Significance
Widespread human impacts on the environment are expected to harm human health, which may in turn alter our interactions with the environment. However, evidence for impacts of environmental changes on health, and for feedbacks between environmental change and health, remains locally specific and context dependent. Using a large, geospatial dataset encompassing the Brazilian Amazon rainforest across 13 y, we identify strong evidence for a feedback between deforestation and malaria: Deforestation significantly increases malaria transmission, while high malaria burden simultaneously reduces forest clearing. Our results put into broader context the contradictory effects of deforestation on malaria found in earlier studies and provide evidence useful to land use policy and public health interventions that provide win–win solutions for conservation and health.
Keywords: Brazil, Plasmodium falciparum, Plasmodium vivax, instrumental variables, environmental change
Abstract
Deforestation and land use change are among the most pressing anthropogenic environmental impacts. In Brazil, a resurgence of malaria in recent decades paralleled rapid deforestation and settlement in the Amazon basin, yet evidence of a deforestation-driven increase in malaria remains equivocal. We hypothesize an underlying cause of this ambiguity is that deforestation and malaria influence each other in bidirectional causal relationships—deforestation increases malaria through ecological mechanisms and malaria reduces deforestation through socioeconomic mechanisms—and that the strength of these relationships depends on the stage of land use transformation. We test these hypotheses with a large geospatial dataset encompassing 795 municipalities across 13 y (2003 to 2015) and show deforestation has a strong positive effect on malaria incidence. Our results suggest a 10% increase in deforestation leads to a 3.3% increase in malaria incidence (∼9,980 additional cases associated with 1,567 additional km2 lost in 2008, the study midpoint, Amazon-wide). The effect is larger in the interior and absent in outer Amazonian states where little forest remains. However, this strong effect is only detectable after controlling for a feedback of malaria burden on forest loss, whereby increased malaria burden significantly reduces forest clearing, possibly mediated by human behavior or economic development. We estimate a 1% increase in malaria incidence results in a 1.4% decrease in forest area cleared (∼219 fewer km2 cleared associated with 3,024 additional cases in 2008). This bidirectional socioecological feedback between deforestation and malaria, which attenuates as land use intensifies, illustrates the intimate ties between environmental change and human health.
Anthropogenic land use change is a major driver of global environmental change (1, 2), reducing biodiversity and carbon storage, changing microclimate, and affecting the burden and distribution of infectious disease (3, 4). Vector-borne diseases—pathogens transmitted by biting arthropods—are particularly sensitive to forest loss and fragmentation through changes in host and vector communities, vector breeding habitat, microclimate suitability for pathogen development, and vector–human contact rates, among others (5, 6). Changes in forest cover can thus have pervasive and multifactorial effects on the ecology of mosquito vectors and the pathogens they transmit, which determine the geographic distribution and burden of some of the most common and debilitating human infectious diseases globally, including malaria and dengue fever.
Following the late 1960s, malaria expanded rapidly in the Amazon basin, reaching over 600,000 cases a year at the turn of the 21st century—primarily Plasmodium vivax and Plasmodium falciparum transmitted by Anopheles (Nyssorhynchus) darlingi—paralleling large-scale deforestation and land use intensification following government-sponsored settlement (7–12). Prior to this, Brazilian malaria control efforts had dramatically reduced malaria incidence from 6 million cases annually in the 1940s to fewer than 50,000 cases in the early 1960s (8). Despite the clear expectation that land use change alters the ecology of Anopheles mosquito vectors and malaria parasites, particularly on settlement frontiers (13, 14), empirical evidence linking environmental change, mosquito vector ecology, and cases of human malaria remains surprisingly ambiguous and even contradictory. Entomological risk for malaria is thought to increase following initial settlement and forest clearing (i.e., in frontier settlements) due to a combination of increased biting rate and available breeding habitat for the primary vector (A. darlingi) (5, 6), increased adult mosquito survival in human-altered landscapes (15), and higher entomological inoculation rates in forest and riverine associated frontier settlements in the Amazon basin (16, 17). However, a direct link between deforestation and human incidence of malaria in the Amazon has not been clearly established (18–20): While forest disturbance and deforestation increased malaria incidence in some studies (10, 18, 21), forest conservation and high forest cover were associated with higher incidence in other studies (19). As a growing human population continues to expand into and clear primary forest across the globe, should we expect increased transmission of human malaria (14, 20, 22, 23)?
The ambiguous empirical evidence surrounding this question could stem from geographic heterogeneity in the relationship between deforestation and malaria and a feedback of malaria burden on rates of forest clearing, among other possible explanations. Using a 13-y (2003 to 2015) dataset of malaria incidence in 795 municipalities encompassing the Brazilian Amazon (Fig. 1) combined with econometric regression methods, we examine evidence for large-scale effects of deforestation on human malaria incidence. With this approach, we overcome 2 primary challenges inherent in making causal inference in observational studies: controlling for unobserved determinants of the outcome of interest and removing bidirectional feedbacks. Geographic, environmental, or population features might also influence disease transmission and obscure the relationship between deforestation and disease (4, 24–26). We address this potentially confounding variation by controlling for municipality-specific characteristics (e.g., size, geographical features) and year-specific shocks shared across the region (e.g., global economic conditions, national policy changes) using a least-squares dummy variable (LSDV) approach. Furthermore, we hypothesize that a bidirectional feedback between deforestation and malaria may occur, whereby deforestation promotes malaria via ecological mechanisms but local epidemics of malaria reduce forest clearing because poor health reduces economic activity (24, 27, 28) and the success of colonization projects in the Amazon (29–31). This is particularly true in the interior of the Amazon where the bulk of smallholder, frontier settlement occurs (32–34). Frontier colonization can fail as a result of high malaria burden because land clearing is conducted by the settlers themselves, which could limit working days devoted to land clearing, rather than hired labor as in the case of large landholders in old-frontier and postfrontier regions of the southern and northeastern Amazon (32–34). We test for statistical evidence of this feedback, then control for it using instrumental variable (IV) regression, which can tease apart unidirectional causal effects (4, 24). Finally, the environmental and sociopolitical landscapes underlying land use change and malaria transmission are heterogeneous, so the relationship between deforestation and malaria might vary over time and space (14). We examine the hypothesized relationships in different data subsets that represent different geographic regions and stages of land use change to address this concern. Using these methods, we address 2 questions: 1) Does deforestation drive malaria transmission in the Brazilian Amazon? and 2) does malaria burden feedback to influence rates of deforestation?
