Significance
Wolbachia are utilized to control agricultural pests and reduce transmission of vector-borne human diseases such as dengue fever. Reproductive manipulation through cytoplasmic incompatibility (CI) is essential to these uses. A causal role in CI has been ascribed recently to a linked pair of Wolbachia genes, cidA and cidB. However, some CI-inducing Wolbachia strains lack the cid operon, implicating additional CI mechanisms. The cinA–cinB operon is paralogous to cidA–cidB and is a strong candidate for a distinct CI system. We show that the cin operon constitutes another toxin–antidote system in which CinB is a nuclease toxin and CinA binds tightly to CinB and can rescue embryo viability. Our data provide important insights into the molecular basis of CI.
Keywords: cytoplasmic incompatibility, Wolbachia, nuclease, toxin
Abstract
Wolbachia are endosymbiotic bacteria that infect nearly half of all arthropod species. This pandemic is due in part to their ability to increase their transmission through the female germline, most commonly by a mechanism called cytoplasmic incompatibility (CI). The Wolbachia cid operon, encoding 2 proteins, CidA and CidB, the latter a deubiquitylating enzyme (DUB), recapitulates CI in transgenic Drosophila melanogaster. However, some CI-inducing Wolbachia strains lack a DUB-encoding cid operon; it was therefore proposed that the related cin operon codes for an alternative CI system. Here we show that the Wolbachia cin operon encodes a nuclease, CinB, and a second protein, CinA, that tightly binds CinB. Recombinant CinB has nuclease activity against both single-stranded and double-stranded DNA but not RNA under the conditions tested. Expression of the cin operon in transgenic male flies induces male sterility and embryonic defects typical of CI. Importantly, transgenic CinA can rescue defects in egg-hatch rates when expressed in females. Expression of CinA also rescues CinB-induced growth defects in yeast. CinB has 2 PD-(D/E)xK nuclease domains, and both are required for nuclease activity and for toxicity in yeast and flies. Our data suggest a distinct mechanism for CI involving a nuclease toxin and highlight the central role of toxin–antidote operons in Wolbachia-induced cytoplasmic incompatibility.
Wolbachia pipientis is an obligate intracellular α-proteobacterium that infects insects and many other arthropods as well as filarial nematodes (1). Since its discovery in Culex pipiens mosquitoes, Wolbachia have been found in every insect order and are estimated to be present in up to 2/3 of all insect species (2, 3). The prevalence of Wolbachia infection depends on its efficient maternal transmission and the ability to manipulate host reproduction to increase the number of germline-infected females. Cytoplasmic incompatibility (CI) is the most frequently encountered host manipulation and can be explained as a Wolbachia-induced modification of sperm that causes embryonic lethality unless rescued in the zygote by a factor provided in females infected with the same Wolbachia strain. In a Wolbachia-infected insect population, infected females are fully capable of producing viable progeny with either infected or uninfected males, while matings between uninfected females and infected males yield mostly inviable embryos. Multiple trials around the world are currently utilizing release of Wolbachia-infected mosquitoes and CI in efforts to reduce transmission of mosquito-borne diseases such as dengue fever (4–7).
The discovery of the Wolbachia CI factor (cif) genes marked a major step in understanding the molecular mechanism of CI (8–13). [Here we use the recently proposed nomenclature in which “cif” is used when discussing CI genes generally and the more specific “cid” or “cin” names when the enzymatic function of particular toxins is known or strongly predicted. The relevant Wolbachia strain is denoted by a superscript (12, 14).] When expressed transgenically along with the cognate CidA in male Drosophila melanogaster, both CidBwPip and CidBwMel induce CI-like postzygotic male sterility through interference with embryonic nuclear division (9–11). Transgenic cidAwMel and cidA–cidBwMel female flies rescue CI caused by male flies infected with the wMel strain of Wolbachia (11). A large-scale population genomic screen of Culex mosquitoes linked crossing-type diversity in CI among mosquitoes infected with different wPip strains to genetic variations in the cidA–cidBwPip operon, further highlighting the important role of the cid genes in CI (13).
Another set of Wolbachia cif factors hypothesized to contribute to CI is the 2-gene cin operon, named after the putative nuclease activity of the CinB protein suggested by sequence analysis (9). The fact that some CI-inducing Wolbachia strains, such as the wNo strain that infects Drosophila simulans, contain only cin but not cid operons and that neither operon is present in wAu, a close relative of wMel that does not induce CI, suggested that the cin operon might also be able to induce CI independent of the cid operon (13, 15, 16). As was true for CidB, CinB was shown to inhibit growth when expressed in yeast (9). Based on in vitro pull-down studies, the cognate protein pairs (A and B) within each cif operon bind specifically to each other (9). The parallels in cognate-binding preference of the Cid and Cin factors further support the possibility that the cinA–cinB operon encodes an independent toxin–antidote pair contributing to CI.
Recent genomic analyses have uncovered natural variation in both cid and cin loci that correlates with CI in different Wolbachia-infected Drosophila species (17, 18). While this supports previous speculations on the possible function of the cinA–cinB operon in CI, the ability of these genes to cause CI has not been experimentally tested. Similarly, while there are distant sequence similarities between CinB and the PD-(D/E)xK superfamily of nucleases, no nuclease activity has been demonstrated (19, 20). Here we show that CinB has DNase activity. Mutation of putative active-site residues in either CinB PD-(D/E)xK domain abolishes activity in vitro and renders the resulting protein nontoxic to yeast. Most importantly, the cinA–cinBwPip operon induces a CI-like phenotype in transgenic flies, and cinAwPip is sufficient for rescue of transgenic CI. Therefore, the cinA–cinB nuclease operon provides a biochemically distinct mechanism for CI and its presence likely accounts for the ability of many Wolbachia strains to induce CI in their hosts despite not carrying an intact cidA–cidB gene pair.
