Significance
Cells require forces generated by the contractile actomyosin cytoskeleton to remodel and shape tissues. In humans, mutations in the myosin II motor are associated with a wide range of diseases, but the cellular mechanisms that underlie disease physiologies are not well understood. Using in vivo imaging and biophysical approaches, we show that engineering human disease mutations in fruit fly myosin II produces proteins with altered organization and dynamics that fail to drive rapid cell movements, resulting in abnormalities in embryo shape. Because many genes and cell behaviors are shared between fruit flies and humans, these results may reveal mechanisms underlying myosin II-related diseases in humans.
Keywords: myosin II, contractility, epithelia, morphogenesis
Abstract
The nonmuscle myosin II motor protein produces forces that are essential to driving the cell movements and cell shape changes that generate tissue structure. Mutations in myosin II that are associated with human diseases are predicted to disrupt critical aspects of myosin function, but the mechanisms that translate altered myosin activity into specific changes in tissue organization and physiology are not well understood. Here we use the Drosophila embryo to model human disease mutations that affect myosin motor activity. Using in vivo imaging and biophysical analysis, we show that engineering human MYH9-related disease mutations into Drosophila myosin II produces motors with altered organization and dynamics that fail to drive rapid cell movements, resulting in defects in epithelial morphogenesis. In embryos that express the Drosophila myosin motor variants R707C or N98K and have reduced levels of wild-type myosin, myosin motors are correctly planar polarized and generate anisotropic contractile tension in the tissue. However, expression of these motor variants is associated with a cellular-scale reduction in the speed of cell intercalation, resulting in a failure to promote full elongation of the body axis. In addition, these myosin motor variants display slowed turnover and aberrant aggregation at the cell cortex, indicating that mutations in the motor domain influence mesoscale properties of myosin organization and dynamics. These results demonstrate that disease-associated mutations in the myosin II motor domain disrupt specific aspects of myosin localization and activity during cell intercalation, linking molecular changes in myosin activity to defects in tissue morphogenesis.
Actomyosin contractility reorganizes groups of cells into functional tissues with precise shapes and structures (1, 2). Contractile assemblies of the nonmuscle myosin II motor protein and actin filaments generate forces that produce cell division, cell shape changes, and cell movements during tissue development (3, 4). The mechanical forces that shape tissues are generated in large part by the motor domain of myosin II, which converts energy from ATP hydrolysis into conformational changes that produce molecular-scale movement and force (3, 5). The myosin motor domain plays dual roles in actomyosin contractility, promoting actin filament sliding and mediating actin filament cross-linking (3, 5). Mutations in nonmuscle myosin II are associated with a broad range of human diseases (2, 6–8). Disease-related mutations occur throughout the protein, although a number of mutations are concentrated in the motor domain (2). Myosin II mutations associated with human diseases are predicted to disrupt essential aspects of myosin function, but the cellular mechanisms that translate altered myosin activity into specific disease pathologies are not well understood. In particular, it is not known how mutations in the motor domain affect cell- and tissue-level patterns of actomyosin activity that allow myosin to carry out its diverse functions in multicellular organisms.
In humans, there are 3 isoforms of nonmuscle myosin II (NM II) that have distinct and partially overlapping functions (3). Mutations in NM IIA, encoded by the MYH9 gene, are linked to autosomal dominant pathologies, including blood platelet dysfunction, deafness, and nephritis, collectively referred to as MYH9-related diseases (2, 3). In addition, mutations in NM IIB have been implicated in such congenital defects as microcephaly, cerebral and cerebellar atrophy, and congenital diaphragmatic hernia (7), and mutations in NM IIC lead to deafness (6). Although the biochemical effects of most disease-causing NM II mutations are not known, 2 MYH9-related disease mutations in the motor domain, R702C and N93K in NM IIA (9–11), cause reduced actin-activated ATPase activity and disrupt motor protein translocation along actin filaments (i.e., actin sliding), but do not affect actin binding in vitro (12–14). Surprisingly, NM IIB motor variants that display impaired actin sliding in vitro are still able to support cytokinesis in cells, suggesting that myosin motor-driven actin sliding is dispensable for cytokinesis (14). Moreover, mouse NM IIB motors that contain the corresponding R709C mutation retain partial function in vivo but lead to defects in neuronal migration, heart development, and body wall closure that are not present in NM IIB null mutant mice and may result from interference with the normal function of NM IIA in affected tissues (15, 16). Therefore, the mechanisms by which myosin motor variants alter essential functions of myosin in cells and tissues in vivo are not well understood.
The use of model systems that are amenable to live imaging and biophysical approaches provides an opportunity to characterize the cellular manifestations of defects resulting from myosin II mutations and investigate how changes in myosin localization, dynamics, and contractile activity influence its cell- and tissue-level functions. The Drosophila myosin II motor domain shares a high level of conservation with human NM II isoforms (72% minimum identity), and Drosophila and human myosin II proteins have similar motor enzymatic activities in vitro (17). Drosophila has been used to elucidate the function and regulation of myosin II (18) during mesoderm invagination (19–21), dorsal closure (22), and axis elongation (23–25), as well as to model MYH9-related diseases associated with mutations in the myosin tail domain, which mediates filament assembly (26, 27). Characterization of myosin II motor variants with disease-associated mutations can help to dissect mechanisms of myosin function in vivo and aid in understanding the etiology of human disease.
