Significance
T cells, essential components of the immune system, are developed in the thymus. Various effector cytokines are produced in the thymus in the steady state, which affect T cell development and tolerance. Interleukin-4 (IL-4) produced by a unique subset of lipid-specific invariant natural killer T (iNKT) cells (NKT2) in the steady state influences a series of immune events to alter thymic development. However, the underling mechanisms for this steady-state IL-4 production are not completely understood. In this study, we show that steady-state IL-4–producing NKT2 cells predominantly localize to the thymic medulla. Furthermore, this steady-state IL-4 production requires continuous T cell receptor (TCR) signaling in NKT2 cells majorly stimulated by thymic macrophages.
Keywords: thymus, IL-4, iNKT cells, NKT2, macrophage
Abstract
Interleukin-4 (IL-4) is produced by a unique subset of invariant natural killer T (iNKT) cells (NKT2) in the thymus in the steady state, where it conditions CD8+ T cells to become “memory-like” among other effects. However, the signals that cause NKT2 cells to constitutively produce IL-4 remain poorly defined. Using histocytometry, we observed IL-4–producing NKT2 cells localized to the thymic medulla, suggesting that medullary signals might instruct NKT2 cells to produce IL-4. Moreover, NKT2 cells receive and require T cell receptor (TCR) stimulation for continuous IL-4 production in the steady state, since NKT2 cells lost IL-4 production when intrathymically transferred into CD1d-deficient recipients. In bone marrow chimeric recipients, only hematopoietic, not stromal, antigen-presenting cells (APCs), provided such stimulation. Furthermore, using different Cre-recombinase transgenic mouse strains to specifically target CD1d deficiency to various APCs, together with the use of diphtheria toxin receptor (DTR) transgenic mouse strains to deplete various APCs, we found that macrophages were the predominant cell to stimulate NKT2 IL-4 production. Thus, NKT2 cells appear to encounter and require different activating ligands for selection in the cortex and activation in the medulla.
The thymus is a primary lymphoid organ that supports T cell development. Nonetheless, even in the steady state, cells of the thymus produce effector cytokines typically associated with acute immune responses, including type I IFN (IFN-α/β), type II cytokines (IL-4/13), and IFN-γ (1–9). These steady-state cytokines shape the thymic microenvironment in myriad ways, which affects T cell development and tolerance (2–8). In particular, steady-state IL-4 in the thymus drives CD8+ single positive thymocytes to differentiate into a memory-like state through up-regulation of eomesodermin (eomes) (3, 4, 7, 8). These IL-4–exposed CD8+ T cells display antigen receptor–independent survival and effector functions due to up-regulation of CD122 and IL-12/18R (and thus are often referred to as “innate” CD8+ T cells) and have trafficking properties similar to memory cells due to up-regulation of chemokine receptors CXCR3 and CD44 (3, 4, 7, 8). Innate CD8+ T cells were shown to have superior function compared to naïve T cells (7, 8, 10–13) and are thought to play a critical role in defense against infections (7, 8, 10–13). Thymic steady-state IL-4 also promotes the development and function of regulatory T cells (Tregs), particularly those that arise through the Foxp3low Treg progenitor pathway (14, 15). In addition, thymic dendritic cells (DCs) are influenced, secreting chemokines CCL17 and CCL22 in response to IL-4 signaling (3), which might further affect thymocyte–DC interactions in the organ (16). Finally, IL-4 signaling in medullary thymic epithelial cells (mTECs) has been shown to promote the thymic emigration of mature T cells (5). Given all of these effects, it is of interest to understand how IL-4 production is driven in the thymus.
Previous reports showed that steady-state IL-4 in the thymus is largely produced by lipid-specific invariant natural killer T (iNKT) cells (3, 7). iNKT cells express a semi-invariant T cell receptor (TCR) characterized by Vα14-Jα18 chains paired to a limited set of Vβ chains in mice (17). iNKT cells specifically recognize lipid antigens presented by the nonpolymorphic major histocompatibility complex (MHC)-I–like molecule CD1d, which contrasts with conventional CD4+ or CD8+ T cells that recognize peptides presented by highly polymorphic MHC molecules (17). Recent reports showed that mature iNKT cells comprise 3 major functionally distinct subsets, termed NKT1, NKT2, and NKT17, according to their expression of transcription factors and cytokine responses upon activation, analogous to the CD4+ T helper sublineages (3, 17). In general, NKT1 cells are positive for T-bet and produce IFN-γ; NKT17 cells are positive for ROR-γt and produce IL-17; while NKT2 cells express a high level of PLZF and produce IL-4. It is these PLZFhigh NKT2 cells that were shown to produce IL-4 in the thymus in the steady state (3, 4). Although “self”-lipids are implicated in the positive selection of iNKT precursors by CD1d+ double-positive (DP) thymocyte antigen-presenting cells (APCs) in the thymic cortex (18–20), it is not known if iNKT cells continue to recognize or respond to self-lipids as they differentiate.
In this study, we sought to explore the factors that drive iNKT cells to produce IL-4 in the steady state. Secretion of cytokines by T cells is usually a result of activation by cytokine and/or TCR signaling, and steady-state IL-4 production by iNKT cells in the thymus might very well fall into this scenario. Indeed, recent findings identified a specialized subset of medullary thymic epithelial cells (called thymic tuft cells) with striking similarity to peripheral mucosal tuft cells, which produce IL-25 to further stimulate the differentiation of NKT2 cells (21, 22). Here we show that NKT2 cells predominantly reside in the thymic medulla, where they receive and require TCR stimulation for their IL-4 production in the steady state. Moreover, this TCR stimulation in NKT2 cells is solely mediated by CD1d expressed by hematopoietic APCs, and macrophages are the predominant cells that trigger steady-state IL-4 production by NKT2 cells. Collectively, our findings show that NKT2 cells integrate TCR and cytokine signals for IL-4 production in the thymic medulla in the steady state.