Results
CinB Is a Nuclease.
CinB has putative PD-(D/E)xK domains located near both the N and C termini of the protein (Fig. 1A). Sequence and secondary structure alignments revealed that these domains have homology to other PD-(D/E)xK nucleases and are highly conserved across the so-called type II and type III Wolbachia cif operons (Fig. 1B) (15, 21, 22). Secondary structure prediction by PSIPRED and protein structure prediction by RaptorX suggest both the N-terminal and C-terminal nuclease domains (NTND and CTND) share a conserved αβββαβ-fold where the 2 α-helices are predicted to sandwich a 4-stranded β-sheet, a conserved feature in almost all PD-(D/E)xK nucleases (Fig. 1B and SI Appendix, Fig. S1) (23).
Fig. 1.
Sequence alignments and structural predictions for CinB. (A) The NTND and CTND of CinBwPip with their predicted catalytic aspartate, glutamate, and lysine residues labeled. CidBwPip also has 2 predicted PD-(D/E)xK nuclease folds upstream of its deubiquitylase domain; these are related to the dual nuclease domains in CinBwPip but lack 2 or 3 of the 3 predicted core catalytic residues. (B) Protein sequence and secondary structure alignments of the NTND and CTND of CinB from various Wolbachia strains as well as several known PD-(D/E)xK nucleases. Predicted α-helical residues are labeled “H” and residues predicted to be part of β-sheets are labeled “E.” The numbers of excluded residues are shown in parentheses. The last residue numbers are shown at the end of each sequence. Catalytic D-E-K residues are highlighted in black. Residues in red are conserved among all 3 groups. Residues in gray are conserved within the respective groups.
Similar to other PD-(D/E)xK nucleases, both CinB nuclease domains also contain a highly conserved set of catalytic residues (highlighted in Fig. 1B and SI Appendix, Fig. S1) (21, 23, 24). In most PD-(D/E)xK nucleases, the negatively charged aspartate and glutamate residues help coordinate up to 3 metal ions that serve as Lewis acids to stabilize the transition state, while lysine functions as a general base for deprotonation of the nucleophilic water molecule (SI Appendix, Fig. S2) (21, 24). Interestingly, CidB also contains 2 potential PD-(D/E)xK folds upstream of its catalytic deubiquitylase (DUB) domain with significant sequence similarities to the NTND and CTND of CinB, respectively (Fig. 1A). However, these domains in CidB lack residues predicted to be essential for catalytic activity (22).
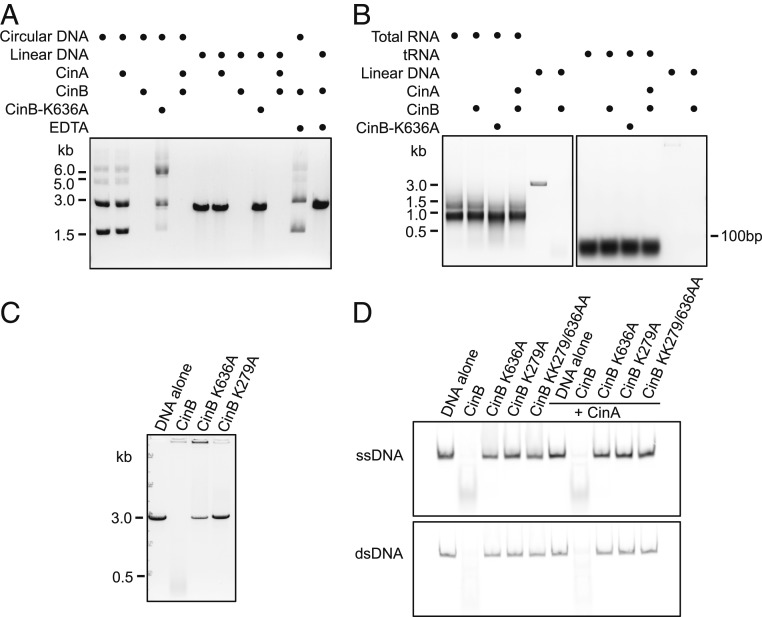
To test if CinB has nuclease activity, we purified recombinant CinBwPip using polyethylenimine to separate DNA from the protein and incubated the purified protein with various DNA and RNA substrates (25). CinBwPip degraded both linearized and circular double-stranded (ds)DNA plasmids. The DNase was activated by magnesium or manganese ions but not calcium and was active over a broad range of pHs (Fig. 2A and SI Appendix, Fig. S3 A, B, E, and F). We further examined the nuclease activity of CinB against shorter single-stranded (ss)DNA and dsDNA substrates and found it cleaves both forms of DNA (either 45 or 70 residues in length; Fig. 2D and SI Appendix, Fig. S3G). Importantly, the catalytic CTND (K636A) or NTND (K279A) mutations each abolished DNase activity (Fig. 2 A, C, and E and SI Appendix, Fig. S3C). By contrast, the purified CidBwPip DUB protein did not exhibit DNase activity even after reinstating key catalytic residues in the CTND of CidBwPip (SI Appendix, Fig. S3D).