Here we analyze the functions of disease-related myosin II motor domain variants in Drosophila. Elongation of the Drosophila body axis is a myosin-dependent process that arises from cell intercalation within the embryonic epithelium (28–31). Myosin II is asymmetrically localized in the plane of the tissue, and forces generated by actomyosin contractility are essential to drive cell intercalation (29–34). To dissect the effects of the motor domain on myosin II function, we used time-lapse imaging and biophysical approaches to analyze the cell- and tissue-level effects of 2 mutations in the nonmuscle myosin II motor domain, which are homologous to the human NM IIA MYH9-related disease mutations R702C and N93K. We show that these proteins, like the wild-type motor, display a planar polarized localization and are capable of generating anisotropic contractile tension in the tissue. However, the myosin motor variants display altered cortical organization and slowed turnover dynamics and fail to promote rapid cell intercalation and axis elongation. These results demonstrate that disease-related mutations in the Drosophila myosin II motor domain produce specific defects in myosin organization, dynamics, and activity during cell intercalation, linking molecular changes in the motor domain to cell behavior and tissue morphogenesis.
Results
Mutations in the Myosin II Motor Domain Disrupt Axis Elongation.
To investigate the effects of disease-associated mutations in myosin II, we generated transgenic Drosophila embryos expressing myosin II heavy chain variants with single amino acid mutations in the motor domain. The wild-type myosin II heavy chain (MyoII-WT, encoded by the zipper gene) and 2 motor mutants homologous to variants involved in human disease, MyoII-R707C and MyoII-N98K (Fig. 1B), were GFP-tagged at the N terminus and expressed in embryos with reduced levels of endogenous myosin using a short hairpin RNA (shRNA) to the zipper 3′ UTR (SI Appendix, Materials and Methods). The transgenes were expressed at similar levels, with approximately half of the myosin pool in these embryos coming from the transgene and the other half from the endogenous wild-type locus (SI Appendix, Fig. S1). These myosin motor variants are predicted to form cofilaments with the endogenous wild-type protein, which may result in dominant-negative effects that could mimic dominant MYH9-related disorders in humans (2, 3, 11). We used these embryos to investigate the effects of myosin motor domain mutations on tissue morphogenesis.
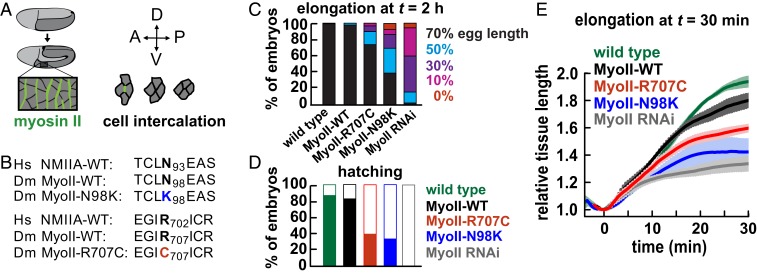
Fig. 1.
Embryos expressing nonmuscle myosin II heavy chain motor variants are defective for axis elongation. (A) The Drosophila germ band epithelium (dark gray) elongates along the AP axis and narrows along the DV axis. Planar polarized myosin II (green) drives oriented cell rearrangements that narrow and elongate the tissue. (B) Point mutations generated in the motor domain of Drosophila MyoII and the corresponding residues in humans. (C) Percentages of embryos that elongated to at least 70%, 50%, 30%, or 10% of total egg length by 2 h after the onset of elongation. Germ band elongation was significantly reduced in MyoII-R707C and MyoII-N98K embryos compared with MyoII-WT (χ2 test, P < 0.05) but was rescued compared with MyoII RNAi alone (χ2 test, P < 0.05). (D) Percentages of embryos that hatched (filled) or did not hatch (white) after 1 d (52 to 129 embryos per genotype in C and D). (E) The AP length of the germ band normalized to the value at t = 0 from particle image velocimetry analysis of confocal movies (3 to 4 embryos per genotype). Transgenes were expressed in a MyoII RNAi background with the gap43:mCherry cell membrane marker. Wild type, gap43:mCherry alone. Data are mean ± SEM between embryos.
We first analyzed the effects of MyoII-R707C and MyoII-N98K on Drosophila axis elongation (Fig. 1A). Embryos expressing zipper shRNA (MyoII RNAi embryos) displayed highly penetrant axis elongation defects and embryonic lethality (Fig. 1 C and D). Expression of the MyoII-WT transgene largely rescued these defects (Fig. 1 C and D), indicating that MyoII-WT functions normally in axis elongation. In contrast, embryos expressing MyoII-R707C or MyoII-N98K in a MyoII RNAi background displayed intermediate defects in axis elongation and embryonic lethality that were partially rescued compared with MyoII RNAi (Fig. 1 C and D). MyoII-N98K embryos were more defective, with 61% of embryos failing to elongate to the wild-type extent, whereas MyoII-R707C embryos had milder defects, with 26% of embryos failing to elongate to the wild-type extent (Fig. 1C). Using particle image velocimetry to quantify tissue deformation in confocal time-lapse movies of embryos expressing the gap43:mCherry cell membrane marker, we found that in contrast to the wild-type germ band epithelium, which extended 1.93 ± 0.03-fold (mean ± SEM) in the first 30 min of elongation, the extent of tissue elongation was reduced to 1.34 ± 0.06-fold in MyoII RNAi embryos (Fig. 1E; t = 30 min). These defects were significantly rescued by the MyoII-WT transgene (1.80 ± 0.05) although not to the wild-type extent, possibly due to lower levels of myosin expression from the transgene (SI Appendix, Fig. S1). Tissue elongation was significantly reduced in MyoII-R707C and MyoII-N98K embryos (1.60 ± 0.02 and 1.42 ± 0.09, respectively) (Fig. 1E). Together, these results demonstrate that the MyoII-R707C and MyoII-N98K motor domain variants fail to support wild-type axis elongation and embryo viability.
