Significance
In the classic androgen biosynthesis pathway, testosterone is converted to 5α-dihydrotestosterone, a step crucially required for normal male genital virilization. Congenital adrenal hyperplasia (CAH) due to P450 oxidoreductase deficiency (PORD) is an inborn disorder that disrupts classic androgen biosynthesis. However, some affected girls present with severe genital virilization at birth. We hypothesized that this is explained by a prenatally active, alternative biosynthesis pathway to 5α-dihydrotestosterone. We show that adrenals and genital skin cooperate to produce androgens via the alternative pathway during the major period of human sexual differentiation and that neonates with PORD still produce alternative pathway androgens during the first weeks of life. This indicates that alternative pathway androgen biosynthesis drives prenatal virilization in CAH due to PORD.
Keywords: fetal androgen biosynthesis, congenital adrenal hyperplasia, alternative androgen pathway, 5α-dihydrotestosterone, human sexual differentiation
Abstract
Androgen biosynthesis in the human fetus proceeds through the adrenal sex steroid precursor dehydroepiandrosterone, which is converted to testosterone in the gonads, followed by further activation to 5α-dihydrotestosterone in genital skin, thereby facilitating male external genital differentiation. Congenital adrenal hyperplasia due to P450 oxidoreductase deficiency results in disrupted dehydroepiandrosterone biosynthesis, explaining undervirilization in affected boys. However, many affected girls are born virilized, despite low circulating androgens. We hypothesized that this is due to a prenatally active, alternative androgen biosynthesis pathway from 17α-hydroxyprogesterone to 5α-dihydrotestosterone, which bypasses dehydroepiandrosterone and testosterone, with increased activity in congenital adrenal hyperplasia variants associated with 17α-hydroxyprogesterone accumulation. Here we employ explant cultures of human fetal organs (adrenals, gonads, genital skin) from the major period of sexual differentiation and show that alternative pathway androgen biosynthesis is active in the fetus, as assessed by liquid chromatography–tandem mass spectrometry. We found androgen receptor expression in male and female genital skin using immunohistochemistry and demonstrated that both 5α-dihydrotestosterone and adrenal explant culture supernatant induce nuclear translocation of the androgen receptor in female genital skin primary cultures. Analyzing urinary steroid excretion by gas chromatography–mass spectrometry, we show that neonates with P450 oxidoreductase deficiency produce androgens through the alternative androgen pathway during the first weeks of life. We provide quantitative in vitro evidence that the corresponding P450 oxidoreductase mutations predominantly support alternative pathway androgen biosynthesis. These results indicate a key role of alternative pathway androgen biosynthesis in the prenatal virilization of girls affected by congenital adrenal hyperplasia due to P450 oxidoreductase deficiency.
Gonadal development depends on chromosomal sex, whereby the 46,XY or 46,XX karyotype, established at fertilization, dictates subsequent development of either testis or ovary (1–3). Gonadal hormones then direct differentiation of either male or female genitalia. In humans, sexual differentiation is established at 7 to 12 wk post conception (wpc) (4).
While secretion of testosterone by fetal testis Leydig cells is thought sufficient to drive virilization of the internal genitalia in the male fetus (5), differentiation of the external genitalia requires the action of 5α-dihydrotestosterone (DHT), which is generated locally from circulating testosterone by the enzyme steroid 5α-reductase type 2 (SRD5A2) (6, 7). By contrast, differentiation of human female genitalia has been regarded as the default of a low-androgen environment.
In humans, the regulation of sexual differentiation is intricately linked to early development of the adrenal cortex (4, 8). Disorders affecting adrenal steroidogenesis commonly affect sexual differentiation, as exemplified by the multiple variants of congenital adrenal hyperplasia (CAH), which result either in inappropriate or disrupted androgen biosynthesis. This consequently causes disorders of sex development (DSDs), which can manifest with external genital virilization in newborn girls (46,XX DSD) or undermasculinization of external genitalia in male neonates (46,XY DSD) (9). The most common variant of CAH, 21-hydroxylase (CYP21A2) deficiency, manifests with 46,XX DSD, while 17α-hydroxylase/17,20-lyase (CYP17A1) deficiency results in 46,XY DSD.
The congenital adrenal hyperplasia variant cytochrome P450 oxidoreductase (POR) deficiency can manifest with both 46,XY DSD and 46,XX DSD (10–12). POR plays a pivotal role as the obligatory electron donor to all microsomal cytochrome P450 enzymes, including CYP21A2 and CYP17A1, the latter catalyzing the biosynthesis of dehydroepiandrosterone (DHEA), the major precursor for testosterone biosynthesis. Consequently, POR deficiency (PORD) results in low circulating androgen concentrations, which readily account for 46,XY DSD, but fails to account for the severe virilization of external genitalia regularly observed in affected 46,XX neonates.