Fig. 2.
CinBwPip has DNase activity in vitro. (A) CinBwPip cleaved both circular and linearized pBluescript SK+ plasmids. In all reactions, 1 μM CinBwPip was incubated with 15 nM DNA for 90 min. In reactions where CinAwPip was present, 10 μM CinAwPip and 1 μM CinBwPip were incubated on ice for 30 min to allow complex formation before adding to the substrate. To stop the reactions, EDTA was added to a 2× molar excess over Mg2+. Samples were run in a 0.8% agarose gel and stained with ethidium bromide. (B) CinBwPip showed no RNase activity against either total RNA extract from D. melanogaster or yeast tRNA; 500 ng of RNA was used in each reaction. Linearized pBluescript SK+ was used as a positive control for nuclease activity. (C) Mutation of either K636 or K279 eliminated CinBwPip cleavage of linearized pBluescript SK+ (∼3 kb). The mutant proteins appeared to still bind DNA based on the signal remaining in the loading wells (Top). (D) CinBwPip cleaved both single- and double-stranded 70-mer DNAs. CinBwPip (1 μM) was incubated with 500 nM Cy5-labeled DNA for 90 min. In reactions including CinAwPip, CinA and CinB proteins were preincubated as above. Samples were run in a 9% polyacrylamide/TBE gel.
We also tested whether CinBwPip could cleave RNA substrates but did not detect activity against either yeast tRNA or a Drosophila total RNA extract under our conditions (Fig. 2B). Thus, CinB is a DNase capable of cleaving both ssDNA and dsDNA. Its activity might be higher against specific DNA sequences or structures, but we have not yet been able to identify such substrates. These in vitro experiments support the idea of CinB as a nuclease that requires both PD-(D/E)xK domains for its activity.
An important aspect of the toxin–antidote model of CI is the binding specificity between the protein pairs within each cif operon as suggested by our previous affinity pull-down experiments (9). Here we used isothermal titration calorimetry (ITC) to determine quantitatively the affinity between CinAwPip and CinBwPip. ITC revealed a Kd of 25 ± 1.3 nM, demonstrating strong binding between the cognate pair (Fig. 3). We tested whether the tight association of CinA with CinB would inhibit CinB nuclease activity. Preincubation of CinBwPip with excess CinAwPip did not reduce DNase activity in our in vitro assays (Fig. 2 A and D), suggesting that CinA rescues cells from CinB-induced toxicity through a distinct mechanism, for example, cellular relocalization. This observation was not surprising inasmuch as coincubation of CidAwPip and CidBwPip also did not limit the DUB activity of CidBwPip (9).
Fig. 3.
ITC binding isotherm for binding of CinAwPip to CinBwPip yielded a Kd of 25 ± 1.3 nM. This tight interaction provides support to the hypothesis that the cognate pair exists as a toxin–antidote system. (Top) Raw injection data over time. DP, differential power. (Bottom) Integrated heats over the course of the reaction. Error bars provided by NITPIC during integration of the raw thermogram represent heat changes for each injection. n = 3.
CinB Nuclease Activity Is Required for Toxicity in Yeast.
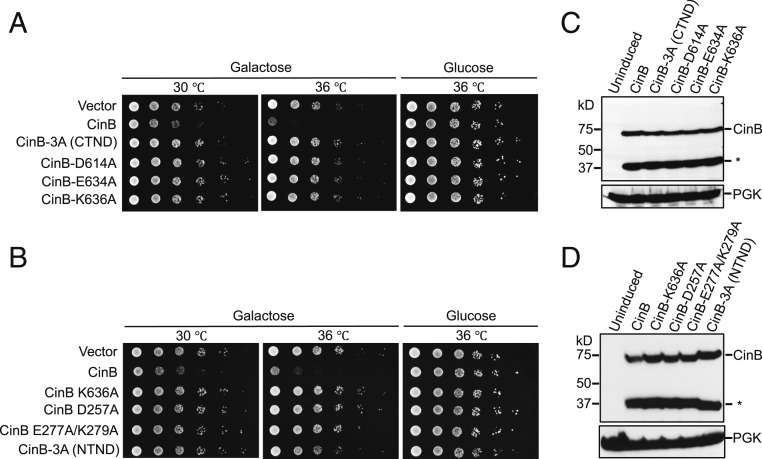
To determine if the toxicity of CinBwPip expression in yeast is due to its nuclease activity, we generated a panel of mutants with mutations in catalytic residues of either the NTND or CTND. Simultaneously changing all 3 CTND catalytic residues to alanines (3A) or individually (D614A, E634A, or K636A) was sufficient for eliminating the CinB-induced growth defect (Fig. 4A). Mutation of NTND catalytic residues also eliminated CinBwPip-induced toxicity, suggesting that the toxin function of CinBwPip requires both nuclease domains to be active (Fig. 4B). Changes in protein levels cannot account for the loss of temperature-dependent lethality due to the mutations in either domain (Fig. 4 C and D and SI Appendix, Fig. S4). These results are consistent with both CinB PD-(D/E)xK domains contributing to nuclease activity (Fig. 2), and indicate that both domains are important for CinB function as measured by its toxicity in yeast.
Fig. 4.