Myosin II Motor Variants Are Planar Polarized.
Planar polarized contractile forces generated by myosin II are required for cell intercalation and axis elongation (29–33). The myosin II motor protein is planar polarized, with higher cortical accumulation at contacts between anterior and posterior cells (AP edges) compared with contacts between dorsal and ventral cells (DV edges) (29, 30). To test whether the reduced elongation in MyoII-R707C and MyoII-N98K embryos results from altered myosin II localization, we analyzed the localization of myosin motor variants using computational methods (35). MyoII-WT is strongly planar polarized during elongation, with an AP enrichment of 2.1 ± 0.2 (absolute ratio) at t = 10 min (Fig. 2 A and B and SI Appendix, Fig. S3 show log2 ratios), consistent with previous studies of myosin planar polarity (24, 29–31, 35, 36). MyoII-R707C and MyoII-N98K displayed a similar planar polarity, with AP enrichments of 2.6 ± 0.3 and 2.5 ± 0.3 (absolute ratios), respectively (Fig. 2 A and B). The planar polarized enrichment of the Bazooka/Par-3 junctional protein at DV edges (23, 29, 31) also occurred normally in MyoII-R707C and MyoII-N98K embryos (SI Appendix, Fig. S2). These results demonstrate that the R707C and N98K mutations do not disrupt myosin planar polarity in this tissue.
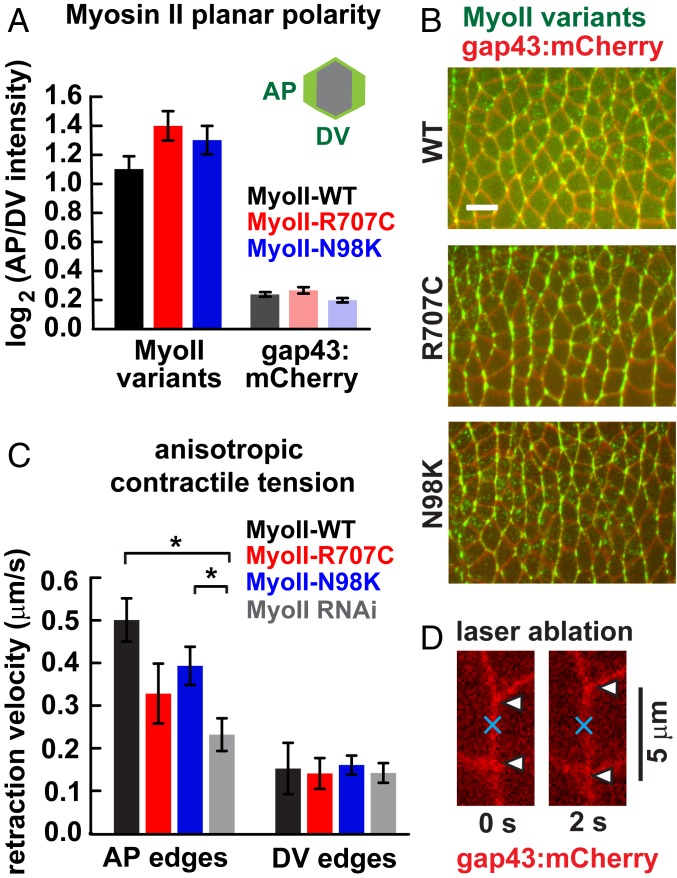
Fig. 2.
Myosin II motor variants are planar polarized and produce anisotropic tension. (A) Planar polarized localization of MyoII-WT, MyoII-R707C, and MyoII-N98K and the gap43:mCherry membrane marker. The log2 ratios of protein intensity at AP edges (oriented at 60° to 90° relative to the AP axis) to DV edges (oriented at 0° to 30°) at t = 10 min are plotted. Ratios were calculated for each cell, and a mean value was obtained for each embryo. Data are mean ± SEM between embryos (3 embryos per genotype, 120 to 370 cells per embryo). MyoII-R707C and MyoII-N98K planar polarity were not significantly different from MyoII-WT. P > 0.1, t test. (B) Stills from movies of MyoII-WT, MyoII-R707C, and MyoII-N98K embryos. (Scale bar: 10 μm.) (C) Mean peak retraction velocities following laser ablation. Peak retraction velocities at AP edges in MyoII-R707C and MyoII-N98K were reduced compared with those in MyoII-WT, but the difference did not reach statistical significance (P > 0.07). The peak retraction velocity at AP edges for MyoII-N98K was rescued compared with MyoII RNAi (P = 0.03). *P < 0.05, t test. Data are mean ± SEM between edges (5 to 7 AP and 3 to 5 DV edges per genotype). The mechanical anisotropy (i.e., the mean AP retraction velocity divided by the mean DV retraction velocity) in MyoII-R707C and MyoII-N98K embryos (2.3 and 2.4, respectively) was intermediate between those of MyoII-WT (3.3) and MyoII RNAi (1.6). (D) AP edge immediately before and 2 s after ablation.