An explanation for this striking and seemingly contradictory genital phenotype in PORD has been lacking. We hypothesized that this apparent paradox could be explained by the existence of an alternative pathway to androgen production that generates DHT from 17α-hydroxyprogesterone (17OHP) during human fetal sexual differentiation, thereby bypassing the classic androgen biosynthesis pathway via DHEA and testosterone, as previously proposed by us (11) and others (13, 14). Elements of this pathway have been characterized in the fetal gonad of the tammar wallaby pouch young (15–17) and fetal opossum urogenital tract (18, 19). 17OHP accumulates in PORD but also in the most common CAH variant, 21-hydroxylase deficiency, and thus could feed into the proposed alternative pathway, if present in the fetus. Indirect biochemical evidence has indicated that the proposed alternative pathway is active postnatally in individuals with CAH due to 21-hydroxylase deficiency (20, 21) and may explain maternal virilization observed in pregnancies affected by PORD (22, 23). However, direct delineation of the putative alternative pathway during human fetal development, and in particular during the major period of sexual differentiation, has been lacking.
Here, we present conclusive evidence for the presence and activity of the alternative pathway in the human fetus, producing potent androgens during the major period of sexual differentiation, and show that human fetal female external genitalia respond sensitively to androgens during the same period. In concert, these findings define an alternative pathway for androgen biosynthesis during the critical period of sexual differentiation in the human fetus that represents an important mechanism to explain the prenatal virilization of female infants affected by CAH.
Results and Discussion
Androgen Biosynthesis in the Human Fetus during Sexual Differentiation.
To ascertain the presence and activity of the hypothesized alternative androgen pathway, we performed incubations with male and female fetal adrenals, gonads, and genital skin, which were collected at 6 to 10 wpc as previously described (8). We separately added deuterated steroid substrates for each step of the alternative pathway and employed liquid chromatography–tandem mass spectrometry (LC-MS/MS) to identify the resulting products (SI Appendix, Fig. S1 and Tables S1 and S2). Experiments were conducted at least in triplicate for each organ of either sex (SI Appendix, Figs. S2 and S3).
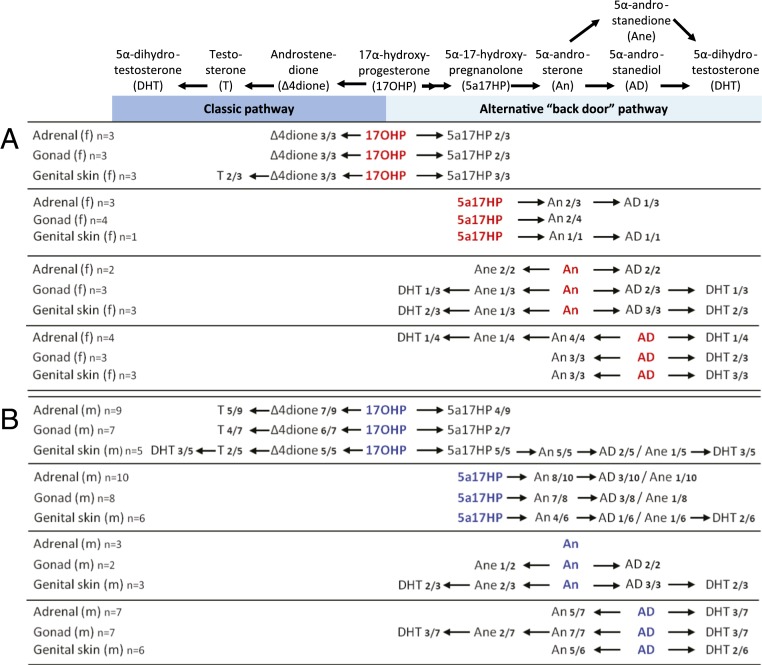
Explant incubations with female tissue showed that some 17OHP entered the classic androgen biosynthesis pathway, yielding androstenedione in adrenal, ovary, and genital skin. However, testosterone was detected in only 2 of 9 female genital skin incubations, and not at all in female adrenals and gonads (Fig. 1A). By contrast, all 3 female tissues (genital skin, ovaries, and adrenals) showed conversion of 17OHP and subsequent intermediates through all steps of the proposed alternative androgen pathway, with the end product, DHT, produced from 5α-androsterone, with either 5α-androstanediol or 5α-androstanedione as intermediates (Fig. 1A).
Fig. 1.
Androgen biosynthesis via classic and alternative androgen pathways as observed in human fetal organ explant cultures from the major period of sexual differentiation. The steroid substrates added to the explant cultures derived from fetal adrenals, gonads, and genital skin (collected at 6 to 10 wpc) are shown in red and blue for fetal female (A) and male (B) tissues, respectively. Conversion products detected and identified by LC-MS/MS are given in black. f, female; m, male; n indicates the number of biological replicates per tissue and fetal sex and how many of the cultures showed detectable synthesis of the indicated products.