CinBwPip toxicity in the S. cerevisiae BY4741 strain. (A and B) All genes were N-terminally FLAG-tagged and cloned into pYES2, a galactose-inducible expression vector. Expression of wild-type CinB caused a temperature-dependent growth defect. Such growth defects were not observed in yeast expressing CinB with predicted inactivating point mutations in either the CTND (A) or NTND (B). All serial dilutions were done in triplicate with independent transformants. (C and D) Relative expression levels of WT and mutant CinB proteins. Equivalent numbers of yeast based on OD600 were lysed, and the lysates were resolved by SDS/PAGE and immunoblotted for CinB by α-FLAG antibody; PGK served as a loading control. A fraction of CinB is proteolytically cleaved in yeast and the N-terminal fragment is labeled with an asterisk (SI Appendix, Fig. S3).
Transgenic cinA–cinBwPip Induces CI and cinAwPip Alone Rescues Transgenic CI in Flies.
To test the ability of the cin operon to induce CI in the absence of Wolbachia infection, we created transgenic D. melanogaster lines containing cinwPip genes by site-directed PhiC31-mediated integration (26). Recent studies utilized 2 distinct strategies to generate transgenic Cid-expressing flies (9–11). Here we attempted both. In the first, cinAwPip and cinBwPip genes were integrated into separate chromosomes (UAS:cinA/UAS:cinA; UAS:cinB/UAS:cinB), while the second strategy utilized a fusion of cinAwPip and cinBwPip genes linked by a T2A viral peptide-coding sequence (UAS:cinA-T2A-cinB/UAS:cinA-T2A-cinB) that causes ribosomal skipping and results in the synthesis of 2 separate proteins from 1 transcript (Fig. 5 A and B). Both the Nanos-Gal4-Tubulin (NGT) driver and maternal triple driver (MTD-Gal4) were used for specific expression of the Gal4 transcription factor in fly germline cells, stimulating transcription of the cin transgenes through their Gal4-responsive upstream activation sequences (27–29). The MTD can increase the transcript levels of cidAwMel by over 1,000-fold relative to expression with the NGT driver, allowing transgenic cidAwMel females to rescue CI induced by male flies infected with wMel (11). The level of CI was determined from the percentage of embryos that hatched into larvae.
Fig. 5.
Expression of the cinA–cinBwPip genes in flies induces CI-like embryo killing and rescue phenotypes. (A and B) Two strategies were used to generate transgenic flies. cinAwPip was inserted into the second chromosome and cinBwPip was inserted into the third chromosome (UAS:cinA/UAS:cinA; UAS:cinB/UAS:cinB) (A), or cinAwPip and cinBwPip were linked by a T2A viral sequence (yellow) and inserted into the third chromosome (UAS:cinA-T2A-cinB/UAS:cinA-T2A-cinB) (B). (C) Crosses with flies transgenic for either cinAwPip or cinBwPip alone expressed using the NGT driver (highlighted in orange). Data in burgundy represent CI-inducing crosses while green represents either rescue or weakening of CI induction by the CinB-K636A mutation. All control crosses are shown in gray. n = 40 to 64. (D) Crosses with flies transgenic for the entire cinA–cinBwPip operon under control of the NGT driver. n = 38 to 58. Error bars in C and D represent SD of the mean; **P < 0.01, ***P < 0.001, ****P < 0.0001 by ANOVA with multiple comparison between all groups. (E) Transgenic CI in crosses with flies transgenic for the entire cinA–cinBwPip operon expressed using the strong maternal triple driver (highlighted in red) could be rescued by transgenic cinAwPip females. Vertical lines represent medians. n = 23 to 57. ****P < 0.0001 by 2-tailed Mann–Whitney U test.
When crossed to wild-type (WT) females, males transgenic for cinAwPip alone under the control of the NGT driver did not affect hatch rates, whereas males transgenic for cinBwPip alone produced an ∼30% hatch-rate reduction (Fig. 5C). This reduction was partially but significantly suppressed if the catalytic lysine-636 residue was mutated to alanine. Consistent with hatch-rate data for transgenic cidAwMel and cidBwMel (10), male flies transgenic for both cinAwPip and cinBwPip (NGT) induced a stronger reduction in hatch rates (∼50 to 60%) when compared with males transgenic for cinBwPip alone (Fig. 5D). Both transgenic strategies generated a similar level of hatch-rate reductions when using the NGT driver. Importantly, the catalytic K636A mutation in CinB significantly weakened (by ∼30%) the effect on hatch rates caused by transgenic UAS:cinA/+ NGT:Gal4/+; UAS:cinB/+ males (Fig. 5D), suggesting the reduction in hatch rates depended on the nuclease activity of CinBwPip.
Transgenic cinA-T2A-cinB males induced a greater hatch-rate reduction (nearly 90%) when under the control of the strong MTD. Importantly, we observed rescue of these low hatch rates by crossing transgenic cinA-T2A-cinBwPip males to females that were transgenic for cinAwPip (Fig. 5E). This strengthens and generalizes the hypothesis of cifA as the antidote gene in the different cif operon systems. Unexpectedly, transgenic UAS:cinA/+; UAS:cinB/+ males from 5 independent homozygous lines all failed to lower hatch rates under the MTD-Gal4 driver when crossed to WT females; this is discussed below.
CI-Like Cytology in Response to Transgenic cinA–cinBwPip.