Results
NKT2 Cells Produce IL-4 in the Steady State and Are Predominantly Located in the Thymic Medulla.
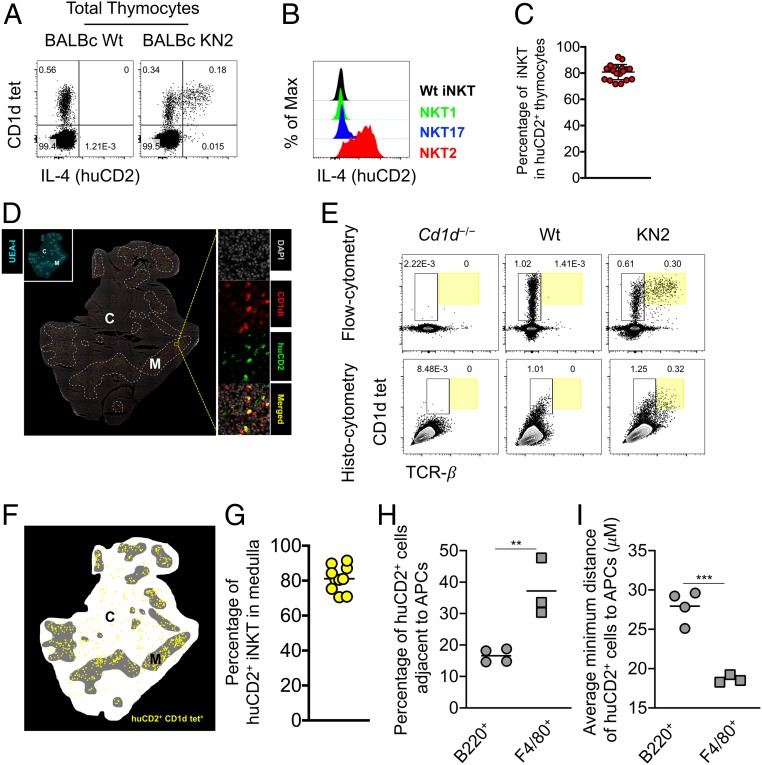
To directly investigate the thymic cells that secrete IL-4 in the steady state, we used an IL-4 reporter mouse strain, KN2 (wherein a human CD2 reporter gene is introduced into the endogenous Il-4 locus). In these mice, IL-4 protein–secreting cells can be faithfully detected through the expression of human CD2 (huCD2) on the cell surface (23). In comparison to wild-type (Wt) BALB/c mice, a distinct population of thymocytes expresses huCD2 in BALB/c KN2 mice (Fig. 1A). Combined with CD1d tetramer staining to specifically detect iNKT cells (SI Appendix, Fig. S1A), we observed that the majority of the IL-4–producing cells were iNKT cells (Fig. 1A). We also used transcription factor staining to distinguish different iNKT subsets (SI Appendix, Fig. S1B), which showed that NKT2 cells were the only cells to express huCD2 highly (Fig. 1 B and C), suggesting that they are the major source of steady-state IL-4 in the thymus, consistent with previous studies (3, 5, 24). To track the localization of IL-4–producing NKT2 cells, we performed in situ CD1d tetramer incubation followed by immunofluorescent staining for huCD2 in thymic sections (SI Appendix, Fig. S2A) (4). The majority of IL-4–producing NKT2 cells (huCD2+ CD1d tet+) were found in the UEA-I+ thymic medulla (Fig. 1D). We then employed quantitative histocytometry (Fig. 1E), which showed more than 80% of IL-4–producing NKT2 cells (huCD2+ CD1d tet+) localized in the UEA-I+ thymic medulla (Fig. 1 F and G). These results demonstrated that NKT2 cells were the major source of steady-state IL-4 in the thymus and predominantly localized to the thymic medulla.
Fig. 1.
NKT2 cells produce IL-4 in the thymic medulla in the steady state. (A) Staining of PBS57-CD1d tetramer and huCD2 in total thymocytes from BALB/c Wt and KN2 mice. Data are representative of 4 experiments. n = 19. (B) Expression of huCD2 in NKT2, NKT17, and NKT1 cells from BALB/c KN2 or total iNKT cells from BALB/c Wt mice. Data are representative of 2 experiments. n = 10. (C) Percentage of iNKT cells of huCD2+ thymocytes from BALB/c KN2 mice. Data are pooled from 4 experiments. n = 19. (D) Immunofluorescence image of thymus of BALB/c KN2 mice (Middle) stained with DAPI, UEA-I, PBS57-CD1d tetramer (CD1dt), and huCD2 to identify IL-4–producing iNKT cells (huCD2+, CD1dt+). White dashed lines outline the medullary region. Data are representative of 3 experiments. n = 10. (E) Each thymic lobe from BALB/c Cd1d−/−, Wt, or KN2 mice was subjected to histocytometry or flow cytometry analysis. Data are representative of 3 experiments. n = 10. (F) Histocytometric analysis of IL-4–producing iNKT cells (huCD2+, CD1dt+). Data are representative of 3 experiments. n = 10. (G) Percentage of huCD2+ iNKT cells localized in the thymic medulla. Data are pooled from 3 experiments. n = 10. Each dot represents an individual mouse, and horizontal bars indicate mean values unless otherwise indicated. (H) Percentage of huCD2+ cells localized adjacent (distance less than 10 μM) to indicated APCs, B220+, or F4/80+ cells. Data are pooled from 2 experiments. n = 4 (B220+), n = 3 (F4/80+). Each dot represents an individual mouse, and horizontal bars indicate mean values. Unpaired t test, **P = 0.0068. (I) Average minimum distance of huCD2+ cells to the nearest APCs, B220+, or F4/80+ cells. Data are pooled from 2 experiments. n = 4 (B220+), n = 3 (F4/80+). Unpaired t test, ***P = 0.0007.