Male fetal tissues readily converted 17OHP along the classic androgen pathway to testosterone and, in the alternative pathway, to 5α-17-hydroxypregnanolone (5α-17HP). In male genital skin, conversion from 17OHP proceeded until the generation of DHT (Fig. 1B), feasibly arising from either route, as DHT represents the end product of both the classic and alternative androgen pathways. However, incubations with the intermediate substrates of the alternative pathway demonstrated stepwise catalysis to DHT by male adrenal, testis, and genital skin (Fig. 1B). Consistent with studies in nonhuman species (24, 25), we did not identify 5α-pregnane-17α-ol-3,20-dione as a significant intermediate of the 2-step conversion of 17OHP to 5α-17HP in tissues from either sex, likely due to its immediate forward conversion.
Taken together, these data show that adrenals, gonads, and genital skin are capable of androgen biosynthesis via both the classic and alternative pathways in both sexes. Our data suggest the presence of an integrated adrenogenital steroidogenic unit capable of producing DHT during the period of sexual differentiation in both male and female fetuses.
We did not study the recently described 11-oxygenated androgen biosynthesis pathway (26, 27), initiated by conversion of androstenedione to 11-hydroxyandrostenedione by CYP11B1 11β-hydroxylase activity, and eventually yielding 11-ketotestosterone, which activates the androgen receptor with similar potency to testosterone. Recent work indicated a significant role of 11-oxygenated androgens in CAH due to 21-hydroxylase deficiency (28) and polycystic ovary syndrome (29). We previously demonstrated the presence and activity of CYP11B1 in the human fetal adrenal during the period of sexual differentiation (8), and hence at least the initial step of the 11-oxygenated androgen pathway is likely to occur. However, urine steroid excretion analysis in infants with PORD (13) did not show increased excretion of 11-hydroxyandrosterone, the major metabolite derived from 11-oxygenated androgens, indicating that this side arm of the classic androgen pathway is unlikely to play a major role in PORD.
Steroidogenic Enzyme Expression during Human Fetal Sexual Differentiation.
The alternative pathway conversion of 17OHP to 5α-17HP requires sequential 5α-reductase and 3α-hydroxysteroid dehydrogenase activities.
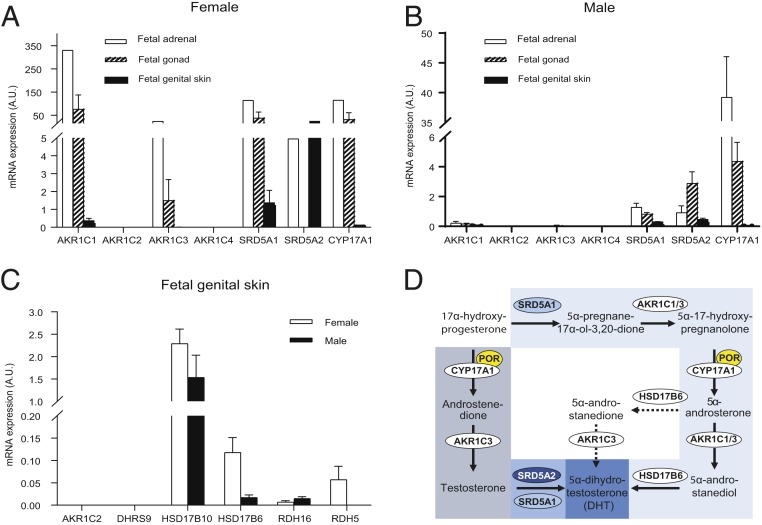
Of the 2 isoforms, the first step is expected to require catalysis by steroid 5α-reductase type 1 (SRD5A1), since SRD5A2 does not convert 17OHP efficiently (30). A study of fetal tissues from 12 to 20 wpc did not detect SRD5A1 in adrenals, gonads, or genital skin (31); a recent study also using fetal tissues from the second trimester of pregnancy (11 to 21 wpc) described detection of SRD5A1 in liver, placenta, testis, and genital tubercle but not in the adrenal, while female gonads were not studied (32). However, studying tissue from the major period of human sexual differentiation, we found mRNA expression of SRD5A1 in adrenals, gonads, and genital skin of both sexes (Fig. 2A). The second step, the conversion of 5α-pregnane-17α-ol-3,20-dione to 5α-17HP, requires 3α-hydroxysteroid dehydrogenase activity, in keeping with the observed expression of AKR1C1 and AKR1C3, both of which encode enzymes capable of catalyzing this reaction (Fig. 2A).