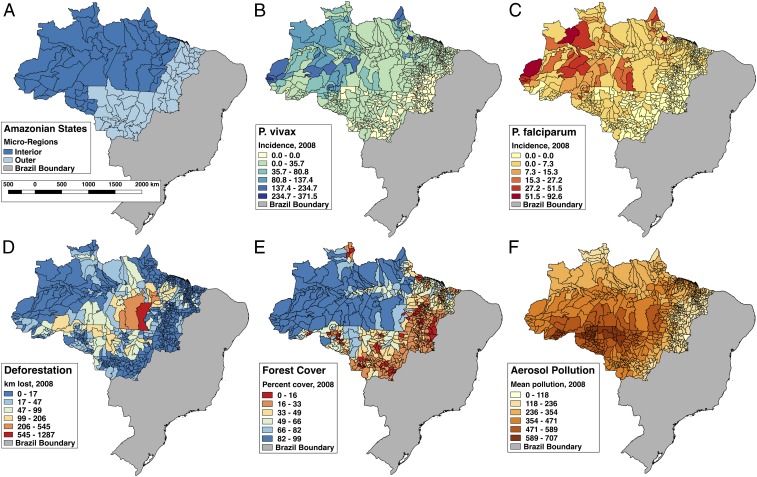
Fig. 1.
Malaria and forest cover are highest in the interior of the Brazilian Amazon, while active deforestation and aerosol pollution peak at the interface between the forest interior and outer Amazonian states. (A) The states encompassing the legal Amazon region with “microregion” boundaries, where the interior region is in dark blue and the outer Amazonian region is in light blue. (B) Plasmodium vivax malaria incidence (cases per 1,000 population). (C) P. falciparum malaria incidence (cases per 1,000 population). (D) Deforestation (square kilometers of forest lost). (E) Forest cover (percentage). (F) Mean September aerosol pollution. Data in B–F are mapped by municipality for the year 2008, the midpoint of the study. Municipality boundaries are from 2010.
Results
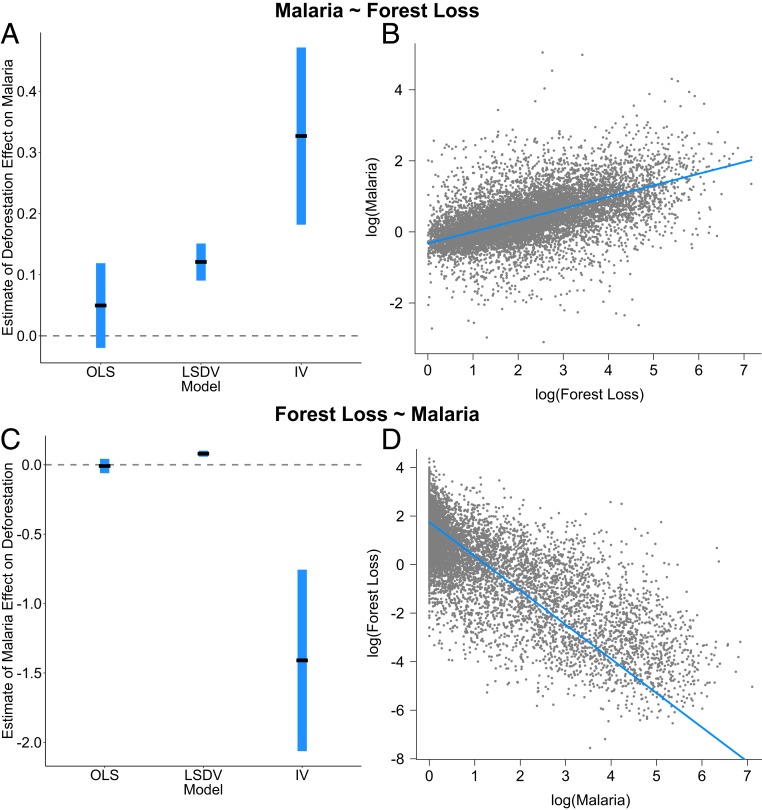
We found that deforestation, specifically measured as annual area of forest lost within a given municipality, significantly increased malaria incidence (β = 0.327, SE = 0.145, P = 0.024* [*indicates statistical significance at the P < 0.05 level]; Fig. 2 and SI Appendix, Table S1 and Fig. S1). The model that used aerosol pollution as an instrument for deforestation predicted that, on average, a 10% increase in deforestation (∼1,567 additional km2 cleared across the Amazon in 2008, the midpoint of the study, for example) would result in a 3.27% increase in malaria incidence (∼9,980 additional cases overall in 2008) (SI Appendix, Table S1). Model diagnostics illustrate that the estimated effects of deforestation on malaria would have been biased and poorly detected without the IV approach (i.e., in the ordinary least squares [OLS] and LSDV models), but that the effect was otherwise robust to multiple model specifications (SI Appendix, Tables S1–S11 and Figs. S1–S7). Additionally, parsing the Amazonian states into “interior,” defined as prefrontier or active frontier, and “outer,” defined as postfrontier (35), we find that deforestation significantly increases malaria incidence in the interior where forest cover remains high, which is particularly evident for P. falciparum (β = 0.716, SE = 0.323, P = 0.027* [*indicates statistical significance at the P < 0.05 level]) (SI Appendix, Table S12). In contrast, we find that the slope of the relationship is not significantly different from zero in the outer Amazonian states where most forest has already been cleared (β = 0.020, SE = 0.014, P = 0.136). These results confirm the hypothesized heterogeneity in the relationship between regions at different stages along the gradient of land use intensification (SI Appendix, Tables S12 and S13). Malaria incidence also increased with precipitation and optimal temperature for transmission in the dry season, when most cases of malaria are transmitted, and decreased with population density and per capita gross domestic product (GDP) (SI Appendix, Table S1). P. falciparum malaria had a stronger relationship with deforestation, while P. vivax malaria exhibited attenuated relationships with deforestation and other predictors, likely because this parasite can relapse months to years after initial infection, decoupling transmission from the onset of disease.
