Significance
A substantial portion of cancer patients treated by immune checkpoint (IC) therapy develop immune-related adverse events (irAEs), which result in significant morbidity and mortality. We sought to understand the mechanisms of these irAEs and to identify biomarkers to predict irAEs to maximize the clinical benefits of IC therapy. We screened plasma samples from patients who developed IC therapy-induced hypophysitis or pneumonitis against a cDNA expression library of the brain or lungs and directly identified autoimmune antibodies correlating with hypophysitis or pneumonitis. Our data suggest that these autoantibodies correlate with hypophysitis or pneumonitis and should be tested as the first predictive biomarkers for early detection, timely treatment, and close monitoring of these 2 potentially fatal irAEs.
Keywords: hypophysitis, pneumonitis, autoimmune antibody, immune checkpoint therapy, immune-related adverse events
Abstract
Immune checkpoint (IC) therapy provides substantial benefits to cancer patients but can also cause distinctive toxicities termed immune-related adverse events (irAEs). Biomarkers to predict toxicities will be necessary to improve management of patients receiving IC therapy. We relied on serological analysis of recombinant cDNA expression libraries to evaluate plasma samples from patients treated with IC therapy and identified autoantibodies, both in pretreatment and on-treatment samples prior to the development of irAEs, which correlate with the development of immune-related hypophysitis (anti-GNAL and anti-ITM2B autoantibodies) and pneumonitis (anti-CD74 autoantibody). We developed an enzyme-linked immunosorbent assay and tested additional patient samples to confirm our initial findings. Collectively, our data suggest that autoantibodies may correlate with irAEs related to IC therapy, and specific autoantibodies may be detected early for the management of irAEs.
Immune checkpoint (IC) therapies targeting cytotoxic T lymphocyte antigen-4 receptor (CTLA-4), programmed death-1 (PD1), and programmed death-ligand 1 (PD-L1) have provided substantial clinical benefit to cancer patients (1, 2). In addition to its intended antitumor effect, IC therapy can also cause distinctive toxicities termed immune-related adverse events (irAEs), such as dermatitis, colitis, hepatitis, hypophysitis, and pneumonitis (3–5). When anti–CTLA-4 and anti-PD1 are combined, 40 to 50% of treated patients develop irAEs of grade 3 or higher, which result in significant morbidity and mortality (6, 7). Therefore, early diagnosis and timely management of these irAEs are essential for maximizing the clinical use and benefits of IC therapy.
Hypophysitis is a serious irAE experienced by a subset of patients who receive IC therapy. It is defined as an inflammatory disease of the pituitary gland, characterized by an infiltration of the pituitary gland with immune cells, mainly lymphocytes and plasma cells, which leads to expansion and atrophy of the pituitary (8–10). A recent study using a mouse model of autoimmune hypophysitis showed that T cells infiltrated the mouse pituitary gland, proliferated, and secreted IFN-γ and interleukin-17 (IL-17). Similarly, T cells were found to infiltrate the pituitary gland in patients affected by autoimmune hypophysitis (11). However, to our knowledge, there have been no systemic studies in patients implicating autoimmune antibodies in the development of hypophysitis in response to IC therapy.
Pneumonitis is another life-threatening adverse event that has been observed in patients who receive IC therapy, and it is defined as inflammation of the lung parenchyma, similar to what is observed in interstitial lung disease (12). The mechanism responsible for IC therapy-induced pneumonitis remains poorly understood, but currently available evidence suggests that ipilimumab-induced pneumonitis involves dysregulated effector and regulatory T cells in the pulmonary interstitium, leading to an inflammatory response (13). On the other hand, the mechanism of anti–PD1-induced pneumonitis is thought to be influenced by PD-L2, which is detected in numerous types of antigen-presenting cells such as macrophages and dendritic cells (14). It is not clear whether autoimmune antibodies are involved in the development of hypophysitis or pneumonitis.
By taking advantage of the high-throughput serological analysis of recombinant cDNA expression (SEREX) technique that enables large-scale screening of autoantibodies in the plasma of IC-therapy–treated patients against the cDNA expression library that expresses over 2 × 105 antigen fragments of a target organ, we carried out experiments to evaluate the role of autoantibodies in IC-therapy–induced irAEs. We identified autoantibodies against guanine nucleotide-binding protein G(olf) subunit alpha (GNAL) and integral membrane protein 2B (ITM2B) that were associated with IC-therapy–induced hypophysitis, and an autoantibody against CD74 that was associated with IC-therapy–induced pneumonitis. We then used recombinant GNAL, ITM2B, and CD74 proteins to design an enzyme-linked immunosorbent assay (ELISA) to confirm the SEREX screening findings in independent discovery and confirmation cohorts of patients. We also found that these identified autoantigens were expressed in target organs, i.e., in the pituitary gland and lungs, respectively.