Fig. 2.
Steroidogenic enzyme expression in human fetal tissue from the major period of sexual differentiation and their proposed role(s) in alternative pathway synthesis. (A and B) mRNA expression (mean ± SEM) in human fetal tissues collected at 6 to 10 wpc as measured by qPCR in female adrenals (n = 1), gonads (n = 2), genital skin (n = 2), and corresponding male tissues (≥3 biological replicates for adrenals, gonads, and genital skin). (C) Fetal genital skin mRNA expression of all enzymes potentially capable of converting 5α-androstanediol to 5α-dihydrotestosterone. Expression data were normalized to ribosomal 18S. (D) Schematic summary of the proposed distinct roles of the identified enzymes in the classic androgen pathway (dark blue) and the alternative androgen synthesis pathway (light blue), both resulting in the synthesis of potent 5α-dihydrotestosterone. Arrows indicate observed conversions in the fetal organ explant cultures; dotted arrows represent reactions only rarely observed.
The next step in the alternative pathway requires CYP17A1 17,20-lyase activity; 5α-17HP is the preferred substrate for this reaction and efficiently converted to 5α-androsterone (33). We detected robust CYP17A1 expression in human fetal adrenals from the major period of sexual differentiation, consistent with previous reports (8, 34) and also in the gonads of both sexes (Fig. 2B). The subsequent reduction of 5α-androsterone to 5α-androstanediol requires 17β-hydroxysteroid dehydrogenase activity, which can be provided by AKR1C3 or AKR1C1, expressed in adrenals, gonads, and genital skin (Fig. 2A).
The final step of the proposed pathway involves the conversion of 5α-androstanediol to DHT, which requires 3β-epimerase (oxidative 3α-HSD) activity. Several enzymes have been considered to catalyze this reaction (i.e., AKR1C2, RDH5, DHRS9, HSD17B10, HSD17B6, and RDH16). However, only HSD17B6 and RDH16 are capable of efficient oxidation of 5α-androstanediol to DHT, as previously demonstrated by transactivation of the androgen receptor following cell-based overexpression (24, 35). We found expression of both HSD17B6 and RDH16 in fetal genital skin of both sexes (Fig. 2C).
In summary, we detected the transcripts encoding all enzymes required to catalyze the alternative androgen pathway (Fig. 2D). Taken together with the steroid conversion studies, these data comprehensively demonstrate that the normal adrenal, gonad, and genital skin are capable of androgen biosynthesis via both the classic and alternative pathways in both sexes. Our data point to an adrenogenital steroidogenic unit that can cooperate to produce DHT via the alternative pathway during the major period of human sexual differentiation.
Androgen Receptor in Female Genitalia from the Start of Sexual Differentiation.
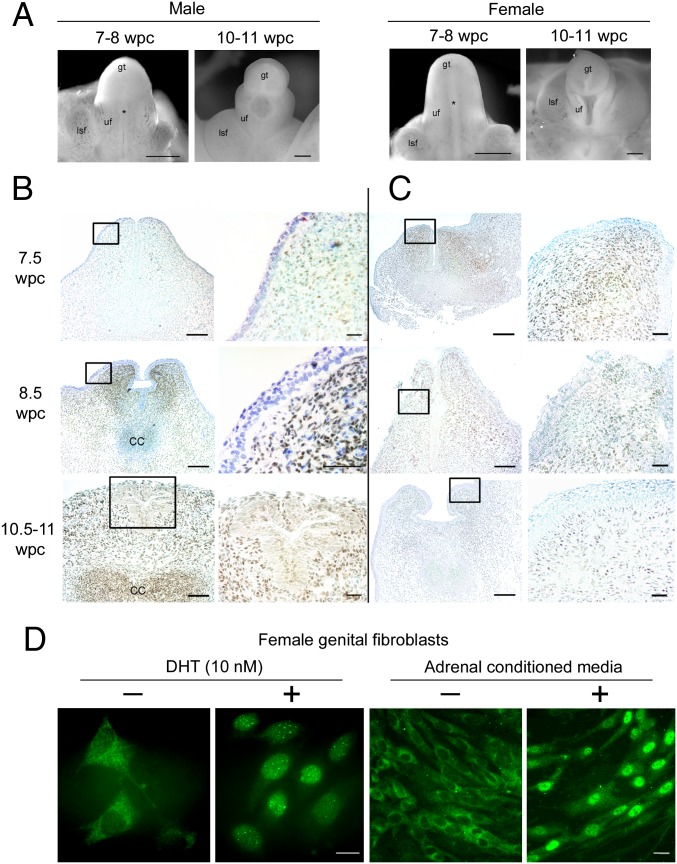
Having demonstrated the capacity for DHT production from steroidogenic precursors in female fetuses, we corroborated its ability to function by examining the presence of the androgen receptor (AR) in female external genitalia from the start of sexual differentiation. Previously, AR expression was documented in 4 female human fetuses from 9 to 18 wpc (36). In our study, we readily detected AR protein in stromal cells in the urethral folds of the external genitalia in both male and female fetuses at the onset of sexual differentiation (Fig. 3 A–C). Its nuclear localization in fixed tissues in both sexes implied AR was ligand-bound. To explore this further, we studied the intracellular localization of AR by immunofluorescence in female external genital fibroblasts taken into primary culture from the same stage of development. In steroid-free media, the external genital cells demonstrated cytoplasmic AR localization. As expected, the addition of 1 nM DHT induced nuclear translocation of AR. Strikingly, the same translocation was observed when using medium conditioned overnight from the corresponding female adrenal gland (Fig. 3D). In combination, these data show AR from the start of sexual differentiation in both male and female external genitalia and demonstrate a functional adrenogenital steroidogenic unit capable of causing AR nuclear translocation.
