modern lineages. Proc. Natl. Acad. Sci. U.S.A. 100, 15335–15340 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruner E., Pereira-Pedro A. S., Bastir M., Patterns of morphological integration between parietal and temporal areas in the human skull. J. Morphol. 278, 1312–1320 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Bruner E., Preuss T. M., Chen X., Rilling J. K., Evidence for expansion of the precuneus in human evolution. Brain Struct. Funct. 222, 1053–1060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott N., Neubauer S., Hublin J.-J., Gunz P., A shared pattern of postnatal endocranial development in extant hominoids. Evol. Biol. 41, 572–594 (2014). [Google Scholar]
- 27.Bruner E., Amano H., de la Cuétara J. M., Ogihara N., The brain and the braincase: A spatial analysis on the midsagittal profile in adult humans. J. Anat. 227, 268–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neubauer S., Hublin J., Gunz P., The evolution of modern human brain shape. Sci. Adv. 4, eaao5961 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zollikofer C. P. E., Bienvenu T., Ponce de León M. S., Effects of cranial integration on hominid endocranial shape. J. Anat. 230, 85–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponce de León M. S., Bienvenu T., Akazawa T., Zollikofer C. P. E., Brain development is similar in Neanderthals and modern humans. Curr. Biol. 26, R665–R666 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Holloway R. L., The human brain evolving: A personal retrospective. Annu. Rev. Anthropol. 37, 1–19 (2008). [Google Scholar]
- 32.Zollikofer C. P. E., De León M. S. P., Pandora’s growing box: Inferring the evolution and development of hominin brains from endocasts. Evol. Anthropol. 22, 20–33 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Falk D., Interpreting sulci on hominin endocasts: Old hypotheses and new findings. Front. Hum. Neurosci. 8, 134 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi Y., Matsui T., Ogihara N., “Inferring cortical subdivisions based on skull morphology” in Digital Endocasts: From Skulls to Brains, Bruner E., Ogihara N., Tanabe H. C., Eds. (Springer Japan, Tokyo, 2018), pp. 33–46. [Google Scholar]
- 35.Iacono M. I., et al. , MIDA: A multimodal imaging-based detailed anatomical model of the human head and neck. PLoS One 10, e0124126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams D. C., Evaluating modularity in morphometric data: Challenges with the RV coefficient and a new test measure. Methods Ecol. Evol. 7, 565–572 (2016). [Google Scholar]
- 37.Zollikofer C. P. E., “Evolution of hominin cranial ontogeny” in Evolution of the Primate Brain, Hofman M. A., Ed. (Elsevier, 2012), chap. 13, pp. 273–292, Falk DBT-P in BR. [DOI] [PubMed] [Google Scholar]
- 38.Holloway R. L., et al. , Endocast morphology of Homo naledi from the Dinaledi Chamber, South Africa. Proc. Natl. Acad. Sci. U.S.A. 115, 5738–5743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holloway R. L., Cerebral brain endocast pattern of Australopithecus afarensis hominid. Nature 303, 420–422 (1983). [DOI] [PubMed] [Google Scholar]
- 40.Neubauer S., Gunz P., Hublin J.-J., Endocranial shape changes during growth in chimpanzees and humans: A morphometric analysis of unique and shared aspects. J. Hum. Evol. 59, 555–566 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Gómez-Robles A., Hopkins W. D., Schapiro S. J., Sherwood C. C., Relaxed genetic control of cortical organization in human brains compared with chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 112, 14799–14804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gómez-Robles A., Hopkins W. D., Sherwood C. C., Modular structure facilitates mosaic evolution of the brain in chimpanzees and humans. Nat. Commun. 5, 4469 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribas G. C., Applied Cranial-Cerebral Anatomy: Brain Architecture and Anatomically Oriented Microneurosurgery (Cambridge University Press, 2018). [Google Scholar]
- 44.Rohlf F. J., Corti M., Use of two-block partial least-squares to study covariation in shape. Syst. Biol. 49, 740–753 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Adams D. C., Collyer M. L., On the comparison of the strength of morphological integration across morphometric datasets. Evolution 70, 2623–2631 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.