Results
Identification of Hypophysitis-Related Autoantibodies.
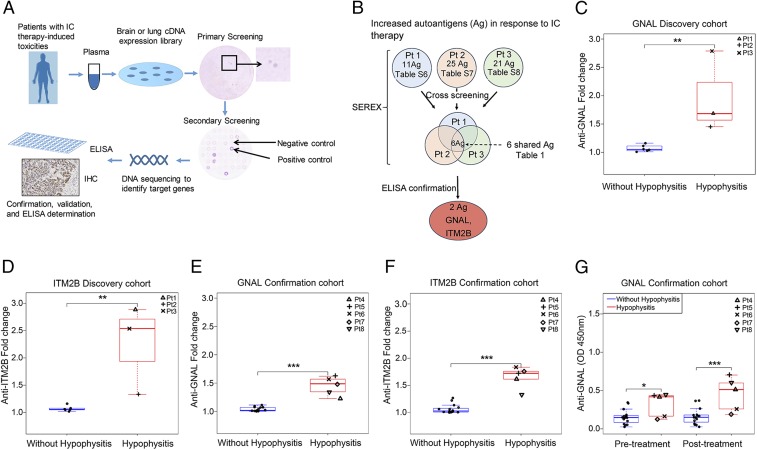
To identify autoantibodies in patients with immune-related hypophysitis, we utilized SEREX (15) as shown in Fig. 1A. All patient samples were collected after appropriate informed consent was obtained on MD Anderson Cancer Center institutional review board-approved protocols (protocol PA13-0291, protocol 2009-0135, and protocol 2009-0378). We tested pre- and posttreatment samples from 3 patients with IC-therapy–induced hypophysitis and designated these 3 patients as our discovery cohort. Information regarding these 3 patients (patients 1, 2, and 3) can be found in SI Appendix, Tables S1 and S2. We identified a total of 83 candidate genes that may encode proteins reactive to autoantibodies in the plasma samples (SI Appendix, Tables S3–S5). Among these 83 genes, 57 displayed higher levels of reactivity in posttreatment plasma as compared to those in pretreatment plasma, suggesting an increase of their corresponding autoantibodies in response to IC therapy (SI Appendix, Tables S6–S8). Cross-screening of positive clones from 1 patient with plasma from the other 2 patients revealed that all 3 patients shared autoantibodies reactive to 6 potential proteins including GNAL, ITM2B, migration and invasion inhibitory protein (MIIP), prostaglandin D2 synthase (PTGDS), APH1A gamma secretase (APH1A), and ERM-like protein (ERMN) (Fig. 1B and Table 1).
Fig. 1.
Plasma anti-GNAL and anti-ITM2B autoantibodies correlate with IC-therapy–induced hypophysitis. (A) Schematic illustration of the SEREX-based identification of IC-therapy–induced toxicity-related autoantibodies and confirmation process. (B) Workflow to identify autoantigens (Ag) by SEREX and to confirm hypophysitis-related autoantibodies. (C) Anti-GNAL and (D) anti-ITM2B autoantibody fold change in response to IC therapy in the plasma of discovery cohort patients (hypophysitis = 3; without hypophysitis = 6). (E) Anti-GNAL and (F) anti-ITM2B autoantibody fold change in the plasma of confirmation cohort patients (hypophysitis = 5; without hypophysitis = 15). (G) Anti-GNAL autoantibody levels in pre- and posttreatment in the confirmation cohorts. Values represent pre- and posttreatment levels, and fold changes represent increase from baseline in response to IC therapy. All boxplots have median center values. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
Shared increased autoantibodies/autoantigens between hypophysitis patients 1, 2, and 3 in response to ipilimumab
| Gene name and description | Molecular functions and associated diseases |
| GNAL, G-protein alpha-activating activity polypeptide olfactory type | Mediates odorant signaling in olfactory epithelium; associated with dystonia 25 |
| ITM2B | Associated with ganglion cell abnormalities, cerebral amyloid angiopathy, and familial British and Danish dementia |
| MIIP | Suppresses tumors by inhibiting cell migration and invasion |
| PTGDS | Involved in variety of central nervous system functions such as sedation, non–rapid-eye-movement sleep, and prostaglandin 2-induced allodynia |
| APH1A gamma secretase | Catalyzes the cleavage of Notch receptor and beta-amyloid precursor protein, associated with Alzheimer’s disease |
| ERMN (ERM-like protein) | Plays a role in late-stage oligodendroglia maturation, myelin/Ranvier node formation |
To determine which of the 6 proteins correlated with hypophysitis, we used patient plasma samples in ELISAs that we developed for these 6 recombinant proteins. We identified autoantibodies to GNAL and ITM2B, but not the other 4 proteins correlated with hypophysitis, using plasma of the 3 patients with IC-therapy–induced hypophysitis. In patients with hypophysitis, we found a median of 1.7-fold increase in autoantibody levels against GNAL in posttreatment samples as compared to pretreatment samples. However, in patients without hypophysitis, we did not observe a significant increase in autoantibody levels against GNAL in posttreatment samples as compared to pretreatment samples. The median fold increase in patients with hypophysitis was significantly higher as compared to that in patients without hypophysitis (Fig. 1C). Similarly, in patients with hypophysitis, we found a median of 2.5-fold increase in autoantibody levels against ITM2B