Fig. 3.
Androgen receptor is present in both male and female genitalia from the onset of sex differentiation. (A) Morphology of male and female external genitalia at the onset of sex differentiation. gt, genital tubercle; lsf, labioscrotal fold; uf, urethral fold. Asterisks indicate patency between urethral folds at 7 to 8 wpc that is partially sealed in males by 10 to 11 wpc. (B and C) Immunohistochemistry in transverse sections through the phallus for AR in male (B) and female (C) external genitalia from the start of human sexual differentiation at 7 to 8 wpc counterstained with toluidine blue. Boxes (Left) are shown at higher magnification (Right). (D) Immunofluorescence for AR in female external genital fibroblasts in the presence (+) or absence (−) of 10 nM DHT (Left) or medium conditioned by overnight incubation with an adrenal gland from the same female fetus (Right). [Scale bars, 500 μm (A); 100 μm (low) and 20 μm (high magnification) (B and C); and 25 μm (D).]
Androgen Biosynthesis in Neonates with Congenital Adrenal Hyperplasia Due to P450 Oxidoreductase Deficiency.
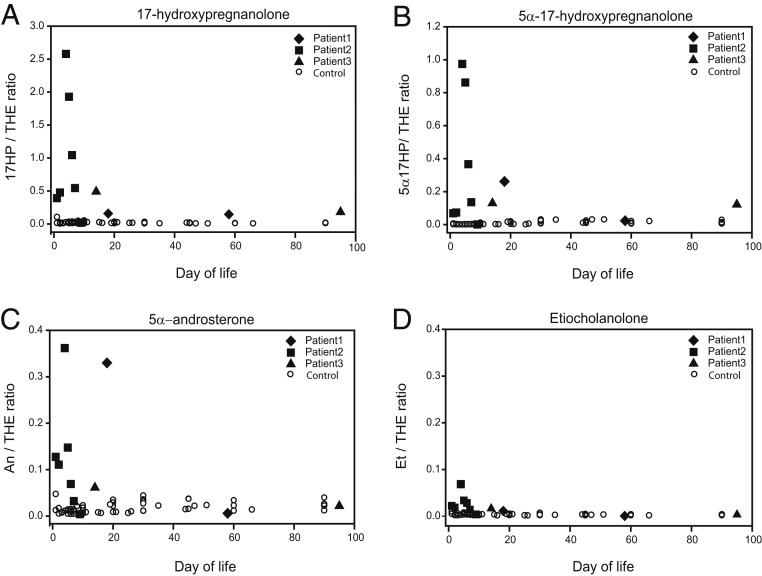
Having shown evidence of the alternative pathway during human sexual differentiation, we next investigated whether we could demonstrate equivalent activity in vivo. To address our hypothesis that excess alternative pathway androgen biosynthesis in fetal life explains female virilization (46,XX DSD) in CAH due to PORD (12, 37), in whom the classic androgen pathway is disrupted, we identified 3 patients with a mutation known to cause 46,XX DSD in affected girls and facilitate normal male appearance of external genitalia in affected boys (POR A287P) (11, 12). All 3 individuals had a 46,XY karyotype; patients 1 and 2 harbored homozygous A287P mutations while patient 3 was compound-heterozygous for POR A287P/G188_V191dup. While postnatal circulating androgens in PORD are low in infancy and beyond (12, 37), we hypothesized that affected individuals would still show evidence of excess alternative pathway androgen biosynthesis in the immediate neonatal period. We analyzed urinary steroid metabolite excretion by gas chromatography–mass spectrometry and detected significant alternative pathway activity in the neonatal period, consistent with previous preliminary findings that argued for the adrenal gland as a major site for alternative pathway activity (13). During the first 3 wk after birth, we documented increased excretion of the 17OHP metabolite 17-HP compared with healthy controls (Fig. 4A), consistent with accumulation of 17OHP, the initial substrate of the alternative androgen pathway. We found significantly increased excretion of 5α-17HP (Fig. 4B), a key intermediate of the alternative pathway; and, finally, there was significantly increased excretion of the major DHT metabolite 5α-androsterone (Fig. 4C). By contrast, etiocholanolone excretion, derived from classic pathway androgen biosynthesis, did not differ between the affected and healthy control groups (Fig. 4D). This result corroborates a previous report on urine steroid metabolite excretion in a 46,XY infant affected by PORD, which also described increased 5α-androsterone but unremarkable etiocholanolone excretion (38). Of note, the POR A287P-homozygous patients in our study had higher excretion of 5α-17HP and 5α-androsterone than the compound-heterozygous patient, who can be expected to have less alternative pathway activity due to the greater impairment of residual POR function. Taken together, these findings indicate increased in vivo alternative pathway androgen biosynthesis in neonates with PORD, which ceases shortly after birth.