Fig. 2.
Forest loss increases malaria, while malaria decreases forest loss. Coefficient estimates and partial residual plots illustrating the effect of deforestation (total municipality forest loss) on total malaria incidence (A and B), and of total malaria incidence on deforestation (C and D). Coefficient estimates are plotted for the ordinary least squares (OLS), least-squares dummy variable (LSDV), and instrumental variable (IV) models (A and C). Model diagnostics indicate that the IV model is most appropriate for both analyses. The IV estimator produces consistent estimates but is less efficient than the OLS and LSDV estimators, which leads to larger SEs in IV estimation than in OLS or LSDV (1 SD is plotted in blue around the point estimate in black). Partial residual plots illustrate the estimated effects of deforestation on total malaria (B) and total malaria on deforestation (D) from the IV models, while controlling for other included independent variables.
We found strong evidence for a bidirectional feedback between deforestation and malaria: While deforestation increased malaria incidence, high malaria incidence in turn decreased the area of forest lost annually (β = −1.410, SE = 0.654, P = 0.031* [*indicates statistical significance at the P < 0.05 level]; Fig. 2 and SI Appendix, Tables S14–S17 and Fig. S8). Detecting this negative feedback of malaria on deforestation required controlling for the simultaneous positive effect of deforestation on malaria using optimal temperature for transmission, a strong instrument for malaria (SI Appendix, Table S15). Without controlling for the bidirectional feedback, the estimated effects of malaria on deforestation were biased and counterintuitive. The negative effect of malaria on deforestation was robust to controlling for omitted variables (via dummy variables) that might also affect deforestation and spatial and temporal autocorrelation of the errors (SI Appendix, Tables S14–S17), across a variety of model specifications (SI Appendix, Tables S18–S25 and Figs. S6, S8, and S9). A 1% increase in malaria incidence (∼3,024 additional cases in 2008, for example) is expected to reduce deforestation by ∼1.41% (∼219 fewer km2 lost in 2008). As expected, the negative effect of malaria incidence on forest clearing is consistent in the interior region (SI Appendix, Tables S26 and S27) where malaria and remaining forest cover are both high (Fig. 1), but disappeared in the outer Amazonian states where most forest has been cleared and malaria incidence is comparatively low (SI Appendix, Tables S27 and S28).
Discussion
The largest remaining tropical rainforest, the Amazon of South America, is under substantial pressure from mining, timber harvest, livestock and agricultural production, forest fires, urbanization, and infrastructure development. Brazil’s government-sponsored settlement of the Amazon region and development of the trans-Amazonian highway has led to substantial deforestation (7, 36) and a simultaneous resurgence in malaria (12), partially reversing the prior success of malaria control programs (8). Despite the recent focus on emerging mosquito-transmitted viruses like Zika and chikungunya, malaria is resurging in a number of regions of South America more broadly, particularly in regions undergoing rapid land conversion and political and economic turmoil (11, 37, 38). Our work provides clear large-scale evidence that deforestation increases malaria, by using econometric techniques that approximate the gold standard of randomized controlled trials with observational data where controlled experiments are impossible. The effects of deforestation on malaria are largest in the early stages of deforestation in the interior of the Amazon as forest edge habitat increases, promoting mosquito vector breeding habitat, survival, and human biting rate (5, 6, 15), but the effects attenuate as forest loss progresses, forest edge area declines, and human settlements become larger and further removed from forest (10, 21). The key implication is that forest clearing has a direct impact on human health, in addition to the loss of other ecosystem services such as species diversity, water quality, and carbon storage, that is quantifiable and predictable at regional and decadal scales.
At the same time, our work provides evidence for a socioecological feedback in which high malaria incidence simultaneously reduces forest clearing. Malaria clearly and consistently reduces economic activity (24, 27, 28, 39, 40) and influences human settlement, including in the Brazilian Amazon (29–31). These effects on economic activity, migration, and settlement could in turn influence rates of land clearing, potentially resulting in the negative feedback of malaria incidence on deforestation that we observed. Our instrument for malaria, optimal transmission temperature, is plausibly exogenous to the larger economic and policy context that determines deforestation. Thus, while numerous other economic, political, social, and environmental factors play a much more substantial role in determining where and how much forest is cleared, here we find evidence that elevated malaria burden may also reduce rates of deforestation.
Our results suggest that deforestation has a significant positive effect on malaria transmission and malaria has a significant negative effect on land clearing, but do not directly identify the underlying mechanisms. Several classes of mechanisms could explain our results. Following frontier settlement and characteristic dynamics of frontier malaria (13), in the early stages of settlement, forest edge habitat promotes malaria transmission by both increasing entomological risk and increasing human–vector contact as a result of economic and human behavioral patterns associated with subsistence agriculture and resource extraction. Over time as a region is settled, forest edge habitat declines, malaria exposure leads to temporary immunity, and housing quality and healthcare access improve (13). In addition, the trajectory of land use change shifts from forest clearing to consolidation and intensification, changing both malaria incidence and deforestation rates (14). Concurrently, changes in political and regulatory practices at corporate, municipal, state, and national levels alter pressures on deforestation and land use change (13). These simultaneous social and ecological forces are characteristic of frontier malaria dynamics and associated sociodemographic changes, and act across different spatial and temporal scales that our approach does not disentangle. Thus, local mechanistic studies are critical for determining the mechanisms, direct and indirect, by which malaria may reduce deforestation.
While one of the strengths of this study is the spatial and temporal scale of analysis, using data from across the entire Brazilian Amazon and spanning over a decade, there is a trade-off with the resolution of analysis. For example, we use aggregate data at the scale of municipalities at an annual time step, which may mask local-scale and intraannual heterogeneity in environmental conditions and malaria transmission. Our statistical approach controls for many possible confounding factors unique to municipalities, regions, and years, and is robust to a variety of different specifications and data subsets. However, as with all observational data studies, we cannot say with certainty that we capture all possible confounding factors that would otherwise preclude a causal interpretation of the results. Thus, local mechanistic studies, which have finer resolution (e.g., settlement-level case reporting data or household-level surveys) that allows them to identify the relative strength of drivers of malaria transmission and land use change locally, and how they differ across regions of the Brazilian Amazon, are necessary complements to the results of this study. Increasing understanding of the underlying mechanistic drivers, as well as local heterogeneity in the strength and significance of the relationships between malaria and deforestation, should be the focus of further scientific inquiry; however, the results of this work may have direct policy relevance. Reducing deforestation in the interior of the Amazon rainforest, particularly in prefrontier regions (35), may have dual benefits for conservation and health by maintaining ecosystem services provided by intact forests and reducing transmission of human malaria. However, the impact of alternative livelihoods to deforestation and subsequent agricultural activities on conservation and health should also be considered in such a policy context.