Myosin II Motor Variants Produce Anisotropic Tension.
As the axis elongation defects in embryos expressing myosin motor variants do not result from a decrease in myosin planar polarity, we tested whether these defects could result from defective forces generated by these motors. To investigate this possibility, we used a UV laser to sever individual cell interfaces (Fig. 2 C and D) (33, 37). The extent of retraction away from the site of ablation correlates with the tension present before ablation, with the peak retraction velocity being proportional to tension and inversely proportional to frictional drag (38). The average peak retraction velocity in MyoII-WT embryos was more than 3 times higher at AP edges (0.50 ± 0.05 μm/s) than at DV edges (0.15 ± 0.06 μm/s) (Fig. 2C), similar to the mechanical anisotropy in wild-type embryos (24, 32, 33). In contrast, in MyoII RNAi embryos, the peak retraction velocity at AP edges was reduced by approximately half (0.23 ± 0.04 μm/s), with no significant change at DV edges (Fig. 2C). In embryos expressing MyoII-R707C or MyoII-N98K, the peak retraction velocities at AP edges (0.33 ± 0.07 and 0.39 ± 0.04 μm/s, respectively) were intermediate between MyoII RNAi and MyoII-WT (Fig. 2C). Because retraction velocity depends on both tension and drag, these results do not address the absolute magnitude of the forces generated in these embryos. However, they do indicate that the myosin II motor variants are capable of generating anisotropic contractile tension in vivo, although perhaps not to the same extent as MyoII-WT.
Myosin II Motor Variants Display Altered Organization at the Cell Cortex.
Despite the proper accumulation of myosin motor variants at AP cell edges, the fine-scale localization of MyoII-R707C and MyoII-N98K at the cell cortex did not appear entirely similar to wild type (Fig. 2B). To investigate whether the R707C and N98K mutations influence myosin organization, we analyzed the subcellular localization of myosin variants along AP edges during axis elongation (Fig. 3 and Movies S1–S3). In control embryos, MyoII-WT was distributed uniformly along the cortex at AP edges (Fig. 3 A and E). In contrast, MyoII-R707C and MyoII-N98K accumulated in punctate aggregates at the cell cortex (Fig. 3 B–D and F–H). The overall organization of filamentous actin (F-actin) was not noticeably altered (SI Appendix, Fig. S4). The mean percentage of AP edges with MyoII aggregates was significantly higher in MyoII-R707C (38 ± 4%) and MyoII-N98K (32 ± 3%) compared to MyoII-WT (16 ± 1%) (Fig. 3D). Myosin aggregates persisted for several minutes (Fig. 3 F–H), and thus appear to be distinct from pulsatile actomyosin structures (25, 39, 40). These results demonstrate that the R707C and N98K motor domain variants of Drosophila myosin II form aberrant aggregates at the cell cortex.
Fig. 3.
Myosin II motor variants display aberrant organization at the cell cortex. (A–C) Stills from movies of embryos expressing GFP-tagged MyoII wild-type or motor variants (green) and gap43:mCherry (red) in a MyoII RNAi background. (A′–C′) Zoomed-in regions of A–C. Note the aberrant foci of MyoII-R707C (B) and MyoII-N98K (C) (arrowheads). (Scale bars: 5 μm.) (D) The percentage of AP edges with MyoII aggregates is higher in MyoII-R707C (38 ± 4%) and MyoII-N98K (32 ± 3%) compared with MyoII-WT (16 ± 1%). P < 0.01, t test. Data are mean ± SEM between embryos (4 to 5 embryos per genotype, 30 to 55 edges per embryo). (E–G) Kymographs of AP edges in MyoII-WT, MyoII-R707C, and MyoII-N98K embryos. (H) MyoII intensity along edges at the indicated time points from the kymographs at left. See also Movies S1–S3.
Myosin II Motor Variants Display Slowed Turnover.
During Drosophila axis elongation, actomyosin structures are highly dynamic (24, 25, 33, 36). In mammals, the NM IIB R709C variant displays a slowed rate of translocation along actin filaments in vitro, due to a reduced rate of ATPase cycling and increased binding time to actin (13, 14). These features are predicted to decrease the turnover of myosin bound to actin, raising the possibility that the homologous mutations in Drosophila myosin II could result in slowed turnover dynamics in vivo. To test if the R707C and N98K mutations influence myosin turnover, we performed fluorescence recovery after photobleaching (FRAP) experiments to analyze the dynamics of GFP-tagged myosin variants at AP edges. MyoII-WT was highly dynamic, with a recovery half time of 17 ± 4 s and a mobile fraction of 69 ± 8% (Fig. 4 A–C), similar to the dynamics of the regulatory light chain (24, 33). In contrast, MyoII-R707C and MyoII-N98K turned over more slowly, with recovery half times of 33 ± 6 s and 37 ± 10 s. In addition, MyoII-N98K and MyoII-R707C were less mobile than MyoII-WT, with mobile fractions of 51 ± 10% for MyoII-R707C and 37 ± 5% for MyoII-N98K over the experimental time scale of 2 min (Fig. 4 A and B). Together, these results indicate that the MyoII-R707C and MyoII-N98K variants produce slowed turnover dynamics of Drosophila myosin II in vivo.