We next determined whether the hatch-rate reduction caused by transgenic expression of the cinA–cinBwPip genes could be traced to embryonic defects similar to those seen in natural CI. The cytology of fly embryos infected with Wolbachia strains known to contain only a cin operon has never been reported. Therefore, we first analyzed embryos from D. simulans infected by wNo, a Wolbachia strain containing a cin but no cid operon, for comparison with the cytological analysis of our transgenic flies (SI Appendix, Figs. S5 and S7) (15, 22). The wNo-infected flies exhibited a range of cytological defects similar to those previously reported for wMel-induced CI with the exception that no regional mitotic failure was observed (Fig. 6 A–E) (10).
Fig. 6.
Embryos from matings with transgenic D. melanogaster males expressing the cinA–cinBwPip operon show CI-like cytology similar to wNo-infected D. simulans. (A–E) Representative images of propidium iodide-stained embryos from incompatible crosses between wNo-infected males and uninfected females showing (A) an unfertilized embryo, (B) a normal embryo after 1 h of development, (C) a normal embryo after 2 h of development, (D) an embryo with early mitotic failure, and (E) an embryo showing anaphase chromatin bridging. (F) Quantification of embryo cytology. wNo (−) and wNo (+) represent D. simulans-uninfected or infected by wNo, respectively. wNo infection was confirmed by PCR amplification of the cinBwNo gene (SI Appendix, Fig. S6). For the transgenic D. melanogaster crosses (Bottom), only the cinA-T2A-cinB crosses under the MTD-Gal4 driver (highlighted in red; the NGT-Gal4 driver is highlighted in orange) strongly phenocopied the natural CI cytology. The number of embryos examined in each cross is shown. Embryos exhibiting normal cytology after 1 to 2 h were grouped together and are shown in teal. ****P < 0.0001 by χ2 test comparing normal (B and C) and abnormal (A, D, and E) cytological phenotypes.
Most importantly, all transgenic crosses that resulted in reduced hatch rates induced CI-like embryonic defects in embryos collected after 1 to 2 h of development (Fig. 5F and SI Appendix, Fig. S6). Similar to the wNo infection control, about half of the embryos from the cross between transgenic cinA-T2A-cinB males under the MTD-Gal4 driver and WT females exhibited an abnormal cytological phenotype. Together, these data establish a role for the Wolbachia cinA–cinB genes as an independent CI-inducing gene system and support the hypothesis that cifA genes are specifically required for the rescue of CI, similar to their ability to suppress cifB toxicity in yeast.
Discussion
Recent studies have revealed the central role of the cidA–cidB operon in both CI induction and rescue (9–11, 20). CidB is a DUB, and this enzymatic activity is crucial for its ability to promote CI (7). Previous findings led to the hypothesis that the cinA–cinB locus, with a pair of genes paralogous to cidA–cidB, might also be involved in Wolbachia-induced CI despite lacking a known DUB domain.
Here we have shown that CinB is a nuclease, specifically a DNase. It remains possible that the apparent lack of RNase activity reflects the absence of a crucial cofactor or appropriate reaction conditions. The DNA-cleaving activity we have detected is of broad specificity but weak, at least under the conditions tested. An unregulated, highly active nuclease would likely be harmful to Wolbachia; we note that expression of CinB in Escherichia coli is not obviously deleterious to growth. It is possible that the enzyme is more potent against particular DNA sequences or structures. For example, during the exchange of protamines for histones that occurs in the male pronucleus in the nascent zygote, transiently uncoated paternal DNA may be prone to forming cruciforms or other structures that are preferred substrates for CinB. Among the earliest signs of CI are chromosome condensation defects in the male pronucleus (30, 31).
Both the NTND and CTND of CinB are highly conserved across many Wolbachia strains. Interestingly, our in vitro enzyme analysis and growth data from Saccharomyces cerevisiae transformants suggest the nuclease activity and toxicity caused by CinB requires both of its nuclease domains to be active. It is possible that the 2 domains feature a mechanism where 1 domain is involved in substrate recognition while the other is responsible for the actual phosphodiester-bond cleavage. Another possibility is that both nuclease domains recognize and cleave DNA substrates in a cooperative manner such that mutation in 1 nuclease domain is sufficient to inhibit the overall function of the protein. Many PD-(D/E)xK nucleases function as homodimers, which also brings together a pair of PD-(D/E)xK domains (21). Structural analysis will be needed to gain a deeper understanding of the exact CinB reaction mechanism.
Our study demonstrates the sufficiency of the cinA–cinB operon for both inducing CI by expression in males and rescuing it through expression (of cinA) in females. Surprisingly, UAS:cinA/+; UAS:cinB/+ males did not induce embryonic lethality when under the control of the MTD-Gal4 driver, unlike UAS:cinA-T2A-cinB/UAS:cinA-T2A-cinB males, in our transgenic CI crosses. It is possible that the operon-like structure of cinA-T2A-cinB better mimics the natural expression ratio of the cinA and cinB genes, which was shown to be important in the cid operon (13). If relative expression of CinA were too high in UAS:cinA/+; UAS:cinB/+ flies under the MTD-Gal4 driver, it could dampen CinB toxicity in CI crosses; alternatively, insufficient CinA during male spermiogenesis might selectively kill sperm precursors with high CinB levels. Another unexpected finding from our transgenic fly analyses was that MTD-driven expression of cinA-T2A-cinB in females caused a high level of embryonic lethality. It is likely that such embryonic lethality is due to the toxicity from expressing the operon at a very high level.