As land conversion for human use continues at a rapid pace to meet the demands of a growing human population for food, fiber, and resources, unintended consequences for ecosystem function, biodiversity, climate, and health are frequently emerging (2). One such consequence may be the emergence, resurgence, and transmission of vector-borne disease (41). Here, we illustrate that deforestation exacerbates malaria transmission in the Brazilian Amazon, particularly in the highly forested regions in the early stages of land conversion. These predictable and quantitative effects could inform forest conservation, land use policy, and public health decisions in Amazonia. Moreover, we identify evidence for a potential negative feedback of malaria burden on deforestation, indicating that deforestation is lower than otherwise expected when malaria burden is high. Importantly, ignoring this statistically well-supported feedback leads to substantial underestimates of the true effect of deforestation on malaria transmission, which may have confounded the results of earlier studies. Teasing apart bidirectional socioecological feedbacks—of deforestation on malaria, and of malaria on deforestation—is important for developing policy to assess trade-offs between forest conservation, human health, and economic development in the Amazon rainforest and globally (35, 42).
Methods
Approach.
Randomized, controlled experiments are widely considered the gold standard for identifying causal relationships. However, ecological processes are often so complex, geographically extensive, long-term, and ethically sensitive that controlled experiments are not possible, which creates several statistical challenges (43). First, if variables that are correlated with the predictor variables and outcome are unobserved or omitted from the analysis, the estimated coefficients on the included predictor variables will be unreliable, due to the induced correlation between the model errors and the predictor variables (i.e., “omitted variable bias”). Similarly, the magnitude and direction of the relationship between the predictor and response variables will be incorrectly estimated if the response and predictor variable of interest influence each other in a bidirectional causal loop (i.e., “simultaneity bias”).
To perform more rigorous causal inference from observational data, we can use regression-based tools from econometrics. As we detail below, we first leverage the longitudinal nature of our data to remove time-invariant heterogeneity unique to municipality (e.g., elevation, latitude, topography) and/or shared by all municipalities in the same year (e.g., soy or timber prices, the global economic recession) using a LSDV approach. Here, the model is identified from deviations in the municipality mean of malaria, after accounting for year-specific shocks shared by all regions. This approach minimizes the potential for omitted variable bias stemming from unobserved time-invariant (or year-specific) characteristics. However, because the Brazilian Amazon is a large region, natural shocks like El Niño events may not have the same impact across the study area. Thus, we include additional covariates (e.g., temperature and precipitation, described below) to account for this regional and temporal heterogeneity. Second, IV regression, a structural equation method (44), can be used to account for simultaneous causality (4, 24, 45). In IV regression, an IV (or “instrument”) is selected that is correlated with the predictor variable of interest, but not independently correlated with the response variable (45). The instrument then provides exogenous variation—variation that is uncorrelated with the model error—in the predictor variable of interest, allowing for consistent estimation of the true effect of the predictor of interest on the outcome variable (SI Appendix, Supplementary Text).
Here, we use these econometric tools to address 2 specific questions: 1) Does deforestation increase malaria incidence in the Brazilian Amazon? 2) If so, is there also evidence that elevated malaria burden reduces forest clearing? It is important to note that, in contrast to many studies in biostatistics, our goal is not to build the model that explains as much variation in the data as possible, but instead to answer these questions as directly, rigorously, and robustly as possible. Therefore, we focus on deforestation as the predictor and malaria incidence as the response to answer the first question and vice versa to answer the second, while controlling for other (potentially biologically interesting) determinants of malaria and deforestation either by including them directly (total forest cover, human population density, per capita GDP, temperature, and precipitation) or by using dummy variables (municipality, year), and using IV estimation to control for potential bidirectional causation between deforestation and malaria. We test different model specifications that include different assumptions and/or data subsets to test the robustness of our results (Estimation Approach).
Data.
Malaria case reports by municipality for the years 2003 to 2015 are available from the System of Epidemiologic Surveillance of Malaria (SIVEP-Malaria) monitoring system in Brazil for all municipalities comprising the 9 states in the Amazon region (46) (http://200.214.130.44/sivep_malaria/). P. vivax and P. falciparum malaria incidence are reported by month and municipality. We calculated annual parasite incidence for each parasite species by summing annual cases, dividing by annual municipality population, and multiplying by 1,000 [(no. cases/population) × 1,000]. We use annual malaria incidence to match the temporal scale of the available deforestation data, as well as the hypothesized timescale over which deforestation is expected to influence malaria.
