Significance
We report the identification of an N-ethyl-N-nitrosourea–induced, nonsynonymous mutation in the fumarylacetoacetate hydrolase (Fah) gene in mice that results in increased plasma tyrosine (chronic tyrosinemia) due to reduced FAH activity in the liver and kidney. Importantly, in contrast to the lethality previously reported with other Fah mutations, our mutant mice survived into adulthood, making them a useful model for examining the long-term behavioral and physiological effects of chronic tyrosinemia.
Keywords: ENU mutagenesis, tyrosinemia, sleep
Abstract
Fumarylacetoacetate hydrolase (FAH) is the last enzyme in tyrosine catabolism, and mutations in the FAH gene are associated with hereditary tyrosinemia type I (HT1 or TYRSN1) in humans. In a behavioral screen of N-ethyl-N-nitrosourea mutagenized mice we identified a mutant line which we named “swingshift” (swst, MGI:3611216) with a nonsynonymous point mutation (N68S) in Fah that caused age-dependent disruption of sleep–wake patterns. Mice homozygous for the mutation had an earlier onset of activity (several hours before lights off) and a reduction in total activity and body weight when compared with wild-type or heterozygous mice. Despite abnormal behavioral entrainment to light–dark cycles, there were no differences in the period or phase of the central clock in mutant mice, indicating a defect downstream of the suprachiasmatic nucleus. Interestingly, these behavioral phenotypes became milder as the mice grew older and were completely rescued by the administration of NTBC [2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione], an inhibitor of 4-hydroxyphenylpyruvate dioxygenase, which is upstream of FAH. Mechanistically, the swst mutation had no effect on the enzymatic activity of FAH, but rather promoted the degradation of the mutant protein. This led to reduced FAH protein levels and enzymatic activity in the liver and kidney (but not the brain or fibroblasts) of homozygous mice. In addition, plasma tyrosine—but not methionine, phenylalanine, or succinylacetone—increased in homozygous mice, suggesting that swst mutants provide a model of mild, chronic HT1.
Hereditary tyrosinemia type I (HT1 or TYRSN1; OMIM:276700) is an autosomal recessive disorder caused by deficiency of fumarylacetoacetate hydrolase (FAH), the last enzyme in the tyrosine catabolic pathway (1–3). FAH deficiency leads to the accumulation of toxic metabolites such as fumaryl–acetoacetate, maleyl–acetoacetate, and succinylacetone. This buildup induces progressive liver disease, secondary renal tubular dysfunction, and neurologic crises that can include changes in mental status, abdominal pain, peripheral neuropathy, and/or respiratory failure (1, 2, 4–6). Based on clinical features, HT1 can be classified into 2 major types: The acute form has symptoms occurring within the first month after birth and usually results in death within the first year and the chronic form shows a slower progression of liver disease (6–8).
Several mouse models of acute HT1 have been described (9). The “albino lethal” mouse has a large deletion on chromosome 7, including the albino locus and the Fah gene (10), while another FAH-deficient mouse was generated by targeted disruption of the Fah gene (11). In addition, 2 N-ethyl-N-nitrosourea (ENU)-induced point mutations also caused FAH deficiency: A missense mutation in exon 6 and a splice mutation causing loss of exon 7 with a subsequent frameshift in the coding region of the Fah gene (12). Phenotypic screens using random mutagenesis have increased our understanding of the genetic basis of many diseases. In contrast with targeted mutagenesis, the unbiased nature of random mutagenesis allows for novel gene discovery and also causes point mutations that may alter gene function in unpredictable ways (13–15).
Although the available models of FAH deficiency have been useful for studying the biochemical and pathological features of HT1, they are perinatally or postnatally lethal due to liver failure, making behavioral and physiological studies impossible without pharmacological intervention (16, 17). Here we report the identification of an ENU-induced, nonsynonymous mutation in the Fah gene. Biochemical analyses indicate that this mutation promotes degradation of the FAH protein, resulting in diminished FAH enzymatic activity in a tissue-specific manner. In contrast to the lethality previously reported in other HT1 mouse models, our mutant mice survived into adulthood, making it possible to examine their behavioral and physiological characteristics. Our studies suggest that FAH deficiency interferes with behavioral entrainment to light–dark cycles downstream of the suprachiasmatic nucleus (SCN), leading to disrupted sleep–wake patterns. Interestingly, the behavioral defects in our model are reversible and are associated with reduced FAH protein levels and FAH activity in the liver and kidney, resulting in chronic tyrosinemia.
Results
Mutagenesis, Screening, and Identification of the swingshift Mutation.