NKT2 Cells Require TCR Signals for Their Production of IL-4 in the Steady State.
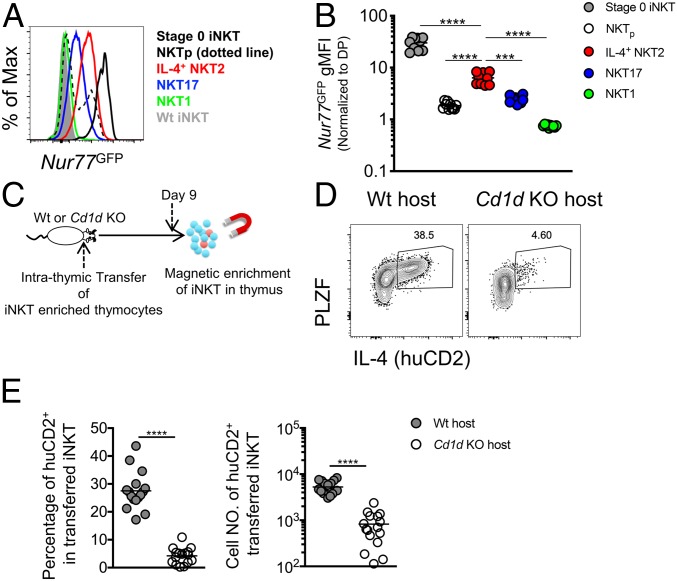
T cells can be activated via stimulation of cytokine and/or TCR signals to produce cytokines (25). A recent report indicated that thymic tuft cell–derived IL-25 promotes the differentiation of NKT2 cells (21). To understand whether NKT2 cells might also receive TCR signals in the steady state, we crossed BALB/c Nur77GFP mice with BALB/c KN2 mice to obtain BALB/c Nur77GFP KN2 mice. In Nur77GFP mice, the level of green fluorescent protein (GFP) reflects TCR signal strength in T cells and is independent of cytokine or inflammatory signals (20, 26). Interestingly, IL-4–producing NKT2 cells had significantly elevated GFP expression compared to NKT1 and NKT17 cells, albeit lower than “stage 0” iNKT cells (Fig. 2 A and B), suggesting that IL-4–producing NKT2 cells indeed receive TCR stimulation in the steady state. This is consistent with the high level of Egr2 observed in PLZFhigh iNKT cells (27). The level was also higher than that on the previously identified CCR7+ iNKT precursor (NKTp) cells (Fig. 2 A and B) (6), which represent an intermediate stage in iNKT cell differentiation that is after stage 0 but prior to development of the NKT1/2/17 stage (6). This finding suggests that iNKT cells are stimulated both in the cortex when undergoing positive selection at stage 0 and in the medulla at the mature NKT2 cell stage.
Fig. 2.
NKT2 cells require TCR signals for steady-state production of IL-4. (A) Representative histogram of Nur77GFP expression on subsets of thymic iNKT cells from BALB/c Nur77GFP or BALB/c Wt mice. Data are representative of 2 experiments. n = 10. (B) Normalized Nur77GFP geometric mean fluorescence intensity (gMFI) (to DP) of thymic stage 0 iNKT, CCR7+ iNKT precursor (NKTp), NKT1, NKT17, and IL-4+ NKT2 cells from BALB/c Nur77GFP mice. Data are pooled from 2 experiments. n = 10. Ordinary 1-way ANOVA, ****P < 0.0001. (C) Experimental scheme showing iNKT-enriched thymocytes from CD45.1+/CD45.2+ BALB/c KN2 mice intrathymically transferred into BALB/c Wt (CD45.2+/CD45.2+ or CD45.1+/CD45.1+) or BALB/c Cd1d KO (CD45.2+/CD45.2+) mice; 9 d later transferred donor iNKT cells were isolated by magnetic enrichment. (D) IL-4 (huCD2) and PLZF in donor iNKT cells 9 d after intrathymic transfer into BALB/c Wt or BALB/c Cd1d KO host mice. Data are representative of 5 experiments. n = 16 (E) Percentage and cell number of huCD2+ cells in transferred donor iNKT cells 9 d after intrathymic transfer. Data are pooled from 5 experiments. n = 16. Unpaired t test, ***P = 0.0002, ****P < 0.0001.
We next tested whether steady-state IL-4 production is dependent on TCR stimulation by transferring iNKT-enriched thymocytes intrathymically into Wt or Cd1d−/− hosts (Fig. 2C). huCD2+ NKT2 cells were dramatically reduced 9 d after intrathymic transfer into Cd1d−/− hosts compared to Wt hosts (Fig. 2 D and E), while NKT1 and NKT17 cell numbers were not affected (SI Appendix, Fig. S3A). Although PLZFhigh iNKT cell numbers were decreased after intrathymic transfer into Cd1d−/− hosts, the degree of this reduction was less profound than that of huCD2+ NKT2 cells (2.08-fold reduction in PLZFhigh iNKT cells compared to 6.37-fold reduction in huCD2+ PLZFhigh iNKT cells) (Fig. 2E and SI Appendix, Fig. S3A), indicating that PLZFhigh NKT2 cells lost IL-4 gene expression when intrathymically transferred into the Cd1d−/− hosts. Altogether, this strongly suggests that NKT2 cells receive and require TCR signals for steady-state IL-4 production, consistent with an earlier study (3). Considering that the majority of IL-4–producing NKT2 cells are localized in the thymic medulla, it implicates medullary APCs in CD1d-dependent stimulation of NKT2 cells.