in posttreatment samples as compared to pretreatment samples. However, in patients without hypophysitis, we did not observe a significant increase in autoantibody levels against ITM2B in posttreatment samples as compared to pretreatment samples. The median fold increase in patients with hypophysitis was significantly higher as compared to that in patients without hypophysitis (Fig. 1D). This finding was verified in a confirmation cohort of 20 patients, consisting of 5 with and 15 without hypophysitis (Fig. 1 E and F). In patients with hypophysitis we found a median 1.49-fold increase for anti-GNAL autoantibodies (Fig. 1E) and a 1.7-fold increase for anti-ITM2B autoantibodies (Fig. 1F) when comparing pre- and posttreatment plasma. These values were significantly higher when compared to patients without hypophysitis (Fig. 1 E and F). These data show that fold change in autoantibodies against GNAL or ITM2B with IC therapy are associated with future development of hypophysitis. The fold changes of anti-GNAL and anti-ITM2B autoantibodies were significantly associated with the development of hypophysitis with a complete separation between values for patients with and without hypophysitis (area under the curve [AUC] = 1; P < 0.001 for each [Table 2]). We also noted a temporal relationship between the autoantibody levels and hypophysitis with increased anti-GNAL and anti-ITM2B autoantibody levels at the onset of symptoms as compared to baseline (SI Appendix, Fig. S1 A–D). However, we do not know whether anti-GNAL and anti-ITM2B autoantibodies decreased to baseline levels at resolution of the hypophysitis due to lack of sample collection at those time points. Interestingly, we found significantly higher levels of autoantibody against GNAL at pre- and posttreatment plasma of patients with hypophysitis compared with those without hypophysitis in the confirmation cohort (Fig. 1G), suggesting that a preexisting autoantibody against GNAL at baseline as well as after treatment may be associated with increased risk of IC-therapy–induced hypophysitis. Similar data were not observed for autoantibody against ITM2B. These findings suggest that anti-GNAL autoantibody warrants further investigation as a potential predictive and on-treatment biomarker for IC-therapy–induced hypophysitis, whereas anti-ITM2B autoantibody may be investigated as a potential on-treatment biomarker for IC-therapy–induced hypophysitis.
Table 2.
Associations between selected markers and adverse outcomes
| Marker | Unit for odds ratio | N | Odds ratio | (95% CI) | AUC for ROC | P value |
| Predicting hypophysitis | ||||||
| GNAL fold change | 0.10 | 20 | 5.13 | (1.95, infinity) | 1.00 | <0.001 |
| GNAL pretreatment | 0.10 | 20 | 2.66 | (1.14, 7.29) | 0.79 | 0.02 |
| GNAL posttreatment | 0.10 | 20 | 2.77 | (1.30, 9.03) | 0.92 | 0.004 |
| ITM2B fold change | 0.10 | 20 | 3.93 | (1.59, infinity) | 1.00 | <0.001 |
| ITM2B pretreatment | 0.10 | 20 | 0.99 | (0.20, 4.31) | 0.55 | >0.99 |
| ITM2B posttreatment | 0.10 | 20 | 3.28 | (0.88, 15.99) | 0.75 | 0.08 |
| Predicting pneumonitis | ||||||
| CD74 fold change | 0.01 | 32 | 1.54 | (1.07, 2.21) | 1.00 | 0.02 |
| CD74 pretreatment | 0.01 | 32 | 1.25 | (1.03, 1.52) | 0.76 | 0.03 |
| CD74 posttreatment | 0.01 | 32 | 1.66 | (1.14, 2.42) | 0.95 | 0.01 |
Due to the separation of values between those with and without the adverse outcome, the odds ratios are very high, and the small sample sizes mean large confidence intervals. The units for the odds ratio show a continuous effect for every increment of that size over the narrow range of values present in our samples (Figs. 1 and 2). For example, a patient with a GNAL fold increase 0.10 higher than another patient is estimated to be 5.1 times more likely to have hypophysitis than the patient with the lower increase. Due to the very small sample size, the confidence interval for this estimate varies widely (1.95, infinity). Similarly, an increase of 0.25 (not shown) is associated with an odds ratio and confidence interval of 59.7 (5.3, infinity). The effect is so large for CD74 that the odds ratio unit is 0.01. An increase of 0.25 (not shown) is associated with an odds ratio and confidence interval of 50,294 (6, 422,160,000). P values set in boldface indicate statistical significance. ROC, receiver operating characteristic.