Annual forest loss and total forest cover are available from the Hansen Global Forest Change (GFC) dataset (version 1.4, 2000 to 2016; accessible through the Google Earth Engine data repository) (47) and were extracted by municipality in Google Earth Engine (GEE) to produce municipality by year measures of total area of forest and total area of forest loss (48). This global data product is available at a 30-m pixel resolution and measures forest loss annually as a change from a forest to nonforest state from 1 y to the next, pixel by pixel. We chose this dataset over the deforestation monitoring program (PRODES) from the Brazilian National Institute for Space Research (INPE) (19, 21, 22), as well as monthly deforestation data products (e.g., INPE DETER and IMAZON SAD datasets), for the following reasons: The Brazilian government began using PRODES as a policing tool in 2008, incentivizing covert deforestation that goes undetected and potentially reducing reliability of its deforestation estimates (49); the monthly datasets for the Amazon from INPE and IMAZON, which are used for monitoring purposes, only detect relatively large-scale deforestation (INPE DETER data reports losses greater than 6.25 ha; IMAZON SAD data reports losses greater than 10 ha during the study period) in contrast with the GFC dataset (0.09-ha resolution). This higher-resolution data better detects small-scale clearing, which may be particularly important to the dynamics of frontier malaria (13) and the deforestation–malaria relationship more generally. However, we repeated published analyses (21) using the PRODES data to ensure our dataset is consistent with recent studies of the effect of deforestation on malaria (SI Appendix, Table S29 and Fig. S10) and that our estimated effect of deforestation on malaria is similar across forest datasets (SI Appendix, Table S30). Additionally, because forest cover and malaria incidence are heterogeneous within municipalities, we evaluated models that specifically quantified forest loss in the vicinity of human settlements using annual forest loss and forest cover from regions of the Brazilian Amazon where population density was at least 1) 1 person/km2 and 2) 5.8 persons/km2 (median municipality population density over the study period) based on United Nations-adjusted gridded population data for 2010 (50) (https://www.worldpop.org/). In addition, in order to more directly control for changes in human population density over time, we obtained annual estimates of municipality population from the Brazilian Institute of Geography and Statistics (IBGE) (https://www.ibge.gov.br/) and divided them by municipality area to calculate average annual population density at the municipality level from 2000 to 2015. We also obtained annual estimates of GDP by municipality from IBGE and calculated annual per capita GDP by municipality using the IBGE population estimates to control for the effects of poverty and economic development.
In addition to characteristics of land cover and human population, malaria transmission is also driven by abiotic and environmental characteristics like temperature and precipitation, which influence vector and pathogen vital rates (5, 6, 15–17, 51). While optimal transmission temperature has not been well resolved for A. darlingi and Plasmodium in the Neotropics, temperature has been illustrated to have a nonlinear, unimodal, relationship with malaria transmission potential (52) and human incidence (53) in Africa with transmission peaking at 25 °C and declining below 22 °C and above 28 °C (52). More generally, mosquito-borne disease transmission responds unimodally to temperature across a suite of 15 medically relevant mosquito species and 11 pathogen species, with optimal temperatures ranging from 23 to 29 °C (54). Although the South American malaria vector Anopheles (Nyssorhynchus) darlingi may differ in its thermal responses for life history and transmission parameters (51), we expect its response to vary by no more than a few degrees from that of the African malaria vector (52) and to be constrained within the range of temperate and tropical vectors studied by Mordecai et al. (54). Therefore, rather than include simple linear measures of average temperature as in earlier studies, we instead calculated optimal temperature for malaria transmission, based on the predictions of the temperature-dependent mechanistic model for the basic reproduction number, R0, for malaria (52), from MODIS satellite imagery in GEE (accessible through the GEE data repository). Specifically, we calculated wet season and dry season temperature suitability by extracting average municipality daytime temperature for each satellite observation (approximately weekly) in each year and calculating the number of observations of optimal temperature [24 to 26 °C (52)] falling in the wet season (January to June) and dry season (July to December), respectively. Since the temperature dependence of Plasmodium transmitted by A. darlingi has not been well resolved in the literature (but see ref. 51), we also considered colder (23 to 25 °C), hotter (26 to 29 °C), and wider (23 to 29 °C) optimal temperature windows in models as robustness checks.
Beyond temperature, precipitation determines available breeding habitat for mosquitoes both by feeding pools and other sources of standing water and, under heavy precipitation, by potentially scouring out and killing larval mosquitoes (55). Thus, we also calculated average precipitation by municipality (approximately weekly) from the Climate Hazards Group Infrared Precipitation with Station Data (56) (accessible through the GEE data repository) in GEE and summed observations of average precipitation in the wet and dry seasons for each municipality and year, producing measures of cumulative precipitation by municipality and year during the wet and dry season, to control for effects of precipitation on malaria that might vary by season.
Finally, we used a measure of aerosol pollution as an instrument for deforestation. We extracted average aerosol optical depth over land by municipality and year in GEE from the MODIS Aqua Monthly Global Product imagery (accessible through the GEE data repository). Aerosol optical depth is a measure of the degree to which light is blocked or scattered by particles in the vertical column of atmosphere over the observation location. Specifically, we extracted aerosol pollution data each year from the middle of the dry season (month of September) (57, 58) when much of the deforestation activity in the Amazon occurs (59), and when recently cleared forest is burned to make way for agricultural activities (59). However, since the timing of the dry season varies from region to region and year to year (60), we also extracted aerosol pollution data for the peak dry season (months of August to October) and sum of annual aerosol pollution as model robustness checks. All data are publicly available and sources are described above. Data extraction and processing was conducted in GEE (48) and R (61).
Estimation Approach.
The first objective of this analysis is to estimate the effect of deforestation on malaria incidence in the Brazilian Amazon. We incorporate data into our models on key variables (described above) to control for their effects on malaria incidence. In addition, to control for unobserved characteristics of municipalities that may also drive malaria, we use municipality dummy variables that control for roughly time-invariant characteristics of municipalities (e.g., perennial water bodies, protected areas, elevation) for which we do not include or have data. We also incorporate year dummy variables to control for year shocks shared by the Amazon basin (e.g., the global recession, changes in international commodity prices for beef or soy, changes in health or forest conservation policy) that may directly or indirectly influence malaria transmission from year to year across the study region.
In addition, it is possible that deforestation and malaria may be simultaneously determined. Earlier studies identify forest disturbance and deforestation as factors that increase malaria incidence (10, 18, 21) and risk (5, 6) in the Amazon. However, there is also evidence that malaria can prevent expansion of new colonization projects in highly endemic regions of the Amazon (29–31), because it impedes consolidation of settlements, reduces economic activity, and exacerbates the challenges of pioneer settlement (29–31). While policies promoting settlement of the Amazon basin in Brazil at the end of the 20th century (32) drove substantial migration to this region despite soaring rates of malaria (8), malaria burden nevertheless may have reduced the level of migration and deforestation from what would have otherwise occurred. Such a bidirectional relationship inhibits statistical understanding of either the effects of deforestation on malaria, or malaria on deforestation. To first identify the effects of deforestation on malaria, we instrument for deforestation using aerosol pollution levels in the dry season, which are highly correlated with rates of deforestation (the strength of the instrument is tested directly in the first-stage regression; see diagnostics in SI Appendix, Tables S2, S10, and S13). There is unlikely to be a relationship between aerosol pollution levels in September and annual malaria transmission, except through deforestation—i.e., aerosol pollution in September should not affect annual malaria incidence when forest loss is held constant. Therefore, the instrument does not suffer from the same problem—correlation with the error—as the original endogenous explanatory variable.