NKT2 Cells Require CD1d in Hematopoietic APCs to Produce IL-4 in the Steady State.
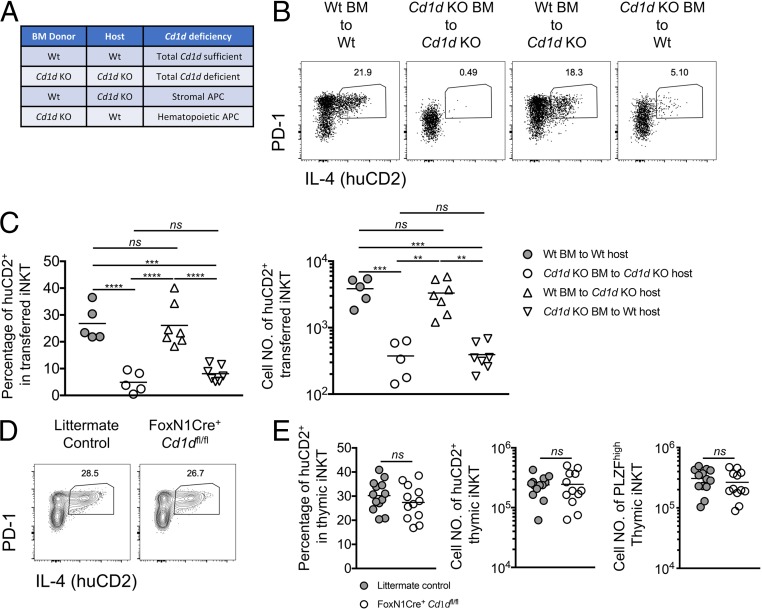
There are multiple lineages of APCs found in the thymic medulla, including stroma-derived APCs (thymic epithelial cells) as well as hematopoietic-origin APCs (B cells, classical dendritic cells, plasmacytoid dendritic cells, and macrophages) (28), all of which express CD1d (SI Appendix, Figs. S4B; also see S6A, and S6C) (29, 30). Therefore, we decided to first distinguish the role of stromal and hematopoietic APCs in supporting NKT2 IL-4 production. For this purpose, we created bone marrow chimeras in which Cd1d was selectively deficient in either stromal or hematopoietic APCs (Fig. 3A). The bone marrow chimeras were used as hosts for intrathymic transfer of iNKT-enriched thymocytes similar to that described above (Figs. 3A and 2C). Strikingly, steady-state IL-4 production in NKT2 cells was dramatically decreased upon intrathymic transfer into chimeras where Cd1d was selectively deficient in hematopoietic cells (Cd1d knockout [KO] bone marrow to Wt) but maintained in chimeras where Cd1d was selectively deficient in stromal cells (Wt bone marrow to Cd1d KO) (Fig. 3 B and C). These results suggest that only hematopoietic APCs stimulate NKT2 cells to produce IL-4 in the steady state.
Fig. 3.
Steady-state IL-4 production in NKT2 requires CD1d solely in hematopoietic APCs. (A) Four types of BALB/c bone marrow chimeras were created. (B) Expression of IL-4 (huCD2) and PD-1 in transferred donor iNKT cells 9 d after intrathymic transfer into the indicated bone marrow chimeras. Data are representative of 4 experiments. n = 5, n = 7. (C) Percentage and cell number of huCD2+ cells in transferred donor iNKT cells 9 d after intrathymic transfer. Data are representative of 4 experiments. n = 5, n = 7. Ordinary 1-way ANOVA; nsP > 0.8 (not significant), **P < 0.003, ***P < 0.001, ****P < 0.0001. (D) Expression of IL-4 (huCD2) and PD-1 in thymic iNKT cells from F1 littermate controls or F1 FoxN1Cre+ Cd1dfl/fl mice. Data are representative of 5 experiments. n = 13, n = 12 (E) Percentage and cell number of huCD2+ cells and cell number of PLZFhigh cells in thymic iNKT cells from the indicated mice. Data are pooled from 5 experiments. n = 13, n = 12. Unpaired t test, nsP = 0.2994 (Left), 0.9180 (Middle), or 0.4132 (Right).
To confirm this result, we sought to target Cd1d deficiency specifically in TECs using FoxN1Cre. However, because NKT2 and NKT17 cells are scarce in B6 mice (3), it was desirable to do this and additional tissue-specific gene deletion experiments in BALB/c mice, but Cd1dfl/fl mice and Cre-recombinase transgenic mice are most readily available on the B6 background. Interestingly, the thymic iNKT cell-subset composition in B6 × BALB/c F1 mice resembled that in BALB/c mice (SI Appendix, Fig. S5 A–C). Thus, we developed a strategy to achieve conditional Cd1d deficiency in B6 × BALB/c F1 mice. Briefly, we backcrossed B6 Cd1dfl/fl to BALB/c KN2 for at least 10 generations to obtain BALB/c Cd1dfl/fl-KN2 (SI Appendix, Fig. S5 A–C). B6 Cre-recombinase transgenic mice were first crossed to B6 Cd1dfl/fl mice to obtain B6 Cre+-Cd1dfl/fl mice. Then the B6 Cre+-Cd1dfl/fl mice were crossed with BALB/c Cd1dfl/fl-KN2 mice to generate F1 Cre-Cd1dfl/fl-KN2 mice (SI Appendix, Fig. S4A). Using this experimental strategy, we generated F1 FoxN1Cre+-Cd1dfl/fl-KN2 to efficiently target Cd1d deficiency in TECs (SI Appendix, Fig. S4 B and C). huCD2 expression in iNKT cells and the cell number of PLZFhigh iNKT cells were comparable between F1 FoxN1Cre+-Cd1dfl/fl-KN2 and littermate controls (Fig. 3 D and E). This was consistent with the results we showed in intrathymic transfer of bone marrow chimeras (Fig. 3 A–C), suggesting that CD1d in TECs is dispensable for stimulating NKT2 cells to produce IL-4 in the steady state.