Identification of Pneumonitis-Related Autoantibodies.
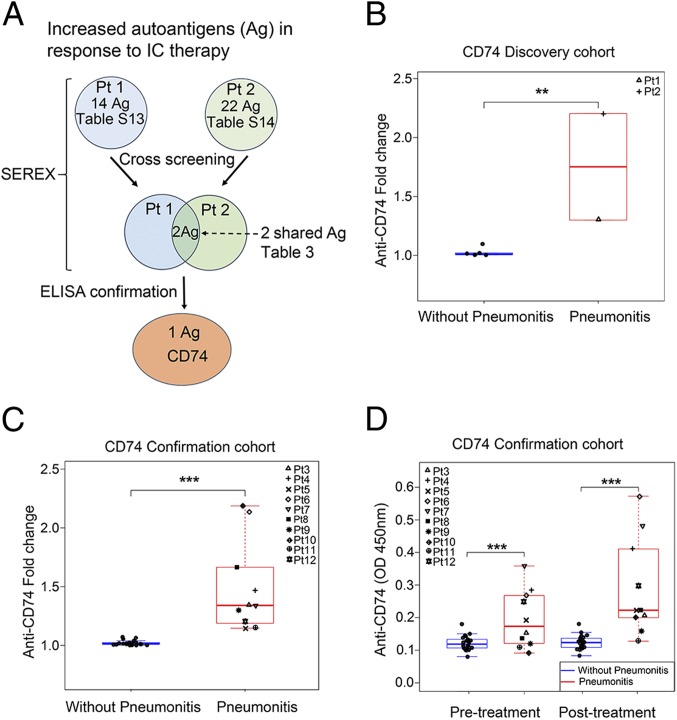
We also carried out SEREX to identify autoantibodies in patients with IC-therapy–induced pneumonitis (Fig. 1A). We tested pre- and posttreatment samples from 2 patients with IC-therapy–induced pneumonitis and designated these 2 patients (patients 1 and 2) as our discovery cohort (SI Appendix, Tables S9 and S10). We identified a total of 99 candidate genes that may encode proteins reactive with autoantibodies (SI Appendix, Tables S11 and S12). Among the 99 genes, 36 displayed increased reactivity in response to IC therapy (SI Appendix, Tables S13 and S14). These 2 patients shared autoantibodies reactive with 2 proteins including CD74 and serum/glucocorticoid-regulated kinase 1 (SGK1) (Fig. 2A and Table 3). To determine which of the 2 proteins correlated with pneumonitis, we used plasma samples from these 2 patients in ELISAs that we developed for these 2 recombinant proteins. We identified autoantibodies against CD74 but not SGK1 correlated to pneumonitis. In patients with pneumonitis, we found a median 1.75-fold increase in autoantibody levels against CD74 in posttreatment samples as compared to pretreatment. However, in patients without pneumonitis, we did not observe a significant increase in autoantibody levels against CD74 in posttreatment samples as compared to pretreatment samples. The median fold increase in patients with pneumonitis was significantly higher as compared to that in patients without pneumonitis (Fig. 2B). This finding was verified in a confirmation cohort of 32 patients, 10 with and 22 without pneumonitis (Fig. 2C). In patients with pneumonitis, we found a median 1.34-fold significant increase of autoantibodies against CD74 when comparing pre- and posttreatment plasma samples. However, in patients without pneumonitis, we did not observe a marked increase in autoantibodies against CD74 when comparing pre- and posttreatment plasma samples (Fig. 2C). These data show that fold change in autoantibodies against CD74 with treatment is associated with development of pneumonitis. According to the exact logistic regression statistical model, the fold change in CD74 measure had a complete separation between values for patients with and without pneumonitis (AUC = 1; P = 0.02 [Table 2]). In addition, we found that autoantibody levels against CD74 in pre- and posttreatment were significantly increased in patients with pneumonitis compared with those without pneumonitis in the confirmation cohort (Fig. 2D). Therefore, anti-CD74 autoantibody may be useful in identifying patients likely to develop pneumonitis, both before starting treatment and after initial treatment.