Second, to investigate the reverse relationship, whether malaria burden has a negative effect on deforestation, which could indicate a bidirectional feedback between deforestation and malaria, we used an IV regression with optimal temperature for transmission in the dry season, which is highly correlated with annual malaria incidence, as an instrument for malaria (see diagnostics in SI Appendix, Tables S15, S22–S24, and S27), but not otherwise related to deforestation. Balanced panel datasets were constructed so that only municipalities with complete data in all years were included in the analysis, with a total of 795 municipalities in the final dataset (SI Appendix, Fig. S11).
Econometric Methods.
We estimate a series of regression models in which we are predicting malaria incidence in municipality i and year t. Malaria incidence is natural log transformed and denoted . We model as a function of the natural log-transformed forest loss in municipality i and year t, , as well as the vector of explanatory control variables listed above (i.e., total forest cover, optimal temperature for transmission in the wet and dry seasons, precipitation in the wet and dry seasons, population density, and per capita GDP). Following earlier studies (21), we also fit models that include linear and squared effects of deforestation to investigate whether there is a nonlinear relationship between deforestation and malaria; however, the quadratic term was not significant and did not substantially influence model results, so this specification was dropped. Control variables are included to reduce bias from omitted variables, although these variables can exhibit high collinearity, which may produce unreliable coefficient estimates. To check the robustness of our core results, we also specify models that limit collinearity in the set of predictor variables by dropping variables with variance inflation factors (VIFs) that exceed 10; however, all control variables were retained in this first model (VIF < 10). The OLS model, which does not include dummy variables or IVs, takes the following form:
| [1] |
where is the model intercept, is the coefficient of interest (the effect of forest loss on malaria incidence), is a vector of control variables, and is the error term. To control for additional omitted or unobserved variables that may bias our regression coefficients, we add year and municipality dummy variables to our base model to construct the LSDV model:
| [2] |
where the intercept now takes a different value for each municipality i (, representing the municipality dummy variable) and year t (, representing the year dummy variable). As an additional robustness check, we used a stepwise process of removing highly collinear predictor variables to explore the consistency of our core results to alternative model specifications.
Finally, to control for possible feedbacks between malaria and deforestation, we specify a 2-stage least-squares IV model where we instrument for deforestation using aerosol pollution. This instrument is relevant because it is highly correlated with deforestation (see first-stage diagnostics in SI Appendix), and unlikely to independently drive malaria transmission. The first-stage model is as follows:
| [3] |
where is the intercept, is the coefficient of interest on aerosol pollution, is the vector of control variables, and is the error term, with municipality and year dummy variables and , respectively. First-stage estimates of deforestation, , are then used to obtain consistent estimates of , our coefficient of interest, in the second-stage regression:
| [4] |
We use cluster-robust SEs (CRSEs), which are robust to arbitrary within-cluster correlation in the errors as well as heteroscedasticity (62). We use microregion clusters (Fig. 1A) in the OLS (Eq. 1), LSDV (Eq. 2), and IV (Eq. 4) models to account for spatial and temporal autocorrelation between municipalities within microregions, which were defined by “physiographic zones” that are similar in terms of social characteristics and natural environment, and represent natural divisions between tributaries of the Amazon River (IBGE, https://www.ibge.gov.br/). CRSEs allow for within-cluster, here within microregion, correlation of observations. The advantages of CRSEs compared to structural approaches is that CRSEs are nonparametric and account for arbitrary forms of autocorrelation between observations within a cluster. We used CRSEs rather than structural approaches to adjusting errors for spatial autocorrelation because the consequence of incorrectly specifying the clusters is less severe (63). In particular, the parameter estimates are insensitive to the cluster specification with CRSEs, and thus remain consistent and unbiased even if the clusters are incorrectly specified (63).
To check the robustness of the relationship between deforestation and malaria, and to identify potential heterogeneity in this relationship, we specified a series of models that included different sets of predictor and response variables and data subsets. We specified models with 1) total malaria, 2) P. vivax malaria, and 3) P. falciparum malaria as the response variables, with data from the full Amazon dataset, and present results of the full model predicting total malaria incidence in the main text. We also specified models with data subsets including 1) the interior states (Acre, Amapá, Amazonas, Pará, Rondônia, and Roraima; Fig. 1F) and 2) the outer Amazonian states (Maranhão, Mato Grosso, and Tocantins; Fig. 1F). Each of these models was run with 1) total forest loss by municipality by year, and 2) forest loss extracted from within inhabited regions of each municipality (at least 1 person/km2, and at least 5.8 people/km2). We specify different models for P. falciparum and P. vivax, as well as total malaria, because although it is the more common malaria parasite in the region, relapse of P. vivax malaria could obscure the effect of deforestation on malaria transmission by causing symptoms months to years following initial infection (64), muddling the link between actual transmission and deforestation. Thus, models of P. falciparum malaria may produce more reliable estimates. Because A. darlingi is the primary vector for both parasite species in this region, we expect their transmission ecology to be similar (but see ref. 15). We ran models on different data subsets, as well as with total deforestation and deforestation in proximity to human settlements, to check the robustness of our results, and to investigate whether effects may differ between the interior and outer Amazonian states. We separate interior and outer Amazonian states because we expect that, in the interior of the Amazon, where the bulk of smallholder, frontier settlement is occurring (32–34), deforestation is likely to have a strong effect on malaria transmission, following patterns of frontier malaria (13). At the same time, high malaria burden in the interior may reduce forest clearing as poor health of frontier settlers has been linked to failure of colonization efforts (29–31). Frontier colonization efforts can fail as a result of high malaria burden as land clearing is conducted by the settlers themselves rather than hired labor, as in the case of agricultural consolidation in the outer Amazonian region where forests have already been extensively cleared primarily by large landholders and land use has intensified for cattle operations and large-scale agricultural production (32–34).