CD11c+ APCs Support Steady-State IL-4 Production in NKT2 Cells in a CD1d-Dependent Manner.
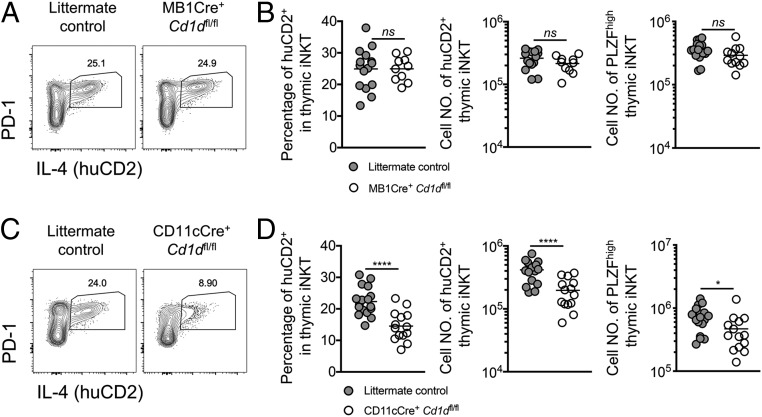
To define hematopoietic APCs in the thymus that are required to stimulate NKT2 cells to produce IL-4 in the steady state, we expanded our F1 Cre+-Cd1dfl/fl strategy (SI Appendix, Fig. S4A). CD1d expression was specifically abrogated in different APCs using animals expressing Cre under various tissue-specific promoters. Effective depletion of CD1d was observed in thymic B cells in F1 MB1Cre+-Cd1dfl/fl mice (SI Appendix, Fig. S6 A and B). However, the frequency and cell number of huCD2+ iNKT and cell number of PLZFhigh iNKT cells were not affected by Cd1d deficiency in B cells (Fig. 4 A and B). Nevertheless, in F1 CD11cCre+-Cd1dfl/fl mice we observed a substantial decrease in huCD2 expression in iNKT cells and a reduction in PLZFhigh iNKT cell number (Fig. 4 C and D), indicating an essential role for CD11c+ APCs in the induction of steady-state IL-4.
Fig. 4.
Steady-state IL-4 production relies on CD1d in CD11c-expressing APCs. (A) Expression of IL-4 (huCD2) and PD-1 in thymic iNKT cells from F1 littermate controls or F1 MB1Cre+ Cd1dfl/fl mice. Data are representative of 5 experiments. n = 15, n = 10. (B) Percentage and cell number of huCD2+ cells and cell number of PLZFhigh cells in thymic iNKT cells from the indicated mice. Data are pooled from 5 experiments. n = 15, n = 10. Unpaired t test, nsP = 0.9852 (Left) or nsP = 0.1456 (Right) (not significant). (C) Expression of IL-4 (huCD2) and PD-1 in thymic iNKT cells from F1 littermate controls or F1 CD11cCre+ Cd1dfl/fl mice. Data are representative of 7 experiments. n = 17, n = 14. (D) Percentage and cell number of huCD2+ cells and cell number of PLZFhigh cells (Right) in thymic iNKT cells from the indicated mice. Data are pooled from 7 experiments. n = 17, n = 14. Unpaired t test, *P = 0.0328, ****P < 0.0001.
Although CD11c is an important marker for dendritic cells, it was previously shown, and confirmed here, that CD11cCre targets multiple lineages of myeloid cells, including classical dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), and macrophages (SI Appendix, Fig. S6 C and D) (31–33). So, although this result strongly suggests that CD11c+ APCs are essential for steady-state NKT2 IL-4 production, either cDCs, pDCs, or macrophages (F4/80+ Mertk+ cells) might be involved.
F4/80+ Mertk+ Macrophages Are Essential for Steady-State IL-4 Production in NKT2 Cells.
We sought to further distinguish the role of cDCs, pDCs, and macrophages in the CD1d-mediated induction of IL-4 by NKT2 cells. For this purpose, we generated F1 Zbtb46Cre-Cd1dfl/fl mice and observed a specific loss of CD1d in cDCs (SI Appendix, Fig. S7 A and B) (31). Nonetheless, the frequency and number of huCD2+ NKT2 cells in the thymus were not affected upon the depletion of Cd1d in cDCs (Fig. 5 A and B), suggesting that the CD1d-mediated interaction between cDCs and iNKT cells is not required for NKT2 steady-state IL-4 production. To evaluate the impact of pDCs on steady-state IL-4, pDCs were effectively depleted using the F1 BDCA2-DTR mice with repeated injection of diphtheria toxin (DT) every other day (SI Appendix, Fig. S8 A–C). After 9 d of DT administration, we observed a mild decrease in huCD2+ iNKT cells in the pDC-depleted F1 BDCA2-DTR mice compared to the F1 littermate control mice (Fig. 5 C and D). This indicates that pDCs may play a supportive role in steady-state IL-4 production.
Fig. 5.