The rescue of transgenic CI by cinAwPip alone is fully consonant with our earlier finding that cinAwPip suppresses cinBwPip-induced toxicity in yeast (9). We have measured a tight physical association between CinA and CinB (Fig. 3). Nevertheless, this interaction did not inhibit the catalytic activity of the nuclease, a result similar to our finding with CidA and CidB where association of the cognate pair failed to suppress the DUB activity of CidB (9). The rescue mechanism might instead be caused by CinA or CidA association changing the cellular localization of the cognate B toxins or their ability to bind their critical targets in vivo.
While details of the CinB nuclease’s mode of action remain to be worked out, our results highlight a mechanism for CI that is likely to be broadly relevant to Wolbachia-induced CI in many different arthropods. An interesting question is why some Wolbachia carry both cin and cid loci, as is true for wPip, or have B genes predicted to encode active nuclease and DUB activities in the same polypeptide (CndB class) (6, 7, 9). The CI loci are usually part of WO prophage regions, and repeats or partial repeats are common (12). Thus, these paralogs may be subject to rapid evolutionary changes that allow shifts between DUB-dominated and nuclease-dominated CI mechanisms in response to host adaptations to the endosymbiont. Finally, PD-(D/E)xK nucleases may have roles in other host–parasite interactions. The selfish genetic element Medea, for instance, which kills embryos expressing Medea maternally but lacking the gene in the zygote, also encodes a putative nuclease of this class (32).
Materials and Methods
Sequence Alignment and Structure Prediction.
Multiple sequence alignments of CinB orthologs from several Wolbachia strains and known PD-(D/E)xK nucleases were generated using the Cluster Omega Multiple Sequence Alignment program from EMBL-EBI followed by manual adjustment (33). Secondary structure predictions and alignments of the protein sequences were performed using the PSIPRED Protein Sequence Analysis Workbench program (34). Structure prediction of CinBwPip was done using the RaptorX Structure Prediction server with a few unstructured regions removed in SI Appendix, Fig. S1 (35).
Western Immunoblotting.
The following antibodies were used: mouse anti-FLAG M2 (Sigma; 1:10,000), mouse 16B12 anti-HA (Covance; 1:1,000), mouse anti-PGK (Molecular Probes; 1:20,000), and horseradish peroxidase (HRP)-conjugated sheep anti-mouse NA931V (GE Healthcare; 1:10,000). Protein samples were resolved by SDS/PAGE and transferred to PVDF membranes (Millipore) for immunoblotting. Proteins were visualized by HRP-based chemiluminescence (36).
Purification of Proteins for in Vitro Nuclease Assays and ITC.
Full-length CinB, catalytically inactive CinB mutants (K279A, K636A, and KK279/636AA), CidB1–761(V686E/R688K), and CinA were expressed as GST fusions from the pGEX6P1 vector in Rosetta DE3 (Novagen) E. coli as previously described (9). To reduce the likelihood of CinB copurification with DNA, we used a protocol to isolate DNA-free protein described by Epling et al. (25) (SI Appendix, Materials and Methods).
Isothermal Titration Calorimetry.
ITC experiments were carried out at 25 °C using a NanoITC (TA Instruments). First, CinAwPip and CinBwPip were dialyzed extensively against a buffer of 50 mM Hepes (pH 7.4) over the course of 2 d with 3 or 4 buffer exchanges. To determine the binding affinity of CinB for CinA, 500 μM CinA was loaded into a syringe and titrated into a 50 μM solution of CinB. A total of 22 injections (2 μL per injection) were performed over the course of the experiment with a spacing of 300 s between injections to ensure a return to baseline prior to the next injection. The data were baseline-corrected using NITPIC and analyzed in SEDPHAT using the 1-site (A + B → AB) binding model. Figure 3 was prepared in GUSSI, which was downloaded from the MBR software page (http://biophysics.swmed.edu/MBR/software.html) (37–39).
Nuclease Assays.
All in vitro nuclease activity assays were performed with full-length CinBwPip (residues 1 to 733) with or without CinAwPip (residues 1 to 446). For DNase and RNase activity assays, 1 μM CinB or CinB mutant proteins was incubated in a reaction buffer containing 20 mM Hepes (pH 8.0), 5 mM MgCl2, 2.5% sucrose, 150 mM NaCl, 0.001% Triton X-100, and 2 mM DTT with 15 nM either linearized or supercoiled pBluescript SK+, 500 nM single-stranded or double-stranded Cy5-labeled DNA [70-mer: Cy5-GCAATTCGATCGTTGACATCTCGCGTGCTCGGTCAATCGGCAGATGCGGAGTGAAGTTCCAACGTTCGGC-3′, previously used for ssDNA cleavage analysis (40, 41); 45-mer: Cy5-GGGTCAACGTGGGCAAAGATGTCCTAGCAAGCCAGAATTCGGCAG-3′, which was tested with the online calculator OligoCalc to confirm that the sequence should not form any strong hairpins; and the respective complements to generate dsDNA], or 500 ng of either yeast tRNA (Thermo Fisher) or D. melanogaster total RNA (40, 41). In reactions where CinA was present, 10 μM CinA was used. All reactions were carried out at 25 °C for 90 min and quenched by adding EDTA to a final concentration of 10 mM unless otherwise noted. For reactions using a linear or circular pBluescript SK+ vector or RNA, samples were run in agarose gels with a range of concentrations between 0.8 and 2.0% containing 0.4 μg/mL ethidium bromide in 1× TAE buffer at 100 V for 40 to 60 min and imaged on a Syngene G:box with GeneTools software. For reactions using Cy5-labeled oligodeoxynucleotides, samples were run in 9% TBE polyacrylamide native gels at 100 V for 45 to 60 min and imaged on a Typhoon FLA 7000 with Typhoon FLA 7000 control software.