Fig. 2.
Plasma anti-CD74 autoantibody correlates with IC-therapy–induced pneumonitis. (A) Workflow to identify autoantigens (Ag) by SEREX and to confirm pneumonitis-related autoantibodies. (B and C) Anti-CD74 autoantibody fold change in response to IC therapy in the plasma of the discovery (B) (pneumonitis = 2; without pneumonitis = 6) and the confirmation (C) (pneumonitis = 10; without pneumonitis = 22) cohorts. (D) Anti-CD74 autoantibody levels in pre- and posttreatment in the confirmation cohort. Values represent pre- and posttreatment levels, and fold changes represent the increase from baseline in response to IC therapy. All boxplots have median center values. **P < 0.01; ***P < 0.001.
Table 3.
Shared increased autoantibodies/autoantigens between pneumonitis patients 1 and 2 in response to IC therapy
| Gene name and description | Molecular functions and associated diseases |
| CD74 | MHC class II chaperone and cell-surface receptor for MIF, associated with lung inflammation, immune diseases, and various cancers |
| SGK1 | Regulates ion channels, membrane transporters, mediates ventilator-induced lung injury, associated with pseudohypoaldosteronism |
Expression of GNAL and ITM2B in Human Pituitary and CD74 in Lung Tissues.
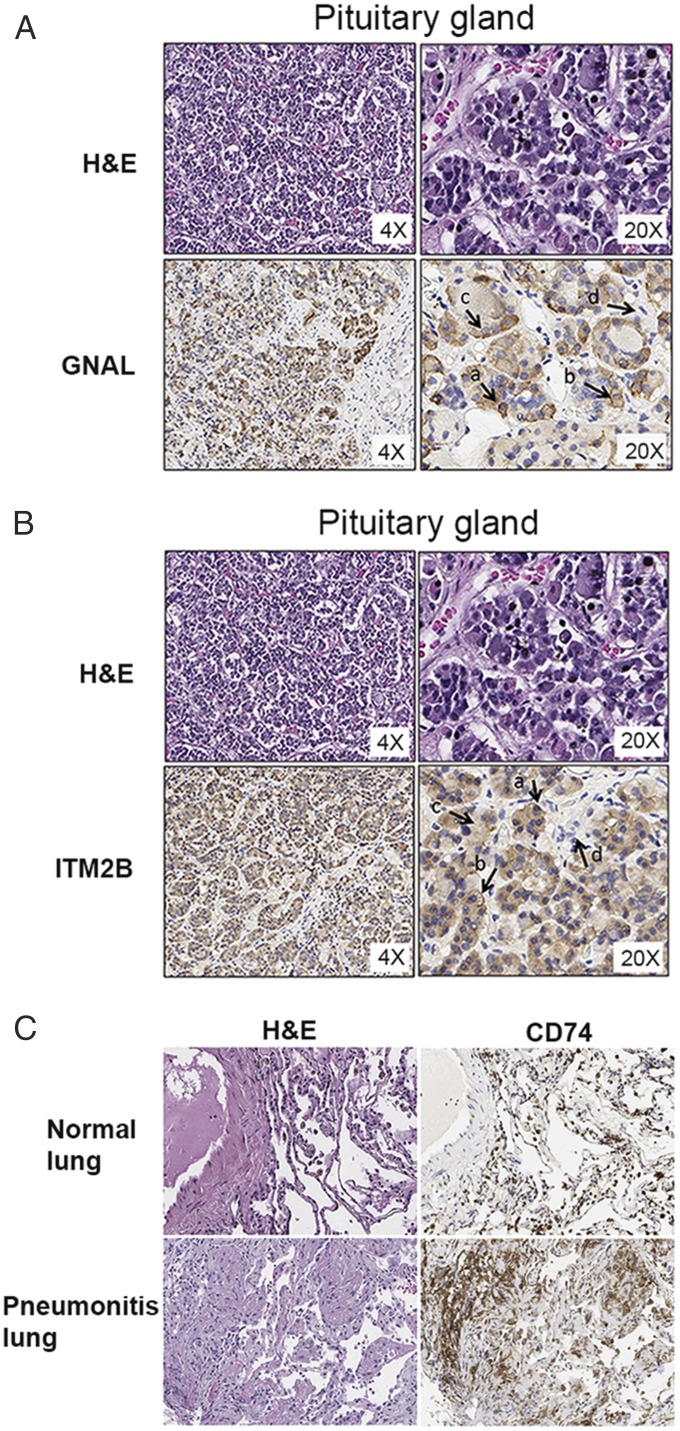
In order for GNAL and ITM2B autoantibodies to play a role in hypophysitis development, the pituitary gland must express the target proteins that react with these 2 autoantibodies. Therefore, we examined whether GNAL and ITM2B proteins are expressed in human pituitary tissue. As expected, immunohistochemical (IHC) analysis showed that both GNAL and ITM2B were expressed in the pituitary glandular epithelium, but not in the stromal component (Fig. 3 A and B). Similarly, in order for CD74 autoantibody to play a role in pneumonitis, the lung tissues must express the target protein that reacts with this autoantibody. Therefore, we examined the expression of CD74 protein in normal human lung tissue and the lung tissue of a patient who developed pneumonitis from IC therapy. We found that normal human lung tissue expresses modest levels of CD74, but CD74 expression levels were dramatically higher in the lung of the patient with IC-therapy–induced pneumonitis (patient 4) (Fig. 3C), suggesting that anti-CD74 could play an important role in the development of pneumonitis.
Fig. 3.
GNAL and ITM2B are expressed in normal human pituitary gland tissue and increased expression of CD74 in the lung of a pneumonitis patient. (A and B) IHC staining of normal human pituitary gland shows GNAL (A) and ITM2B (B) expression on the glandular epithelium. Arrows indicate the expression levels: (a) strong, (b) moderate, (c) light, and (d) negative. (C) IHC staining of human lung shows considerably higher expression of CD74 in the lung of a patient with IC-therapy–induced pneumonitis than that in a normal lung.