Macrophages/phagocytes are essential for steady-state IL-4 production in NKT2 cells. Expression of IL-4 (huCD2) and PD-1 in thymic iNKT cells from F1 littermate controls and Zbtb46Cre+ Cd1dfl/fl mice (A) or the following F1 littermate controls and DTR strains after a 9-d course of DT treatment: BDCA2-DTR+/− (C) and LysMCre+ Csf1rLsL−DTR+/− (E). Expression of eomes in CD8SP thymocytes (G); representative data are shown. Percentage and cell number of huCD2+ cells, cell number of PLZFhigh cells in thymic iNKT cells, or percentage and number of eomes+ CD8SP thymocytes from the indicated mice are shown in B, D, F, and H. Data are representative of 7 experiments with n = 20, n = 9 (A and B); 3 experiments with n = 14, n = 12 for C and D; 7 experiments with n = 20, n = 25 (E and F); and 6 experiments with n = 27, n = 22 (G and H). Unpaired t test, nsP > 0.5 (not significant), *P < 0.025, ***P = 0.0002, ****P < 0.0001. (I) Correlation of percentage or cell number of huCD2+ cells in thymic iNKT cells with the cell number of F4/80+ Mertk+ thymic macrophages in F1 littermate controls and F1 LysMCre+ Csf1rLsL−DTR+/−–treated mice. Linear regression was used to calculate goodness of fit (R2).
Finally, we examined the role of macrophages by generating LysMCre-Csf1rLsL−DTR mice (34) on an F1 background. We effectively ablated F4/80+ Mertk+ macrophages with a 9-d course of DT administration every other day in these mice (SI Appendix, Fig. S8 D and E). Moreover, flow cytometric analysis of various myeloid cells and lymphocytes in the thymus (SI Appendix, Fig. S9A) showed that cells of other lineages were not affected (SI Appendix, Fig. S8F). Ablation of macrophages under these conditions also did not disrupt the overall structure of the thymus (medulla-to-cortex ratio) or the medullary localization of thymic iNKT cells (SI Appendix, Fig. S10 A–C). Strikingly, however, depletion of the relatively small population of F4/80+ Mertk+ macrophages in the thymus led to a substantial decrease in the cell number of PLZFhigh iNKT cells and of IL-4 production by NKT2 cells (Fig. 5 E and F). NKT2-derived IL-4 was previously shown to condition CD8+ T cells to become memory phenotype, characterized by the expression of the transcription factor eomes (3, 7). Consistent with a loss of NKT2-derived IL-4, we observed a marked decrease in eomes+ CD8+ T cells in the thymus after depletion of macrophages (Fig. 5 G and H). In addition, the abundance of IL-4–producing NKT2 cells was tightly correlated with the cell number of thymic macrophages (Fig. 5I). We further tracked the localization of IL-4–producing cells (huCD2+) together with B220+ (B cells and pDCs) or F4/80+ cells (macrophages) (SI Appendix, Fig. S11 A and B). There were many more IL-4–producing cells localized adjacent to F4/80+ cells (37.2%) compared to the B220+ cells (16.6%) (Fig. 1H). Moreover, IL-4–producing cells, on average, were localized closer to F4/80+ cells than to B220+ cells (Fig. 1I and SI Appendix, Fig. S11C). Together, these results show that thymic macrophages (F4/80+ Mertk+ cells) are critical for stimulating thymic NKT2 cells to produce IL-4 in the steady state.
Discussion
This study and previous studies show that NKT2 cells are the major source of IL-4 in the thymus in the steady state (3, 5, 7, 35). This type II cytokine–enriched environment strongly influences thymocyte development through the induction of large numbers of innate CD8+ T cells (3, 7), although the biological rationale for this is not yet clear. It may enhance neonatal immunity, as thymic IL-4 is more abundant early in life (3). Alternatively, thymic IL-4 may enhance tolerance of conventional T cells to self-antigens that are uniquely produced during type II immune responses, especially those derived from IL-4–induced genes.
In this study, we further demonstrated that IL-4–producing NKT2 cells reside in the thymic medulla and require continuous TCR signaling for their steady-state production of IL-4, suggesting a cognate interaction between NKT2 cells and thymic APCs in the medulla in the steady state. Our data also suggest that both the function of NKT2 cells (IL-4 production and PD-1 expression) and, to a lesser extent, their differentiation, survival, or maintenance (high expression of PLZF) require TCR stimulation. Both the selective activation of a subset of iNKT cells and their medullary location are surprising findings that raise additional questions.
The Medulla Is an Important Site of iNKT Cell Differentiation.
iNKT cells are initially selected in the most immature, stage 0, state through interactions among DP thymocytes in the thymic cortex (18, 36, 37). Nonetheless, the thymic medulla has been suggested to be crucial for the continued development of iNKT cells, as mature thymic iNKT cells (CD44+) were greatly reduced in Relb−/− TEC kidney capsule grafts, which specifically lack mTECs (38). In line with this, we recently showed that CCR7+ iNKT cells represent a multipotent precursor for all 3 iNKT effector subsets (NKT1, NKT2, and NKT17) and that CCR7 facilitates migration of this precursor from the cortex to the medulla (6). Collectively, these findings put forward the notion that initial selection happens in the cortex while further differentiation into thymic effector subsets is reinforced in the medulla. Indeed, IL-15 produced by medullary thymic epithelial cells was essential for the generation of T-bet+ NKT1 cells (38, 39) and medullary thymic tuft cells were shown to influence the differentiation of thymic NKT2 cells, possibly through production of IL-25 (21).
Medullary Macrophages Activate iNKT Cells.