Yeast Methods.
The yeast growth assays shown were done in the BY4741 strain (42). The 2-μm plasmid pYES2 (URA3) utilizes a GAL1 promoter and a CYC1 terminator and was used for galactose-inducible CinB expression in yeast. Yeast growth assays and immunoblotting were performed as previously described (9) (SI Appendix, Materials and Methods).
Drosophila Hatch-Rate and Cytology Analyses.
Each CI candidate gene (cinA, cinB, cinB-K636A, and cinA-T2A-cinB) was inserted into the pUASp-attB vector by standard cloning techniques without codon optimization. In brief, the ORFs of the genes were amplified by PCR and cloned into the pBluescript SK+ vector followed by restriction digestion and religation of the genes into the pUASp-attB vector. All plasmids were verified by fully sequencing the inserted genes before they were sent to BestGene for microinjection of D. melanogaster embryos (9). Fly background 9744 was chosen for all gene constructs for site-directed attP/B integration on the third chromosome by PhiC31 integrase (26) with the exception that background 9723 was chosen for site-specific integration of cinA on the second chromosome. Integrations were confirmed independently by PCR amplifying the candidate genes (43, 44). D. melanogaster stocks were verified to be uninfected with native Wolbachia isolates by PCR amplification of the cidAwMel gene (SI Appendix, Fig. S6). The MTD-Gal4 line from the Bloomington Stock Center was found to be infected by wMel and was treated by addition of 20 μg/mL tetracycline to the growth medium for 3 generations. Once the infection was confirmed to be cleared by PCR amplification of the cidAwMel gene, flies were reared on untreated media for at least 3 additional generations to allow for mitochondrial recovery (45). Both uninfected and wNo-infected D. simulans were also verified by PCR amplification of the cinBwNo gene (SI Appendix, Fig. S6). Flies were reared on standard cornmeal-based solid media and maintained at room temperature. During virgin female collection, stocks were maintained at 18 °C overnight and room temperature the following day. All transgenic flies were maintained as homozygous lines.
Parental flies were generated by crossing either NGT-Gal4 or MTD-Gal4 virgin females with cin transgenic males (27, 29). Only the males emerging between 0 and 30 h from these crosses were collected and used in CI analyses (46). All flies used in both hatch-rate and cytological analyses were aged for 2 to 4 d. Hatch-rate analysis was performed and embryos for cytological analyses were prepared similarly as previously described (9, 10) (SI Appendix, Materials and Methods). The samples were stained with either Hoechst 33342 at 1:1,000 in PBTA or 1 μg/mL propidium iodide (9, 10). Stained embryos were mounted on glass slides and sealed under coverslips by nail polish. Imaging was done either on a Zeiss Axioskop microscope with AxioCam MRm camera using 10× and 40× objective lenses or a Zeiss LSM 880 Airyscan/NLO confocal microscope with internal PMT using a 20× objective lens. Software used to capture and analyze the images were AxioVision Rel. 4.8 or Zen (blue edition), respectively.
Statistical Analyses.
All statistical analyses were done in GraphPad Prism 7. Hatch-rate analyses were performed by either using 1-way ANOVA with pairwise comparison after removal of outliers identified by the ROUT method with Q = 1% (Fig. 5 C and D) or unpaired 2-tailed Mann–Whitney U test (Fig. 5E and SI Appendix, Fig. S4). Pairwise χ2 test was used in cytological analyses to compare normal and defect cytological phenotypes.
Data Availability.
Quantitative analyses for this study are provided in Dataset S1.
Supplementary Material
Acknowledgments
We thank Dr. John Carlson for providing lab space for some of the early Drosophila work, Drs. Christian Schlieker, Dieter Söll, and Anna Pyle for use of instruments, and Dr. Jason Berk for his comments and edits on the manuscript. This work was funded by Cellular and Molecular Biology Training Program-supported T32GM007223 and T32GM007223-S1 (to H.C.) and NIH R01 Grants GM053756 and GM046904 (to M.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914571116/-/DCSupplemental.