Discussion
Tremendous efforts have been made to identify potential predictive biomarkers for IC-therapy–induced irAEs. These efforts include evaluation of immune cell phenotyping (16), cytokine assays (17), immune repertoire analysis (18), genetic variability of immune regulation (19), gastrointestinal microbiome diversity (20), and autoantibodies (21), etc. Among these potential biomarkers, autoantibodies have garnered particular interest as they can be easily detected from minimally invasive blood collection. A recent study investigating the autoantibody repertoire of a small number of patients with advanced metastatic melanoma treated with ipilimumab demonstrated that patients who developed irAEs were characterized by increases in the repertoire of autoantibodies directed against both self and cancer antigens at time points that preceded the development of the toxicity (21). Another recent study identified increased levels of specific B cell populations, including antibody-producing plasmablasts, in patients receiving combination IC therapy treatment. Interestingly, these changes preceded and correlated with both the frequency and the timing of irAEs (22). In fact, a few pituitary autoantibodies, including anti-thyrotrophs, anti-corticotrophs, and anti-gonadotrophs, have been reported to be increased in patients with hypophysitis (23).
GNAL is expressed primarily in the olfactory neuroepithelium, brain, spleen, lung, heart, pancreas, and testis (24). GNAL binds to D1 dopamine and A2A adenosine receptors, which leads to the activation of adenylyl cyclase and the cAMP-signaling pathway. In the pituitary, cAMP has been established as a key signal molecule that controls responsiveness to mitogens and secretagogues. It stimulates both cell proliferation and hormone synthesis and/or secretion including thyroid stimulating hormone (TSH) (25–29). Therefore, autoantibodies against GNAL may contribute to the development of hypophysitis and may lead to a decrease in TSH, an early marker of IC-therapy–induced hypophysitis (30). ITM2B is expressed in human brain, retina, heart, placenta, kidney, pancreas, and liver tissues (31–33). ITM2B stimulates the secretion of insulin-degrading enzyme in the brain and causes the stimulation and release of the pituitary hormone ACTH through the elimination of the inhibitory effect of guanylate cyclase GC (34–36). Therefore, autoantibody against ITM2B may contribute to a decrease of ACTH, another hallmark of IC-therapy–induced hypophysitis.