In addition to providing cytokines that impact the differentiation or retention of iNKT cells, we showed here that NKT2 cells in particular are activated by CD1d-expressing APCs in the medulla. This is consistent with an earlier study that showed CD1d is required for maturation of thymic iNKT cells beyond the positive selection of stage 0 iNKT cells (40), albeit not for their retention. We further demonstrated that thymic macrophages are the predominant APCs required for NKT2 cells to produce IL-4 through a CD1d-mediated interaction. Thymic macrophages are distributed throughout the cortex and medulla (41, 42), and are believed to serve as scavengers that play an essential role in clearing apoptotic thymocytes. However, previous reports also showed that thymic macrophages express both MHC-I and MHC-II and may possess antigen-presenting activity (41–44). Interestingly, macrophages are enriched in genes involved in lipid metabolism (45). Moreover, recent studies showed that macrophages are required to stimulate iNKT cells to produce IL-4 in the context of sterile liver injury (46) and influenza infection (47). Those CD1d-dependent interactions facilitate recovery from injury and optimal B cell immunity, respectively. Collectively, these data indicate that macrophages can serve as important APCs to present lipid antigens to iNKT cells under certain circumstances. However, the nature of these lipid antigens, and whether they are distinct from the lipid antigens that mediate positive selection of iNKT cells in the thymic cortex, remains to be elucidated.
NKT2 Cells Are Selectively Activated in the Thymus.
Not only do NKT2 cells produce the highest levels of IL-4 among mature thymic iNKT cells, they also express the highest level of Nur77GFP, indicating that they are perceiving stronger TCR signaling. This is consistent with the elevated Egr2 level in PLZFhigh iNKT cells (27). This TCR signal is essential for IL-4 production, as it ceased after adoptive transfer in the Cd1d KO hosts. High TCR signal strength also favors the differentiation of thymic NKT2 cells (PLZFhigh iNKT); as such, cells were substantially decreased in mice with weakened TCR signaling (SKG, YYAA mice) (27, 48). How do NKT2 cells perceive CD1d/self-lipid ligands differently from NKT1 and NKT17 when they all utilize a “semi-invariant” TCR? One possibility is that Vβ usage alters the fine specificity of the iNKT cells. Indeed, the 3 iNKT subsets have distinct and stable Vβ repertoires (3, 27, 49), with NKT2 cells (PLZFhigh iNKT) showing higher TCR density and preferential usage of Vβ7 (3, 27, 50). In a model system where retrogenic mice expressed various iNKT TCR β chains, it was demonstrated that the frequency of different iNKT subsets was governed by the half-life of TCR-Ag-CD1d interaction (51). Similarly, another study using somatic nuclear transfer to generate mouse strains bearing different monoclonal iNKT TCRs showed higher TCR avidity correlated with higher frequency of PLZFhigh iNKT cells (49). An earlier study suggested Vβ7+ iNKT cells might have a higher avidity for CD1d/self-lipid ligands than iNKT cells bearing other Vβs (52). Thus, it is possible that biased TCR Vβ gene usage leads to differential TCR signaling events that further impact the differentiation of iNKT subsets. Another possibility is that the TCR level dictates the perceived stimulus. NKT2 cells express the highest level of surface TCR, followed by NKT17 cells, and NKT1 cells have quite low levels. Indeed, TCR density was directly shown to influence the differentiation of iNKT subsets (53). It was also recently suggested that SLAM family receptors (SFRs) facilitate iNKT development by reducing TCR strength after position selection, and that SFR deficiency skews the subset distribution, most strongly affecting NKT1 cells (54).
In conclusion, we showed that IL-4–producing NKT2 cells residing in the thymic medulla are the major source of steady-state IL-4 in the thymus. The steady-state production of IL-4 in NKT2 cells depends on continuous TCR signaling mediated by hematopoietic APCs, predominantly thymic macrophages. Moreover, considering the previous reports discussed above, all these data suggest a model for the differentiation steps of thymic iNKT cells wherein 1) the initial positive selection among DP thymocytes generates the most immature, stage 0, iNKT cells in the thymic cortex; 2) upon the up-regulation of PLZF and CCR7, the iNKT multipotent precursors migrate from the cortex to the medulla; and 3) the differentiation of iNKT precursors into effector subsets (NKT1, NKT2, and NKT17) is influenced by various factors in the thymic medulla.
Materials and Methods
Mice.
The cell-type specificities of various Cre and DTR transgenic mouse strains used in this study are shown in the SI Appendix, Table S1. All animal work was conducted in compliance with the protocols approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Statistical Analysis.
Unpaired 2-tailed t tests and 1-way ANOVA were used for data analysis and calculation of P values in Prism software (GraphPad).
Supplementary Material
Acknowledgments
We thank Ms. Jane Ding for expert technical assistance and Dr. Hristo Georgiev for critical reading of the manuscript, as well as all present and past members of the Hogquist and Jameson lab for insightful discussions. This work was supported by NIH grants AI39560 (K.A.H.) and F30 AI131483, T32 AI007313, and T32 GM008244 (E.R.B.); and a University of Minnesota dissertation fellowship (H.W.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910412116/-/DCSupplemental.