Fig. 4.
Myosin II motor variants display slowed turnover dynamics. (A) The percentage of prebleach fluorescence intensity recovered after photobleaching of a 1-μm region of GFP-tagged MyoII at AP cell edges (6 to 9 edges in 3 embryos per genotype). (B) MyoII-R707C and MyoII-N98K had reduced mobile fractions at t = 120 s compared with MyoII-WT. P < 0.05, t test. (C) MyoII-R707C and MyoII-N98K had increased recovery half times compared with MyoII-WT. P < 0.05, t test. Half times were calculated as the time to reach 50% of the final recovered intensity at t = 120 s. Data are mean ± SEM between edges. *P < 0.05.
Myosin II Motor Variants Fail to Promote Rapid Cell Intercalation.
Myosin-generated forces in the Drosophila embryo drive cell intercalation, which elongates the germ band epithelium along the head-to-tail axis. During intercalation, the contraction of AP edges brings 4 or more cells together at a common vertex, and these vertices resolve directionally through the formation of new DV edges (30, 31). We reasoned that the altered dynamics and organization of MyoII-R707C and MyoII-N98K could affect axis elongation by altering the frequency or directionality of cell movements during intercalation. In particular, we hypothesized that MyoII-R707C and MyoII-N98K could produce slowed cell intercalation as an outcome of the slowed turnover dynamics. To investigate this possibility, we analyzed cell intercalation by measuring the rates of 2 steps in the process: edge contraction and vertex resolution (Fig. 5 A and B).
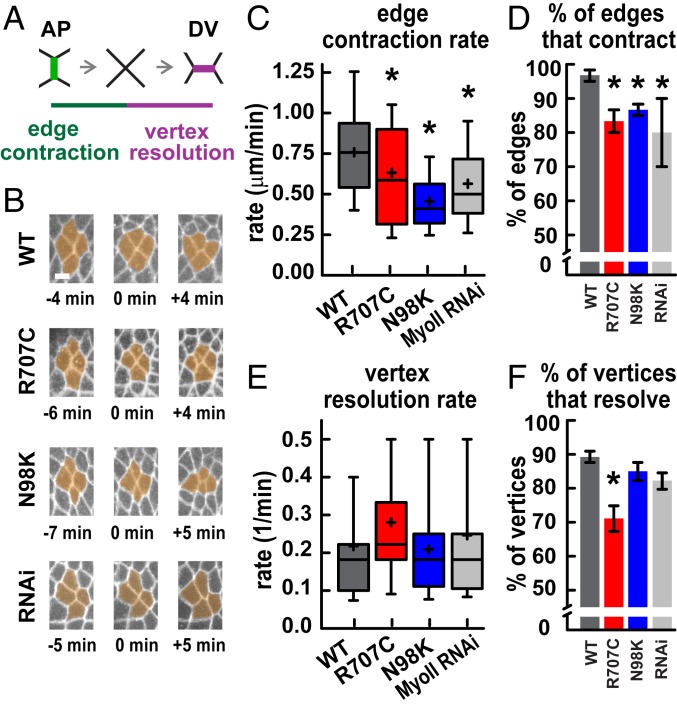
Fig. 5.
Myosin II motor variants produce slowed cell intercalation. (A) Schematic of cell intercalation. (B) Stills from movies showing cell rearrangements, with time indicated relative to vertex formation. (Scale bar: 5 μm.) (C) AP edge contraction rates were decreased in MyoII-R707C, MyoII-N98K, and MyoII RNAi embryos compared with MyoII-WT. *P < 0.05, t test. Edge contraction was slower in MyoII-N98K than MyoII RNAi (P = 0.05) (t test). (D) The percentage of AP edges that completed contraction was decreased in MyoII-R707C, MyoII-N98K, and MyoII RNAi embryos compared with MyoII-WT. *P < 0.05, χ2 test. (E) Vertex resolution rates were not significantly different in all genotypes. P > 0.1, t test (τ = time for a vertex to resolve to form a new edge >1 μm in length; 1/t = vertex resolution rate). (F) The percentage of vertices that resolved directionally to form DV edges was decreased in MyoII-R707C compared with MyoII-WT. *P < 0.05, χ2 test. In C and E, the boxes indicate the 25th to 75th percentiles, with a line at the median, and the whiskers indicate the 10th to 90th percentiles. Mean, plus sign. In D and F, data are mean ± SEM between embryos. In C and D, there are 3 embryos per genotype and 20 edges per embryo; in E and F, there are 4 to 5 embryos per genotype and 15 to 24 vertices per embryo.
In MyoII-WT, the median edge contraction rate was 0.76 μm/min (Fig. 5C). The contraction rate was decreased to 0.59 μm/min in MyoII-R707C and to 0.42 μm/min in MyoII-N98K (Fig. 5C). The MyoII-N98K contraction rate was slightly slower than in MyoII RNAi alone (0.50 μm/min), perhaps because MyoII-N98K may interfere with the activity of the endogenous wild-type myosin protein. In MyoII-WT, nearly all AP edges contracted to form vertices (97 ± 2%), whereas fewer edges completed contraction in MyoII RNAi (80 ± 10%) (Fig. 5D). These defects were not significantly rescued in MyoII-R707C (83 ± 3%) or MyoII-N98K (87 ± 2%). Together, these results demonstrate that edge contraction is disrupted in MyoII-R707C and MyoII-N98K embryos.