Fig. 4.
Urinary steroid excretion in 3 46,XY neonates with POR deficiency (closed symbols) in comparison with 9 sex- and age-matched healthy controls (open symbols). POR-deficient neonates harbored the A287P mutation, which in the homozygous state is associated with normal male genitalia in boys and genital virilization (46,XX DSD). We included data from 3 46,XY neonates with PORD; 2 harbored homozygous POR mutations (A287P/A287P), and 1 harbored compound-heterozygous mutations (A287P/G188_V191dup). Longitudinal urine collections were carried out during the first 3 mo of life and analyzed by gas chromatography–mass spectrometry. Depicted are the urinary excretion of (A) the 17OHP metabolite 17-hydroxypregnanolone (17HP) and the 2 alternative pathway intermediates (B) 5α-17-hydroxypregnanolone (5α-17HP) and (C) 5α-androsterone (An), in comparison with (D) etiocholanolone (Et), which is only generated via the classic androgen pathway. All steroids are shown relative to tetrahydrocortisone (THE), an abundant adrenal-derived steroid metabolite, as the denominator.
These findings were further supported by in vitro 17,20-lyase activity assays employing yeast microsomes cotransformed with human CYP17A1 and either wild-type or mutant POR. The results demonstrated that the residual activity of POR A287P was higher in the alternative androgen pathway than in the classic pathway. POR A287P demonstrated significantly higher activity than POR mutant H628P, which in the homozygous state is associated with severe male undervirilization (46,XY DSD) and normal female genital phenotype (SI Appendix, Fig. S1 A–C). Additional experiments with yeast microsomes cotransformed with CYP19A1 and wild-type or mutant POR demonstrated that neither mutant affected aromatase activity (SI Appendix, Fig. S1D), thus excluding impaired aromatization of classic pathway androgens as a driver of prenatal virilization.
In conclusion, we have provided in vitro, ex vivo, and in vivo evidence for the existence and activity of an alternative pathway for the synthesis of the most potent androgen, DHT, during early human development. Our data demonstrate that, through cooperation of an adrenogenital steroidogenic unit, the alternative androgen pathway yields active androgen synthesis in the female fetus, with excess activity driving female virilization, 46,XX DSD, in CAH due to P450 oxidoreductase deficiency. Given that the alternative pathway substrate 17OHP also accumulates in 21-hydroxylase deficiency, it is conceivable that alternative pathway androgens contribute to prenatal virilization in this most common CAH variant.
Materials and Methods
Collection of Human Embryonic and Fetal Material.
Ethical approval for these studies was granted by the North West Haydock Research Ethics Committee of the UK Health Research Authority (approval no. 18/NW/0096). The collection and staging of human embryonic and fetal material were carried out with informed consent, as described previously (8, 39), using Carnegie classification and fetal foot length to provide a direct assessment of developmental age as days or weeks post conception (dpc or wpc), respectively, and male fetal material was identified by SRY expression, as previously described (1). We analyzed organs and tissue from 30 fetuses: 25 male and 5 female; median age 55 dpc (range 44 to 84 dpc).
RNA Extraction, Reverse Transcription, and Quantitative PCR.
Total RNA was extracted from whole organs using the TRI Reagent system (Sigma-Aldrich). RNA integrity and concentrations were assessed using a NanoDrop spectrophotometer. Reverse transcription was carried out employing a standard protocol. mRNA expression levels were quantified using an ABI 7500 sequence detection system (PerkinElmer Applied Biosystems), employing the Applied Biosystems “assay on demand” probe and primers for specific amplification of SRD5A1, SRD5A2, CYP17A1, AKR1C1, AKR1C2, AKR1C3, AKR1C4, HADH2/HSD17B10, HSD17B6, RDH5, DHRS9, and RDH16 (for further details, see SI Appendix).