Finally, following evidence for endogeneity between deforestation and malaria obtained above, which suggests a possible effect of malaria on deforestation, we specified a series of models in the opposite direction to directly investigate this relationship. In addition to an OLS model and LSDV model with municipality and year dummy variables, as described above, we specify an IV model where we use our metric for temperature suitability for transmission in the dry season, , as an instrument for malaria incidence in municipality i and time t to investigate the possible effect of malaria burden on deforestation in municipality i and time t. The first-stage IV model is as follows:
| [5] |
where is the intercept, is the coefficient of interest on optimal temperature for transmission, is the error term, with municipality and year dummy variables and , respectively, and is a vector of explanatory control variables, including total forest cover (standing forest available to clear), per capita GDP, population density, and precipitation in the wet and dry season, which could limit access and complicate logging operations through resulting flooding in the wet season.
We then obtain a theoretically consistent estimate of , our coefficient of interest, in the second-stage regression using predicted values of malaria, , from the first stage:
| [6] |
Different models were specified with forest loss in 1) the full Amazon, 2) the interior states, and 3) the outer Amazonian states as the response variables and total malaria as the predictor variable of interest (we also present model results for P. vivax and P. falciparum in the SI Appendix, as well as results of models using the same forest data subsets as above: 1) total forest loss by municipality by year, and 2) forest loss extracted from within inhabited regions of each municipality). All models were specified and run in R (version 3.4.2) (61) using the AER package (65).
Supplementary Material
Acknowledgments
We acknowledge Ashley Larsen, David Relman, Mercedes Pascual, Mauricio Santos-Vega, Eric Lambin, Ryan Abman, Teevrat Garg, and Andrew Plantinga for insights that helped to shape this study and formulation of the manuscript, as well as helpful comments and insights from 2 anonymous reviewers. A.J.M. was supported by a National Science Foundation Postdoctoral Research Fellowship in Biology (1611767) and a Stanford University Center for International Security and Cooperation Science Postdoctoral Fellowship. E.A.M. was supported by National Science Foundation Ecology and Evolution of Infectious Diseases Grant DEB-1518681, the Stanford Woods Institute for the Environment–Environmental Ventures Program, and the Hellman Faculty Scholarship.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905315116/-/DCSupplemental.
References
- 1.Song X.-P., et al. , Global land change from 1982 to 2016. Nature 560, 639–643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley J. A., et al. , Global consequences of land use. Science 309, 570–574 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Aragão L. E. O. C., et al. , Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1779–1785 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald A. J., Larsen A. E., Plantinga A. J., Missing the people for the trees: Identifying coupled natural-human system feedbacks driving the ecology of Lyme disease. J. Appl. Ecol. 56, 354–364 (2019). [Google Scholar]
- 5.Vittor A. Y., et al. , Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am. J. Trop. Med. Hyg. 81, 5–12 (2009). [PMC free article] [PubMed] [Google Scholar]
- 6.Vittor A. Y., et al. , The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 74, 3–11 (2006). [PubMed] [Google Scholar]
- 7.Fearnside P. M., Deforestation in Brazilian Amazonia: History, rates, and consequences. Conserv. Biol. 19, 680–688 (2005). [Google Scholar]
- 8.Oliveira-Ferreira J., et al. , Malaria in Brazil: An overview. Malar. J. 9, 115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefani A., et al. , Land cover, land use and malaria in the Amazon: A systematic literature review of studies using remotely sensed data. Malar. J. 12, 192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaves L. S. M., Conn J. E., López R. V. M., Sallum M. A. M., Abundance of impacted forest patches less than 5 km2 is a key driver of the incidence of malaria in Amazonian Brazil. Sci. Rep. 8, 7077 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallum M. A. M., et al. , Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian region of Brazil. Malar. J. 18, 117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffing S. M., Tauil P. L., Udhayakumar V., Silva-Flannery L., A historical perspective on malaria control in Brazil. Mem. Inst. Oswaldo Cruz 110, 701–718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Castro M. C., Monte-Mór R. L., Sawyer D. O., Singer B. H., Malaria risk on the Amazon frontier. Proc. Natl. Acad. Sci. U.S.A. 103, 2452–2457 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeza A., Santos-Vega M., Dobson A. P., Pascual M., The rise and fall of malaria under land-use change in frontier regions. Nat. Ecol. Evol. 1, 108 (2017). [DOI] [PubMed] [Google Scholar]
- 15.de Barros F. S. M., Honório N. A., Arruda M. E., Survivorship of Anopheles darlingi (Diptera: Culicidae) in relation with malaria incidence in the Brazilian Amazon. PLoS One 6, e22388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker B. S., et al. , Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar. J. 12, 178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lainhart W., et al. , Evidence for temporal population replacement and the signature of ecological adaptation in a major Neotropical malaria vector in Amazonian Peru. Malar. J. 14, 375 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn M. B., Gangnon R. E., Barcellos C., Asner G. P., Patz J. A., Influence of deforestation, logging, and fire on malaria in the Brazilian Amazon. PLoS One 9, e85725 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valle D., Clark J., Conservation efforts may increase malaria burden in the Brazilian Amazon. PLoS One 8, e57519 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker Lima J. M., Vittor A., Rifai S., Valle D., Does deforestation promote or inhibit malaria transmission in the Amazon? A systematic literature review and critical appraisal of current evidence. Philos. Trans. R Soc. Lond. B Biol. Sci. 372, 20160125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos A. S., Almeida A. N., The impact of deforestation on malaria infections in the Brazilian Amazon. Ecol. Econ. 154, 247–256 (2018). [Google Scholar]