Productive cell intercalation requires the formation of new edges between cells that were previously separated along the DV axis, a process that occurs with high spatial fidelity (30, 31). In MyoII-WT embryos, a majority of vertices in which 4 or more cells meet (89 ± 2%) resolved directionally to create new DV edges, promoting cell rearrangement (Fig. 5F). The fraction of vertices that resolved directionally was reduced in MyoII-R707C embryos (71 ± 5%) but was not significantly affected in MyoII-N98K or MyoII-RNAi embryos (Fig. 5F). As vertex resolution requires myosin activity (24, 41, 42), the lack of a defect in this process in MyoII-RNAi embryos is likely due to the fact that these embryos express some endogenous wild-type myosin protein (SI Appendix, Fig. S1). The presence of vertex resolution defects in MyoII-R707C embryos but not in MyoII-N98K embryos is surprising, as MyoII-N98K was more defective for the other phenotypes analyzed. We speculate that resolution defects might be related to other tissue-level properties, such as epithelial folding, that could influence the outcome of vertex resolution.
We also investigated the possibility that MyoII-R707C and MyoII-N98K could influence other cell behaviors during axis elongation. Ectopic tissue folds were often present in MyoII RNAi, MyoII-R707C, and MyoII-N98K embryos (SI Appendix, Fig. S5A), creating local deformations in the tissue. MyoII-R707C and MyoII-N98K embryos also displayed increased cell elongation parallel to the DV axis compared with MyoII-WT, with no significant change in apical cell area (SI Appendix, Fig. S5 B and C). These cell shape defects were not observed in MyoII RNAi embryos and may be due to dominant negative effects of the mutant transgenes. Tissue folds and altered cell shapes could result from autonomous changes within the germ band or nonautonomous effects of extrinsic forces arising from other myosin-dependent processes in the embryo, such as ventral furrow formation, posterior midgut invagination, or amnioserosa morphogenesis (41, 43, 44). Taken together, these findings demonstrate that the myosin II motor domain variants display slowed cell edge contraction and altered cell shape, which may contribute to their failure to support normal cell intercalation and tissue elongation.
Discussion
The Drosophila nonmuscle myosin II motor domain mutations in this study are homologous to mutations associated with MYH9-related diseases and deafness in humans (6, 9–11), but the mechanisms underlying these disease manifestations are poorly understood. Here we show that introducing these motor domain mutations into Drosophila myosin II produces motors that are properly planar polarized and generate anisotropic contractile tension in the developing embryo. However, these myosin II motor domain variants display slowed turnover and increased aggregation, and are less effective at promoting cell edge contraction, resulting in fewer cell rearrangements and reduced axis elongation. Tissue elongation and embryo viability in MyoII-R707C and MyoII-N98K embryos are intermediate between MyoII-WT and MyoII RNAi, suggesting that these variants retain partial myosin activity. Yet the phenotypes of MyoII-R707C and MyoII-N98K embryos are partially distinct from and not strictly intermediate between MyoII-WT and MyoII RNAi. Whereas the motor domain variants are correctly planar polarized, they display little to no rescue of edge contraction. Moreover, MyoII-R707C and MyoII-N98K display slowed turnover and form aberrant foci along cell edges that are not observed in MyoII-WT. MyoII-N98K generally has more deleterious effects in Drosophila compared with MyoII-R707C, similar to the effects of the corresponding mutations on mammalian NM IIA motor activity in vitro (12), potentially reflecting somewhat stronger effects of this mutation on myosin function. These results indicate that molecular properties of the motor domain influence mesoscale features of myosin organization, dynamics, and activity, resulting in defects in cell intercalation and epithelial morphogenesis. These studies shed light on the requirements for myosin motor activities in distinct aspects of a highly conserved myosin-driven process, and on how mutations in the myosin motor domain are translated into defects at the tissue level during development.
Mutations in the myosin motor domain could specifically affect motor activity by altering the myosin ATPase cycle, or they could alter myosin localization or dynamics indirectly through effects on protein folding or altered interactions with F-actin. For example, if myosin variants do not interact properly with F-actin, this could mimic a partial loss of activity. The analogous mutations in mammalian motors do not disrupt actin binding but instead produce slowed ATPase cycling, increased actin binding time, and disrupted actin sliding in vitro (12–14). MyoII-R707C and MyoII-N98K exhibit a proper planar polarized localization but nonetheless fail to drive wild-type cell intercalation in embryos in which half of the myosin pool is expressed from the mutant transgene and the other half is expressed from the endogenous wild-type locus. In one model, different thresholds of motor activity may be necessary for myosin planar polarity and cell intercalation. Partial activity of the MyoII-R707C and MyoII-N98K motors could be sufficient for myosin localization but insufficient to support rapid and directional cell rearrangement. Alternatively, myosin motor activity could be dispensable for its planar polarized localization. This interpretation would suggest that myosin planar polarity, which requires phosphorylation of the myosin regulatory light chain (24), may not require the full activity of the motor. As analogous mutations in mammalian myosin produce motors with reduced actin sliding in vitro (12–14), this raises the possibility that actin binding may be sufficient for myosin planar polarity, whereas actin sliding is required for cell intercalation. Future studies of the role of the motor domain in myosin planar polarity and the biochemical effects of mutations in Drosophila myosin II are needed to distinguish between these models.