Tissue Explant Culture and Steroid Identification by Tandem Mass Spectrometry.
Whole-organ tissue explants (fetal adrenals, gonads, and genital skin) were cultured in DMEM/F12 (PAA Laboratories) supplemented with 2% Ultroser SF (i.e., steroid-free; BioSepra) and 1% ITS+ (BD) at 37 °C in humidified 5% CO2 and 95% air for 64 h. Genital skin was cultured as monolayers and used for experiments at passage 4.
Identification of steroid products from the explant cultures was achieved using LC-MS/MS. Steroids were positively identified by comparison of retention time and MS/MS mass transitions with authentic steroid standards (SI Appendix, Table S1). Two mass transitions were used to positively identify each steroid, referred to as quantifier and qualifier ions, respectively; the resolution of a series of authentic steroid standards is shown in SI Appendix, Fig. S2, alongside further method details.
For steroid conversion assays, tissue explants were incubated with precursor steroids purchased from Steraloids and Sigma-Aldrich. For explant cultures assessing the conversion of 17-hydroxyprogesterone, 5α-androsterone, and 5α-androstanediol, we used deuterated steroids (for details, see SI Appendix, Table S2). Representative results of steroid detection following explant culture incubations are shown in SI Appendix, Figs. S3 and S4 for female and male tissues.
Immunohistochemistry.
Immunohistochemistry, immunoblotting, and immunofluorescence were carried out as reported previously (40), using monoclonal mouse anti-AR (1:100; LabVision).
Urine Steroid Metabolite Excretion Analysis.
Urine samples were collected longitudinally from birth until 90 d of life in 3 neonates affected with PORD (2 homozygous for POR A287P, and the other 1 compound-heterozygous for POR A287P/G188_V191dup) and compared with those collected from 9 healthy controls. These were matched for sex, age, and gestational age at birth and collected during the same time window. The parents of PORD patients and healthy controls provided written informed consent prior to urine collection. The study protocol was reviewed and approved by the Research Ethics Committee of the University College London Institute of Child Health/Great Ormond Street Hospital NHS Trust (REC reference 05/Q0508/24).
Urinary steroid hormone profiles were determined by gas chromatography–mass spectrometry analysis as described previously (41). The final analytical samples are the methyloxime-trimethylsilyl derivatives of steroids enzymatically released from sulfate and glucuronide conjugation. Analytes quantified by selected ion monitoring were normalized to tetrahydrocortisone, the most abundant steroid metabolite consistently excreted throughout life, with no significant difference in urinary tetrahydrocortisone concentrations identified between PORD patients (n = 3; 13 urine samples; median 285 µg/L, range 56 to 1,256 µg/L) and healthy controls (n = 9; 48 urine samples; median 306 µg/L, range 59 to 1,663 µg/L).
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust (Senior Research Fellowship WT088566 to N.A.H.; Investigator Award in Science 209492/Z/17/Z to W.A.), Medical Research Council UK (Program Grant 0900567 to W.A.), Gerald Kerkut Trust (PhD Studentship to D.J.A.), European Commission (Marie Curie Intra-European Fellowship PIEF-GA-2008-221058 to N.R.), Deutsche Forschungsgemeinschaft (Heisenberg Professorship 325768017, CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” to N.R.), and Society for Endocrinology UK (Lab Visit Award to N.R.). W.A. receives support from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (Grant BRC-1215-20009). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care UK. We thank Beverly A. Hughes, University of Birmingham, for technical support. We thank our research nurses and study participants for tissue collection.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.B.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906623116/-/DCSupplemental.