Similarly, agonistic anti-CD74 antibody has been reported to act through CD74, an intracellular chaperone molecule for MHCII, but can be expressed on the cell membrane of immune cells including macrophages (independent from MHC II) to serve as a high-affinity receptor for macrophage migration-inhibitory factor (MIF) (37, 38) to stimulate expression of inflammatory mediators (39). Overexpression of CD74 has been reported in human interstitial pneumonitis (40) and mouse acute lung injury (41). In addition, a recent report demonstrated that distinct baseline antibody profiles may be associated with severe irAEs in melanoma patients treated with IC therapy (42). Despite these findings, no validated biomarkers have been found to date to predict irAEs.
We show that 1) anti-GNAL and anti-ITM2B autoantibodies are associated with the development of IC-therapy–induced hypophysitis and that anti-CD74 autoantibody is associated with IC-therapy–induced pneumonitis development; 2) anti-GNAL autoantibody has the potential to act as both a predictive and an on-treatment biomarker for IC-therapy–induced hypophysitis, whereas anti-ITM2B autoantibody has the potential to act as an on-treatment biomarker for IC-therapy–induced hypophysitis; and 3) anti-CD74 autoantibody has the potential to act as both a predictive and an on-treatment biomarker for IC therapy-induced pneumonitis. Our findings warrant further investigation in larger, prospective studies. If confirmed in larger studies and validated in future studies as predictive biomarkers, these autoantibodies will serve an important role to enable early detection, close monitoring, and timely treatment of hypophysitis or pneumonitis with immunosuppressive and other supportive therapies and thus maximize clinical benefits of IC therapy for cancer patients.
Supplementary Material
Acknowledgments
This research work is supported in part by the MD Anderson Cancer Center Immunotherapy Platform; the MD Anderson Physician Scientist Award (J.G.); Khalifa Physician Scientist Award (J.G.); Andrew Sabin Family Foundation Fellows Award (J.G.); Doris Duke Charitable Foundation Career Development Award (J.G.); and the Wendy and Leslie Irvin Barnhart Fund (J.G.). In addition, we thank Ashura Khan, Lisa Pruitt, Amelia Brown, and Cesar Ortiz for technical support; P.S. and J.P.A. are members of the Parker Institute for Cancer Immunotherapy.
Footnotes
Competing interest statement: J.P.A. has ownership in Jounce, Neon, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Hummingbird, Dragonfly, Lytix, and Tvardi Therapeutics and serves as a consultant for Jounce, Kite Pharma, Neon, Amgen, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Tvardi Therapeutics, Lytix, Hummingbird, and Dragonfly. P.S. has ownership in Jounce, Neon, Constellation, Oncolytics, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Lytix, Hummingbird, and Dragonfly and serves as a consultant for Constellation, Jounce, Kite Pharma, Neon, BioAtla, Pieris Pharmaceuticals, Oncolytics Biotech, Merck, BioMx, Forty-Seven, Polaris, Apricity, Marker Therapeutics, Codiak, ImaginAb, Hummingbird, Lytix, and Dragonfly. J.G. serves as a consultant for ARMO Biosciences, AstraZeneca, CRISPR Therapeutics, Jounce, Nektar, Polaris, Pfizer, and Symphogen. C.G.D. and J.P.A. are coauthors on a 2016 workshop report.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908079116/-/DCSupplemental.
References
- 1.Sharma P., Allison J. P., The future of immune checkpoint therapy. Science 348, 56–61 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Sharma P., Allison J. P., Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 161, 205–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi F. S., et al. , Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J. S., Kähler K. C., Hauschild A., Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Gao J., et al. , Review of immune-related adverse events in prostate cancer patients treated with ipilimumab: MD Anderson experience. Oncogene 34, 5411–5417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J., et al. , Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer R. J., et al. ; CheckMate 214 Investigators , Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupi I., et al. , From pituitary expansion to empty sella: Disease progression in a mouse model of autoimmune hypophysitis. Endocrinology 152, 4190–4198 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutenberg A., Buslei R., Fahlbusch R., Buchfelder M., Brück W., Immunopathology of primary hypophysitis: Implications for pathogenesis. Am. J. Surg. Pathol. 29, 329–338 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Caturegli P., Autoimmune hypophysitis: An underestimated disease in search of its autoantigen(s). J. Clin. Endocrinol. Metab. 92, 2038–2040 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Lin H. H., et al. , In situ activation of pituitary-infiltrating T lymphocytes in autoimmune hypophysitis. Sci. Rep. 7, 43492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabchi S., Messier C., Blais N., Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr. Opin. Oncol. 28, 269–277 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Pardoll D. M., The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latchman Y., et al. , PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Hoeppner L. H., Dubovsky J. A., Dunphy E. J., McNeel D. G., Humoral immune responses to testis antigens in sera from patients with prostate cancer. Cancer Immun. 6, 1 (2006). [PubMed] [Google Scholar]