The aberrant aggregation of myosin motor variants at the cell cortex suggests that the distribution of myosin motors requires the wild-type motor domain. Myosin aggregation has also been described for Drosophila myosin II tail domain mutants (27) and a subset of mutations that affect phosphorylation of the myosin regulatory light chain (20, 24). Actomyosin networks exist in at least 3 higher-order states: stable aggregates, dynamic pulsatile foci, and uniform networks. Purified and reconstituted actomyosin networks can spontaneously coarsen into stable aggregated structures in vitro when actin filaments are cross-linked or when ATP concentrations are decreased (45, 46). Reducing ATP concentration increases the time myosin spends strongly bound to actin and slows ATPase cycling of the motor, similar to the effects of MYH9-related disease motor domain mutations on human and mouse myosin II in vitro (12–14). These results raise the possibility that myosin motor domain variants could influence the transition between different actomyosin network structures. Stable myosin aggregates are not commonly observed in cells, but pulsatile actomyosin foci that dynamically assemble and dissipate are present in many epithelia undergoing dynamic shape changes (25, 39, 40). In vivo studies indicate a role for actin turnover in producing dynamic pulsing (47). An in silico model predicts that transitions between actomyosin states depend not only on the concentration of myosin motors and actin cross-links, but also on the balance between actin turnover and myosin activity (48). Our findings suggest that disease-associated mutations in the myosin II motor domain favor an aggregated state of myosin in vivo and demonstrate an important role for myosin motor activity in controlling myosin organization. Interestingly, the aggregated structures formed by MyoII-R707C and MyoII-N98K are reminiscent of the myosin-rich Döhle-like bodies observed in neutrophils of humans affected by MYH9-related disorders associated with mutations in the motor and tail domains of NM IIA (11, 49). Going forward, it will be interesting to explore how intrinsic molecular features of the myosin motor influence the higher-order organization of myosin structures and the cell- and tissue-level functions of myosin in normal and disease states.
Methods
GFP-tagged wild-type or mutant myosin II transgenes were expressed from the sqh promoter. Expression of the endogenous nonmuscle myosin II heavy chain was reduced using an shRNA directed to the zipper 3′ UTR (TRiP# GL00623) (50) that was expressed using the Gal4-UAS system with a maternal α-tubulin Gal4-VP16 driver. Cell outlines were visualized with a sqh-gap43:mCherry transgene (51). Embryos were generated at 18 °C and analyzed at room temperature. Embryos were imaged on a PerkinElmer UltraView VOX spinning disk confocal microscope (Figs. 1E, 2 A and B, and 5 and SI Appendix, Figs. S3 and S5), a Zeiss LSM880 laser scanning confocal microscope (Figs. 3 and 4, SI Appendix, Figs. S2 and S4, and Movies S1–S3), and a PerkinElmer RS5 spinning disk confocal microscope with a Micropoint laser (Fig. 2 C and D). Time-lapse movies were analyzed with SEGGA software in MATLAB (35) (Fig. 2A and SI Appendix, Figs. S3 and S5), with PIVlab version 1.41 in MATLAB (52) (Fig. 1E), and by manual analysis (Fig. 5). Details are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Nicole Guillery and Mohammed Tarek Islam for assistance with immunofluorescence and image analysis, Dan Kiehart for the GFP-Zipper plasmid and Zipper antibody, Adam Martin for sqh-gap43:mCherry fly stocks, the Bloomington Drosophila Stock Center (NIH Grant P40OD018537) and the Transgenic RNAi Project (TRiP) at Harvard Medical School (National Institute of General Medical Sciences [NIGMS] Grant R01 GM084947) for transgenic RNAi fly stocks, Frederik Wirtz-Peitz for the pSqh-attB vector, Maria Bustillo for help with movie acquisition, Dene Farrell and Ori Weitz for computational methods, and Bob Adelstein, Jim Sellers, and Sarah Heissler for helpful discussions. This work was supported by NIH/NIGMS Grant R01 GM102803 (to J.A.Z.). K.E.K. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund, a Clare Boothe Luce Professorship, and a Packard Fellowship. J.A.Z. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909227116/-/DCSupplemental.