References
- 1.MacLaughlin D. T., Donahoe P. K., Sex determination and differentiation. N. Engl. J. Med. 350, 367–378 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Sekido R., Lovell-Badge R., Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Eggers S., Ohnesorg T., Sinclair A., Genetic regulation of mammalian gonad development. Nat. Rev. Endocrinol. 10, 673–683 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Hanley N. A., Arlt W., The human fetal adrenal cortex and the window of sexual differentiation. Trends Endocrinol. Metab. 17, 391–397 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Park S. Y., Jameson J. L., Minireview: Transcriptional regulation of gonadal development and differentiation. Endocrinology 146, 1035–1042 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Leshin M., Griffin J. E., Wilson J. D., Hereditary male pseudohermaphroditism associated with an unstable form of 5α-reductase. J. Clin. Invest. 62, 685–691 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson J. D., Griffin J. E., Russell D. W., Steroid 5α-reductase 2 deficiency. Endocr. Rev. 14, 577–593 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Goto M., et al. , In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J. Clin. Invest. 116, 953–960 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krone N., Arlt W., Genetics of congenital adrenal hyperplasia. Best Pract. Res. Clin. Endocrinol. Metab. 23, 181–192 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flück C. E., et al. , Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat. Genet. 36, 228–230 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Arlt W., et al. , Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: Analytical study. Lancet 363, 2128–2135 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Krone N., et al. , Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J. Clin. Endocrinol. Metab. 97, E257–E267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homma K., et al. , Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: Implication for the backdoor pathway to dihydrotestosterone. J. Clin. Endocrinol. Metab. 91, 2643–2649 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Flück C. E., Pandey A. V., Steroidogenesis of the testis—New genes and pathways. Ann. Endocrinol. (Paris) 75, 40–47 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Wilson J. D., et al. , 5α-androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology 144, 575–580 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Auchus R. J., The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 15, 432–438 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Shaw G., et al. , Role of the alternate pathway of dihydrotestosterone formation in virilization of the Wolffian ducts of the tammar wallaby, Macropus eugenii. Endocrinology 147, 2368–2373 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Wilson J. D., et al. , Ontogeny and pathway of formation of 5α-androstane-3α,17β-diol in the testes of the immature brushtail possum Trichosurus vulpecula. Reprod. Fertil. Dev. 17, 603–609 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Wilson J. D., Renfree M. B., Auchus R. J., Pask A. J., Shaw G., Formation of 5α-reduced androgens in the testes and urogenital tract of the grey short-tailed opossum, Monodelphis domestica. Reprod. Fertil. Dev. 21, 649–654 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Kamrath C., Hochberg Z., Hartmann M. F., Remer T., Wudy S. A., Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: Evidence from urinary steroid hormone analysis. J. Clin. Endocrinol. Metab. 97, E367–E375 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Jones C. M., et al. , Modified-release and conventional glucocorticoids and diurnal androgen excretion in congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 102, 1797–1806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shackleton C., Marcos J., Arlt W., Hauffa B. P., Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): A disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am. J. Med. Genet. A. 129A, 105–112 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Reisch N., et al. , Prenatal diagnosis of congenital adrenal hyperplasia caused by P450 oxidoreductase deficiency. J. Clin. Endocrinol. Metab. 98, E528–E536 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauman D. R., Steckelbroeck S., Williams M. V., Peehl D. M., Penning T. M., Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha,17beta-diol to 5alpha-dihydrotestosterone: A potential therapeutic target for androgen-dependent disease. Mol. Endocrinol. 20, 444–458 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Tsujimura T., Mizutani S., Matsumoto K., Pathway from progesterone to 5α-reduced C19 steroids not involving androstenedione and testosterone in immature mouse testes in vitro. Endocrinology 96, 515–518 (1975). [DOI] [PubMed] [Google Scholar]
- 26.Rege J., et al. , Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 98, 1182–1188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storbeck K. H., et al. , 11β-hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: A putative role in castration resistant prostate cancer? Mol. Cell. Endocrinol. 377, 135–146 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Turcu A. F., et al. , Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur. J. Endocrinol. 174, 601–609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Reilly M. W., et al. , 11-oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 840–848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frederiksen D. W., Wilson J. D., Partial characterization of the nuclear reduced nicotinamide adenine dinucleotide phosphate: Delta 4-3-ketosteroid 5α-oxidoreductase of rat prostate. J. Biol. Chem. 246, 2584–2593 (1971). [PubMed] [Google Scholar]
- 31.Thigpen A. E., et al. , Tissue distribution and ontogeny of steroid 5 α-reductase isozyme expression. J. Clin. Invest. 92, 903–910 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Shaughnessy P. J., et al. , Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. 17, e3000002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta M. K., Guryev O. L., Auchus R. J., 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch. Biochem. Biophys. 418, 151–160 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Melau C., et al. , Characterization of human adrenal steroidogenesis during fetal development. J. Clin. Endocrinol. Metab. 104, 1802–1812 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauman D. R., Steckelbroeck S., Peehl D. M., Penning T. M., Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology 147, 5806–5816 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Shapiro E., Huang H. Y., Wu X. R., Uroplakin and androgen receptor expression in the human fetal genital tract: Insights into the development of the vagina. J. Urol. 164, 1048–1051 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Huang N., et al. , Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 76, 729–749 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono H., et al. , Longitudinal serum and urine steroid metabolite profiling in a 46,XY infant with prenatally identified POR deficiency. J. Steroid Biochem. Mol. Biol. 178, 177–184 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Piper K., et al. , Beta cell differentiation during early human pancreas development. J. Endocrinol. 181, 11–23 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Pritchett J., et al. , Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology 56, 1108–1116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shackleton C., et al. , Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am. J. Med. Genet. A. 128A, 223–231 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.