- 22.Hahn M. B., et al. , Conservation efforts and malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 90, 591–594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valle D., Response to the critique by Hahn and others entitled “Conservation and malaria in the Brazilian Amazon”. Am. J. Trop. Med. Hyg. 90, 595–596 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonds M. H., Dobson A. P., Keenan D. C., Disease ecology, biodiversity, and the latitudinal gradient in income. PLoS Biol. 10, e1001456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emerson K. J., Conn J. E., Bergo E. S., Randel M. A., Sallum M. A. M., Brazilian Anopheles darlingi root (Diptera: Culicidae) clusters by major biogeographical region. PLoS One 10, e0130773 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley D. H., et al. , Geographic distribution, evolution, and disease importance of species within the Neotropical Anopheles albitarsis Group (Diptera, Culicidae). J. Vector Ecol. 39, 168–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs J., Malaney P., The economic and social burden of malaria. Nature 415, 680–685 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Gallup J. L., Sachs J. D., The economic burden of malaria. Am. J. Trop. Med. Hyg. 64 (suppl. 1–2), 85–96 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Sawyer D., Economic and social consequences of malaria in new colonization projects in Brazil. Soc. Sci. Med. 37, 1131–1136 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Sawyer D. R., Malaria on the Amazon frontier: Economic and social aspects of transmission and control. Southeast Asian J. Trop. Med. Public Health 17, 342–345 (1986). [PubMed] [Google Scholar]
- 31.Singer B. H., de Castro M. C., Agricultural colonization and malaria on the Amazon frontier. Ann. N. Y. Acad. Sci. 954, 184–222 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Tritsch I., Le Tourneau F.-M., Population densities and deforestation in the Brazilian Amazon: New insights on the current human settlement patterns. Appl. Geogr. 76, 163–172 (2016). [Google Scholar]
- 33.Pacheco P., Actor and frontier types in the Brazilian Amazon: Assessing interactions and outcomes associated with frontier expansion. Geoforum 43, 864–874 (2012). [Google Scholar]
- 34.Schielein J., Börner J., Recent transformations of land-use and land-cover dynamics across different deforestation frontiers in the Brazilian Amazon. Land Use Policy 76, 81–94 (2018). [Google Scholar]
- 35.Rodrigues A. S. L., et al. , Boom-and-bust development patterns across the Amazon deforestation frontier. Science 324, 1435–1437 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Barona E., Ramankutty N., Hyman G., Coomes O. T., The role of pasture and soybean in deforestation of the Brazilian Amazon. Environ. Res. Lett. 5, 024002 (2010). [Google Scholar]
- 37.Sanchez J. F., et al. , Unstable malaria transmission in the Southern Peruvian Amazon and its association with gold mining, Madre de Dios, 2001–2012. Am. J. Trop. Med. Hyg. 96, 304–311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grillet M. E., et al. , Venezuela’s humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect. Dis. 19, e149–e161 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Bonds M. H., Keenan D. C., Rohani P., Sachs J. D., Poverty trap formed by the ecology of infectious diseases. Proc. Biol. Sci. 277, 1185–1192 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acemoglu D., Johnson S., Robinson J., The colonial origins of comparative development: An empirical investigation. Am. Econ. Rev. 91, 1369–1401 (2001). [Google Scholar]
- 41.Lambin E. F., Tran A., Vanwambeke S. O., Linard C., Soti V., Pathogenic landscapes: Interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr. 9, 54 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robalino J. A., Land conservation policies and income distribution: Who bears the burden of our environmental efforts? Environ. Dev. Econ. 12, 521–533 (2007). [Google Scholar]
- 43.Larsen A. E., Meng K., Kendall B. E., Causal analysis in control–Impact ecological studies with observational data. Methods Ecol. Evol. 10, 924–934 (2019). [Google Scholar]
- 44.Grace J. B., Structural Equation Modeling and Natural Systems (Cambridge University Press, 2006). [Google Scholar]
- 45.Wooldridge J. M., Econometric Analysis of Cross Section and Panel Data (MIT Press, Cambridge, MA, ed. 1, 2002). [Google Scholar]
- 46.Tucker Lima J. M., et al. , A social-ecological database to advance research on infrastructure development impacts in the Brazilian Amazon. Sci. Data 3, 160071–160079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen M. C., et al. , High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Gorelick N., et al. , Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27 (2017). [Google Scholar]
- 49.Richards P., Arima E., VanWey L., Cohn A., Bhattarai N., Are Brazil’s deforesters avoiding detection? Conserv. Lett. 10, 470–476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorichetta A., et al. , High-resolution gridded population datasets for Latin America and the Caribbean in 2010, 2015, and 2020. Sci. Data 2, 150045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu V. M., et al. , Regional variation in life history traits and plastic responses to temperature of the major malaria vector Nyssorhynchus darlingi in Brazil. Sci. Rep. 9, 5356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mordecai E. A., et al. , Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 16, 22–30 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Shah M. M., et al. , Malaria smear positivity among Kenyan children peaks at intermediate temperatures as predicted by ecological models. Parasit. Vectors 12, 288 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mordecai E. A., et al. , Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690–1708 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tompkins A. M., Ermert V., A regional-scale, high resolution dynamical malaria model that accounts for population density, climate and surface hydrology. Malar. J. 12, 65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Funk C., et al. , The climate hazards infrared precipitation with stations—a new environmental record for monitoring extremes. Sci. Data 2, 150066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costa M. H., Pires G. F., Effects of Amazon and Central Brazil deforestation scenarios on the duration of the dry season in the arc of deforestation. Int. J. Climatol. 30, 1970–1979 (2009). [Google Scholar]
- 58.Morton D. C., et al. , Amazon forests maintain consistent canopy structure and greenness during the dry season. Nature 506, 221–224 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Vosti S. A., Braz E. M., Carpentier C. L., d’Oliveira M. V. N., Witcover J., Rights to forest products, deforestation and smallholder income: Evidence from the Western Brazilian Amazon. World Dev. 31, 1889–1901 (2003). [Google Scholar]
- 60.Brando P. M., et al. , Seasonal and interannual variability of climate and vegetation indices across the Amazon. Proc. Natl. Acad. Sci. U.S.A. 107, 14685–14690 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Core Team R, R: A Language and Environment for Statistical Computing (Version 3.4.2, R Foundation for Statistical Computing, Vienna, Austria, 2018). https://www.R-project.org/. Accessed 21 November 2017.
- 62.Angrist J. D., Pischke J.-S., Mostly Harmless Econometrics: An Empiricist’s Companion (Princeton University Press, Princeton, NJ, 2009). [Google Scholar]
- 63.Anselin L., Spatial Econometrics: Methods and Models (Springer Science and Business Media, 2013). [Google Scholar]
- 64.White N. J., Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 10, 297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleiber C., Zeileis A., Applied Econometrics with R (Springer, New York, NY, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.