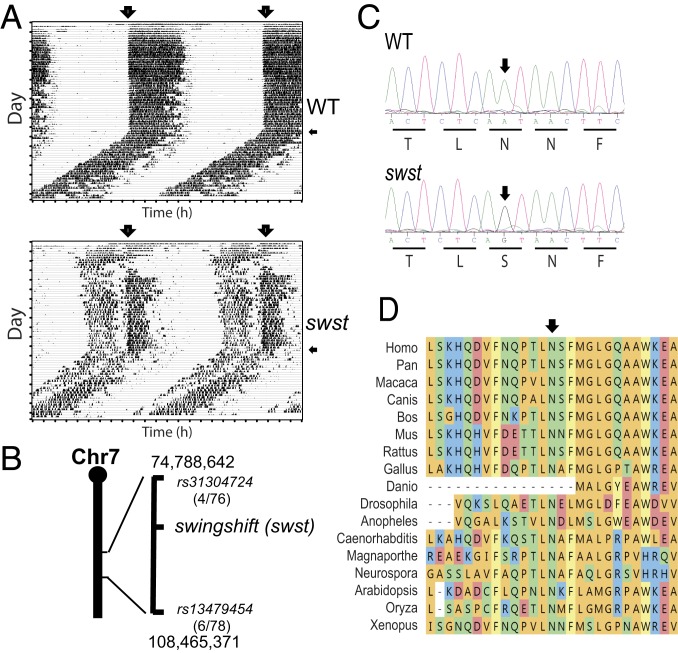
Using a recessive approach, we screened ENU-mutagenized mice for abnormal circadian behaviors. One of the mutant mouse lines was characterized by early activity onset (Fig. 1A), and we named this mutant “swingshift” (swst, MGI:3611216). Using C57BR/sdJ and C57L/J strains, we mapped swst to a 33.7-Mb interval on chromosome 7 between rs31304724 (4 recombinants/76 meioses) and rs13479454 (6 recombinants/78 meioses) (Fig. 1B). This region contains 511 Refgenes according to the mouse genome assembly GRCm38/mm10. To identify a candidate gene, we performed exome sequencing using genomic DNA from one homozygous mouse and found a nonsynonymous point mutation within the coding region of Fah. There was a single base transition from A to G at chromosome 7:84,601,144 in exon 3 of Fah in swst mice, which was confirmed by sequencing the flanking region using DNA samples from 4 homozygous and 3 wild-type (WT) mice (Fig. 1C). The point mutation converts the highly conserved amino acid residue 68 from an asparagine to a serine (N68S; Fig. 1 C and D).
Fig. 1.
Identification of the swst mutation in the Fah gene in mice. (A) Representative wheel-running actograms for WT (Upper) and mutant littermates (swst, Lower). Recordings were started with 12:12 LD cycles (lights on indicated with large arrows) followed by a period of constant darkness (indicated with small arrows). The swst mutant mice had disrupted sleep–wake patterns. (B) The swst mutation maps to a 33.7-Mb region on chromosome 7. (C) Sequencing of genomic DNA confirmed a point mutation in the Fah gene. An A-to-G transition converts amino acid residue 68 from asparagine to serine (N68S) in the mouse FAH protein. (D) Alignment of FAH protein indicates that asparagine 68 is highly conserved.
swst Mutant Mice Have Disrupted Sleep–Wake Patterns but a Normal Circadian Clock.
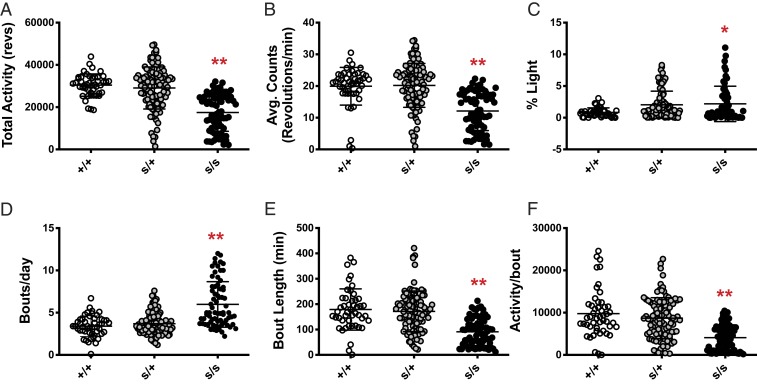
In order to investigate genotype–phenotype associations, we quantified the swst behavioral phenotype using total activity (wheel revolutions per day) from the 6th to the 15th day and the percentage of activity during the light phase (% Light) in 12:12 light–dark (LD) cycles. When compared with heterozygous and WT mice, homozygous mutant mice had lower total activity (Fig. 2 A and B; P < 0.0001) even though they had increased activity during the light phase (Fig. 2C; P < 0.01). Moreover, homozygous mutants had more activity bouts per day (Fig. 2D; P < 0.0001), while bout length and activity level per bout were reduced (Fig. 2 E and F; P < 0.0001). Taken together, these results point to a failure of entrainment of sleep–wake rhythms with light–dark cycles in swst mutant mice, while the lack of phenotype in the heterozygotes confirms the autosomal recessive inheritance of the swst mutation.