References
- 1.Serbus L. R., Casper-Lindley C., Landmann F., Sullivan W., The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42, 683–707 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J. H., How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen J. H., Barr A. R., The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 22, 242–250 (1973). [DOI] [PubMed] [Google Scholar]
- 4.Callaway E., Rio fights Zika with biggest release yet of bacteria-infected mosquitoes. Nature 539, 17–18 (2016). [DOI] [PubMed] [Google Scholar]
- 5.O’Neill S. L., The use of Wolbachia by the World Mosquito Program to interrupt transmission of Aedes aegypti transmitted viruses. Adv. Exp. Med. Biol. 1062, 355–360 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Gilbert J. A., Melton L., Verily project releases millions of factory-reared mosquitoes. Nat. Biotechnol. 36, 781–782 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Zheng X., et al. , Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Beckmann J. F., Fallon A. M., Detection of the Wolbachia protein WPIP0282 in mosquito spermathecae: Implications for cytoplasmic incompatibility. Insect Biochem. Mol. Biol. 43, 867–878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckmann J. F., Ronau J. A., Hochstrasser M., A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2, 17007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LePage D. P., et al. , Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shropshire J. D., On J., Layton E. M., Zhou H., Bordenstein S. R., One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 115, 4987–4991 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckmann J. F., et al. , The toxin-antidote model of cytoplasmic incompatibility: Genetics and evolutionary implications. Trends Genet. 35, 175–185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonneau M., et al. , Culex pipiens crossing type diversity is governed by an amplified and polymorphic operon of Wolbachia. Nat. Commun. 9, 319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckmann J. F., et al. , Caution does not preclude predictive and testable models of cytoplasmic incompatibility: A reply to Shropshire et al. Trends Genet. 35, 399–400 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsey A. R. I., et al. , Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in prophage WO of Wolbachia. Genome Biol. Evol. 10, 434–451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton E. R., Harris S. R., Parkhill J., Sinkins S. P., Comparative genome analysis of Wolbachia strain wAu. BMC Genomics 15, 928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper B. S., Vanderpool D., Conner W. R., Matute D. R., Turelli M., Wolbachia acquisition by Drosophila yakuba-clade hosts and transfer of incompatibility loci between distantly related Wolbachia. Genetics 212, 1399–1419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meany M. K., et al. , Loss of cytoplasmic incompatibility and minimal fecundity effects explain relatively low Wolbachia frequencies in Drosophila mauritiana. Evolution 73, 1278–1295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shropshire J. D., et al. , Models and nomenclature for cytoplasmic incompatibility: Caution over premature conclusions—A response to Beckmann et al. Trends Genet. 35, 397–399 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Shropshire J. D., Bordenstein S. R., Two-by-one model of cytoplasmic incompatibility: Synthetic recapitulation by transgenic expression of cifA and cifB in Drosophila. PLoS Genet. 15, e1008221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knizewski L., Kinch L. N., Grishin N. V., Rychlewski L., Ginalski K., Realm of PD-(D/E)XK nuclease superfamily revisited: Detection of novel families with modified transitive meta profile searches. BMC Struct. Biol. 7, 40 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie J. J., et al. , A tangled web: Origins of reproductive parasitism. Genome Biol. Evol. 10, 2292–2309 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steczkiewicz K., Muszewska A., Knizewski L., Rychlewski L., Ginalski K., Sequence, structure and functional diversity of PD-(D/E)XK phosphodiesterase superfamily. Nucleic Acids Res. 40, 7016–7045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pingoud A., Fuxreiter M., Pingoud V., Wende W., Type II restriction endonucleases: Structure and mechanism. Cell. Mol. Life Sci. 62, 685–707 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Epling L. B., Grace C. R., Lowe B. R., Partridge J. F., Enemark E. J., Cancer-associated mutants of RNA helicase DDX3X are defective in RNA-stimulated ATP hydrolysis. J. Mol. Biol. 427, 1779–1796 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groth A. C., Fish M., Nusse R., Calos M. P., Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166, 1775–1782 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rørth P., Gal4 in the Drosophila female germline. Mech. Dev. 78, 113–118 (1998). [DOI] [PubMed] [Google Scholar]
- 28.White-Cooper H., Tissue, cell type and stage-specific ectopic gene expression and RNAi induction in the Drosophila testis. Spermatogenesis 2, 11–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrella L. N., Smith-Leiker T., Cooley L., The Ovhts polyprotein is cleaved to produce fusome and ring canal proteins required for Drosophila oogenesis. Development 134, 703–712 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Callaini G., Dallai R., Riparbelli M. G., Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J. Cell Sci. 110, 271–280 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Landmann F., Orsi G. A., Loppin B., Sullivan W., Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathog. 5, e1000343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzen M. D., et al. , The maternal-effect, selfish genetic element Medea is associated with a composite Tc1 transposon. Proc. Natl. Acad. Sci. U.S.A. 105, 10085–10089 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanz C., et al. , The EMBL Nucleotide Sequence Database. Nucleic Acids Res. 33, D29–D33 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones D. T., Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Li W., Liu S., Xu J., RaptorX-Property: A web server for protein structure property prediction. Nucleic Acids Res. 44, W430–W435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mruk D. D., Cheng C. Y., Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis 1, 121–122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roe D. R., Cheatham T. E. III, PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Keller S., et al. , High-precision isothermal titration calorimetry with automated peak-shape analysis. Anal. Chem. 84, 5066–5073 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houtman J. C., et al. , Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: Application to adaptor protein complexes in cell signaling. Protein Sci. 16, 30–42 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogart K., Andrews J., “Extraction of total RNA from Drosophila” (CGB Tech. Rep. 2006-10, The Center for Genomics and Bioinformatics, Indiana University, Bloomington, IN, 2006).
- 41.Komori K., et al. , Biochemical characterization of the hjc Holliday junction resolvase of Pyrococcus furiosus. Nucleic Acids Res. 28, 4544–4551 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brachmann C. B., et al. , Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Livak K. J., Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107, 611–634 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckmann J. F., Fallon A. M., Decapitation improves detection of Wolbachia pipientis (Rickettsiales: Anaplasmataceae) in Culex pipiens (Diptera: Culicidae) mosquitoes by the polymerase chain reaction. J. Med. Entomol. 49, 1103–1108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chatzispyrou I. A., Held N. M., Mouchiroud L., Auwerx J., Houtkooper R. H., Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 75, 4446–4449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada R., Floate K. D., Riegler M., O’Neill S. L., Male development time influences the strength of Wolbachia-induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics 177, 801–808 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Quantitative analyses for this study are provided in Dataset S1.