References
- 1.Heisenberg C. P., Bellaïche Y., Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Ma X., Adelstein R. S., The role of vertebrate nonmuscle Myosin II in development and human disease. Bioarchitecture 4, 88–102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vicente-Manzanares M., Ma X., Adelstein R. S., Horwitz A. R., Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green R. A., Paluch E., Oegema K., Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 28, 29–58 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Sellers J. R., Myosins: A diverse superfamily. Biochim. Biophys. Acta 1496, 3–22 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Donaudy F., et al. , Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4). Am. J. Hum. Genet. 74, 770–776 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuzovic L., et al. , A human de novo mutation in MYH10 phenocopies the loss of function mutation in mice. Rare Dis. 1, e26144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pecci A., et al. , MYH9-related disease: A novel prognostic model to predict the clinical evolution of the disease based on genotype-phenotype correlations. Hum. Mutat. 35, 236–247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley M. J., Jawien W., Ortel T. L., Korczak J. F., Mutation of MYH9, encoding non-muscle myosin heavy chain A, in May-Hegglin anomaly. Nat. Genet. 26, 106–108 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Seri M., et al. ; May-Heggllin/Fechtner Syndrome Consortium , Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. Nat. Genet. 26, 103–105 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Seri M., et al. , MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore) 82, 203–215 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Hu A., Wang F., Sellers J. R., Mutations in human nonmuscle myosin IIA found in patients with May-Hegglin anomaly and Fechtner syndrome result in impaired enzymatic function. J. Biol. Chem. 277, 46512–46517 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Kim K. Y., Kovács M., Kawamoto S., Sellers J. R., Adelstein R. S., Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J. Biol. Chem. 280, 22769–22775 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Ma X., et al. , Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc. Natl. Acad. Sci. U.S.A. 109, 4509–4514 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X., Kawamoto S., Hara Y., Adelstein R. S., A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons. Mol. Biol. Cell 15, 2568–2579 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X., Adelstein R. S., A point mutation in Myh10 causes major defects in heart development and body wall closure. Circ. Cardiovasc. Genet. 7, 257–265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heissler S. M., Chinthalapudi K., Sellers J. R., Kinetic characterization of the sole nonmuscle myosin-2 from the model organism Drosophila melanogaster. FASEB J. 29, 1456–1466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiehart D. P., Feghali R., Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103, 1517–1525 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawes-Hoang R. E., et al. , folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Vasquez C. G., Tworoger M., Martin A. C., Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J. Cell Biol. 206, 435–450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasquez C. G., Heissler S. M., Billington N., Sellers J. R., Martin A. C., Drosophila non-muscle myosin II motor activity determines the rate of tissue folding. Elife 5, e20828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke J. D., Montague R. A., Kiehart D. P., Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr. Biol. 15, 2208–2221 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Simões S., et al. , Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev. Cell 19, 377–388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasza K. E., Farrell D. L., Zallen J. A., Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 111, 11732–11737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munjal A., Philippe J. M., Munro E., Lecuit T., A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351–355 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Franke J. D., Montague R. A., Rickoll W. L., Kiehart D. P., An MYH9 human disease model in flies: Site-directed mutagenesis of the Drosophila non-muscle myosin II results in hypomorphic alleles with dominant character. Hum. Mol. Genet. 16, 3160–3173 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Li T., et al. , The E3 ligase Ubr3 regulates Usher syndrome and MYH9 disorder proteins in the auditory organs of Drosophila and mammals. Elife 5, e15258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irvine K. D., Wieschaus E., Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120, 827–841 (1994). [DOI] [PubMed] [Google Scholar]
- 29.Zallen J. A., Wieschaus E., Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Bertet C., Sulak L., Lecuit T., Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Blankenship J. T., Backovic S. T., Sanny J. S., Weitz O., Zallen J. A., Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459–470 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Rauzi M., Verant P., Lecuit T., Lenne P. F., Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401–1410 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Gonzalez R., Simoes S., Röper J. C., Eaton S., Zallen J. A., Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauzi M., Lenne P. F., Lecuit T., Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Farrell D. L., Weitz O., Magnasco M. O., Zallen J. A., SEGGA: A toolset for rapid automated analysis of epithelial cell polarity and dynamics. Development 144, 1725–1734 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tetley R. J., Blanchard G. B., Fletcher A. G., Adams R. J., Sanson B., Unipolar distributions of junctional Myosin II identify cell stripe boundaries that drive cell intercalation throughout Drosophila axis extension. Elife 5, e12094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farhadifar R., Röper J. C., Aigouy B., Eaton S., Jülicher F., The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Hutson M. S., et al. , Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145–149 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Martin A. C., Kaschube M., Wieschaus E. F., Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Gonzalez R., Zallen J. A., Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Phys. Biol. 8, 045005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collinet C., Rauzi M., Lenne P. F., Lecuit T., Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol. 17, 1247–1258 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Yu J. C., Fernandez-Gonzalez R., Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. Elife 5, e10757 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler L. C., et al. , Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol. 11, 859–864 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Lye C. M., et al. , Mechanical coupling between endoderm invagination and axis extension in Drosophila. PLoS Biol. 13, e1002292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soares e Silva M., et al. , Active multistage coarsening of actin networks driven by myosin motors. Proc. Natl. Acad. Sci. U.S.A. 108, 9408–9413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murrell M. P., Gardel M. L., F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl. Acad. Sci. U.S.A. 109, 20820–20825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jodoin J. N., et al. , Stable force balance between epithelial cells arises from F-actin turnover. Dev. Cell 35, 685–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mak M., Zaman M. H., Kamm R. D., Kim T., Interplay of active processes modulates tension and drives phase transition in self-renewing, motor-driven cytoskeletal networks. Nat. Commun. 7, 10323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunishima S., et al. , Immunofluorescence analysis of neutrophil nonmuscle myosin heavy chain-A in MYH9 disorders: Association of subcellular localization with MYH9 mutations. Lab. Invest. 83, 115–122 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Ni J. Q., et al. , A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin A. C., Gelbart M., Fernandez-Gonzalez R., Kaschube M., Wieschaus E. F., Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thielicke W., Stamhuis E. J., PIVlab: Towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. J. Open Res. Softw. 2, e30 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.