References
- 1.Lienenklaus S., et al. , Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J. Immunol. 183, 3229–3236 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Xing Y., Wang X., Jameson S. C., Hogquist K. A., Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat. Immunol. 17, 565–573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y. J., Holzapfel K. L., Zhu J., Jameson S. C., Hogquist K. A., Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y. J., et al. , Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity 43, 566–578 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White A. J., et al. , A type 2 cytokine axis for thymus emigration. J. Exp. Med. 214, 2205–2216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H., Hogquist K. A., CCR7 defines a precursor for murine iNKT cells in thymus and periphery. eLife 7, e34793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreich M. A., Odumade O. A., Jameson S. C., Hogquist K. A., T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 11, 709–716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreich M. A., et al. , KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity 31, 122–130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.C. Reynolds J., et al. , Bioluminescent reporting of in vivo IFN-gamma immune responses during infection and autoimmunity. J. Immunol. 202, 2502–2510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jameson S. C., Lee Y. J., Hogquist K. A., Innate memory T cells. Adv. Immunol. 126, 173–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A., et al. , IL-4 induced innate CD8+ T cells control persistent viral infection. PLoS Pathog. 11, e1005193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renkema K. R., et al. , IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. J. Exp. Med. 213, 1319–1329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White J. T., Cross E. W., Kedl R. M., Antigen-inexperienced memory CD8+ T cells: Where they come from and why we need them. Nat. Rev. Immunol. 17, 391–400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen D. L., et al. , Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 20, 195–205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang B. H., et al. , PLZF(+) innate T cells support the TGF-β-dependent generation of activated/memory-like regulatory T cells. Mol. Cells 39, 468–476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Z., Lancaster J. N., Sasiponganan C., Ehrlich L. I., CCR4 promotes medullary entry and thymocyte-dendritic cell interactions required for central tolerance. J. Exp. Med. 212, 1947–1965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Hogquist K. A., How lipid-specific T cells become effectors: The differentiation of iNKT subsets. Front. Immunol. 9, 1450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egawa T., et al. , Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity 22, 705–716 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Kain L., et al. , The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity 41, 543–554 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran A. E., et al. , T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller C. N., et al. , Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559, 627–631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Moltke J., Ji M., Liang H. E., Locksley R. M., Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohrs K., Wakil A. E., Killeen N., Locksley R. M., Mohrs M., A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23, 419–429 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai D., et al. , KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J. Exp. Med. 208, 1093–1103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins M. K., et al. , In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19, 23–45 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Au-Yeung B. B., et al. , A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 111, E3679–E3688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuttle K. D., et al. , TCR signal strength controls thymic differentiation of iNKT cell subsets. Nat. Commun. 9, 2650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breed E. R., Lee S. T., Hogquist K. A., Directing T cell fate: How thymic antigen presenting cells coordinate thymocyte selection. Semin. Cell Dev. Biol. 84, 2–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmer M. I., et al. , A cell-type specific CD1d expression program modulates invariant NKT cell development and function. J. Immunol. 176, 1421–1430 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Forestier C., et al. , T cell development in mice expressing CD1d directed by a classical MHC class II promoter. J. Immunol. 171, 4096–4104 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Loschko J., et al. , Absence of MHC class II on cDCs results in microbial-dependent intestinal inflammation. J. Exp. Med. 213, 517–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai L., et al. , Distinct APCs explain the cytokine bias of alpha-galactosylceramide variants in vivo. J. Immunol. 188, 3053–3061 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abram C. L., Roberge G. L., Hu Y., Lowell C. A., Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 408, 89–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreiber H. A., et al. , Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J. Exp. Med. 210, 2025–2039 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgiev H., Ravens I., Shibuya A., Förster R., Bernhardt G., CD155/CD226-interaction impacts on the generation of innate CD8(+) thymocytes by regulating iNKT-cell differentiation. Eur. J. Immunol. 46, 993–1003 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Bendelac A., Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gapin L., Matsuda J. L., Surh C. D., Kronenberg M., NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2, 971–978 (2001). [DOI] [PubMed] [Google Scholar]
- 38.White A. J., et al. , An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J. Immunol. 192, 2659–2666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui G., et al. , Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl. Acad. Sci. U.S.A. 111, 1915–1920 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNab F. W., et al. , The influence of CD1d in postselection NKT cell maturation and homeostasis. J. Immunol. 175, 3762–3768 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Wood G. W., Macrophages in the thymus. Surv. Immunol. Res. 4, 179–191 (1985). [DOI] [PubMed] [Google Scholar]
- 42.Soga H., et al. , Heterogeneity of mouse thymic macrophages: I. Immunohistochemical analysis. Arch. Histol. Cytol. 60, 53–63 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Esashi E., Sekiguchi T., Ito H., Koyasu S., Miyajima A., Cutting edge: A possible role for CD4+ thymic macrophages as professional scavengers of apoptotic thymocytes. J. Immunol. 171, 2773–2777 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Unanue E. R., Antigen-presenting function of the macrophage. Annu. Rev. Immunol. 2, 395–428 (1984). [DOI] [PubMed] [Google Scholar]
- 45.Remmerie A., Scott C. L., Macrophages and lipid metabolism. Cell. Immunol. 330, 27–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liew P. X., Lee W. Y., Kubes P., iNKT cells orchestrate a switch from inflammation to resolution of sterile liver injury. Immunity 47, 752–765.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Gaya M., et al. , Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 172, 517–533.e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao M., et al. , Altered thymic differentiation and modulation of arthritis by invariant NKT cells expressing mutant ZAP70. Nat. Commun. 9, 2627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clancy-Thompson E., et al. , Monoclonal invariant NKT (iNKT) cell mice reveal a role for both tissue of origin and the TCR in development of iNKT functional subsets. J. Immunol. 199, 159–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Georgiev H., Ravens I., Benarafa C., Förster R., Bernhardt G., Distinct gene expression patterns correlate with developmental and functional traits of iNKT subsets. Nat. Commun. 7, 13116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruz Tleugabulova M., et al. , Discrete TCR binding kinetics control invariant NKT cell selection and central priming. J. Immunol. 197, 3959–3969 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Schumann J., Mycko M. P., Dellabona P., Casorati G., MacDonald H. R., Cutting edge: Influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J. Immunol. 176, 2064–2068 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Joseph C., et al. , TCR density in early iNKT cell precursors regulates agonist selection and subset differentiation in mice. Eur. J. Immunol. 49, 894–910 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Lu Y., et al. , SLAM receptors foster iNKT cell development by reducing TCR signal strength after positive selection. Nat. Immunol. 20, 447–457 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.