- 16.Ermann J., Rao D. A., Teslovich N. C., Brenner M. B., Raychaudhuri S., Immune cell profiling to guide therapeutic decisions in rheumatic diseases. Nat. Rev. Rheumatol. 11, 541–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarhini A. A., et al. , Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 3, 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subudhi S. K., et al. , Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl. Acad. Sci. U.S.A. 113, 11919–11924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi N. A., et al. , Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Routy B., et al. , The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Da Gama Duarte J., et al. , Autoantibodies may predict immune-related toxicity: Results from a Phase I study of intralesional Bacillus Calmette-Guérin followed by ipilimumab in patients with advanced metastatic melanoma. Front. Immunol. 9, 411 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das R., et al. , Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwama S., et al. , Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra45 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Régnauld K. L., Leteurtre E., Gutkind S. J., Gespach C. P., Emami S., Activation of adenylyl cyclases, regulation of insulin status, and cell survival by G(alpha)olf in pancreatic beta-cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R870–R880 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Burton F. H., Hasel K. W., Bloom F. E., Sutcliffe J. G., Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature 350, 74–77 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Kim G. L., et al. , Generation of immortal cell lines from the adult pituitary: Role of cAMP on differentiation of SOX2-expressing progenitor cells to mature gonadotropes. PLoS One 6, e27799 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertuit M., Barlier A., Enjalbert A., Gérard C., Signalling pathway alterations in pituitary adenomas: Involvement of Gsalpha, cAMP and mitogen-activated protein kinases. J. Neuroendocrinol. 21, 869–877 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Peverelli E., Mantovani G., Lania A. G., Spada A., cAMP in the pituitary: An old messenger for multiple signals. J. Mol. Endocrinol. 52, R67–R77 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K., et al. , cAMP response element-binding protein-binding protein mediates thyrotropin-releasing hormone signaling on thyrotropin subunit genes. J. Biol. Chem. 275, 33365–33372 (2000). [DOI] [PubMed] [Google Scholar]
- 30.De Sousa S. M. C., et al. , Fall in thyroid stimulating hormone (TSH) may be an early marker of ipilimumab-induced hypophysitis. Pituitary 21, 274–282 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Audo I., et al. , The familial dementia gene revisited: A missense mutation revealed by whole-exome sequencing identifies ITM2B as a candidate gene underlying a novel autosomal dominant retinal dystrophy in a large family. Hum. Mol. Genet. 23, 491–501 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Pittois K., Deleersnijder W., Merregaert J., cDNA sequence analysis, chromosomal assignment and expression pattern of the gene coding for integral membrane protein 2B. Gene 217, 141–149 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Vidal R., et al. , A stop-codon mutation in the BRI gene associated with familial British dementia. Nature 399, 776–781 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Antoni F. A., Hunter E. F., Lowry P. J., Noble J. M., Seckl J. R., Atriopeptin: An endogenous corticotropin-release inhibiting hormone. Endocrinology 130, 1753–1755 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Bernstein H. G., et al. , Regional and cellular distribution patterns of insulin-degrading enzyme in the adult human brain and pituitary. J. Chem. Neuroanat. 35, 216–224 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Kilger E., et al. , BRI2 protein regulates β-amyloid degradation by increasing levels of secreted insulin-degrading enzyme (IDE). J. Biol. Chem. 286, 37446–37457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henne C., Schwenk F., Koch N., Möller P., Surface expression of the invariant chain (CD74) is independent of concomitant expression of major histocompatibility complex class II antigens. Immunology 84, 177–182 (1995). [PMC free article] [PubMed] [Google Scholar]
- 38.Leng L., et al. , MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197, 1467–1476 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starlets D., et al. , Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 107, 4807–4816 (2006). [DOI] [PubMed] [Google Scholar]
- 40.de Souza Costa V. H., Jr, et al. , Immunohistochemistry analysis of pulmonary infiltrates in necropsy samples of children with non-pandemic lethal respiratory infections (RSV; ADV; PIV1; PIV2; PIV3; FLU A; FLU B). J. Clin. Virol. 61, 211–215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu G., et al. , Relationship between elevated soluble CD74 and severity of experimental and clinical ALI/ARDS. Sci. Rep. 6, 30067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gowen M. F., et al. , Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J. Transl. Med. 16, 82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.