Fig. 2.
The swst mutant mice have altered activity levels. (A) Homozygous mutant mice (s/s, n = 71) exhibited decreased total activity (wheel revolutions per day) as compared to heterozygous (s/+, n = 102) and WT littermates (+/+, n = 49). (B) This decrease in total activity in s/s mice was reflected in their reduced overall average activity per minute, although s/s mice had increased activity during the light phase (C). (D) The average number of activity bouts per day was increased in s/s mice, while bout length (E) and average activity per bout (F) were decreased. There were no differences between s/+ and +/+ littermates for any measure. *P < 0.01; **P < 0.0001.
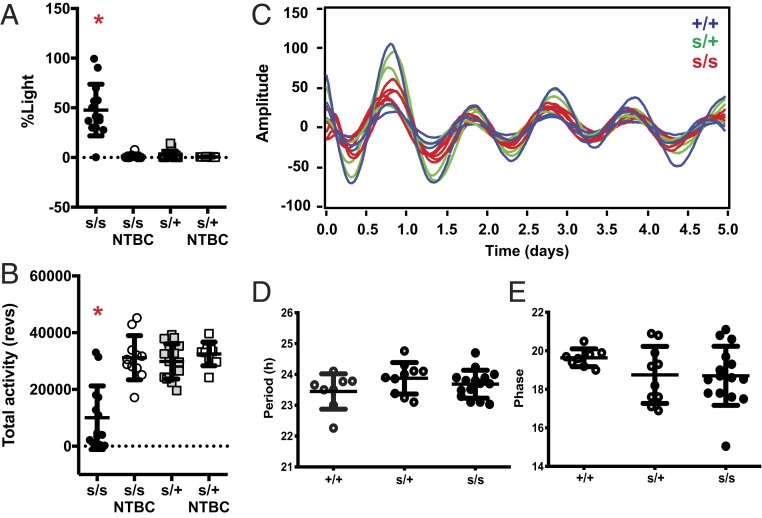
To further verify that the identified swst mutation was causative for the observed behavioral changes, we next sought to rescue the phenotype (18). Hereditary tyrosinemia is commonly treated with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), an inhibitor of 4-hydroxyphenylpyruvate dioxygenase upstream of FAH (19, 20) that has also been used to successfully rescue lethality in Fah knockout mice (16, 17). When swst mutant mice were given NTBC in their drinking water, their behavioral phenotype was completely rescued (see SI Appendix, Fig. S1 for representative actograms). Both the percentage of activity during the light phase (% Light, assessed from days 9 to 13 in 12:12 LD cycles) and the total activity in NTBC-treated swst homozygous mice were restored to levels similar to heterozygous mice (Fig. 3 A and B; swst homozygous [s/s] untreated different from all other groups, P < 0.0001). These findings indicate that swst is allelic with Fah and further support the hypothesis that the behavioral defects observed in swst mice are due to the mutation that we found in the Fah gene.
Fig. 3.
Behavioral alterations in swst mice can be reversed with NTBC treatment but are not due to defects in the SCN. (A) NTBC treatment completely rescued the percentage of light activity in homozygous mutant mice (s/s, n = 11 to 14) without altering behavior in heterozygous mice (s/+, n = 9 to 16). (B) NTBC treatment in s/s mice increased total activity to a level comparable to that of s/+ mice, while NTBC treatment in s/+ mice had no effect on total activity. (C) Real-time bioluminescence recording of SCN explants from s/s (red, n = 11), s/+ (green, n = 6), and +/+ (blue, n = 8) littermates heterozygous for the mPer2-Luc knock-in allele. (D and E) Circadian period (D) and phase (E). No differences were detected among the different genotypes. *P < 0.0001, untreated s/s mice different from all other groups.
Altered sleep–wake rhythms are commonly associated with malfunctions of the circadian master clock located in the SCN. Therefore, to investigate whether the swst mutation causes disrupted circadian behavior by altering SCN function, we crossed the swst mice to PER2::Luciferase reporter mice and cultured SCN explants to monitor their central clock (Fig. 3C). Surprisingly, we failed to detect any differences in period (Fig. 3D) or phase (Fig. 3E) of the central clock of mutant mice. Thus, the swst mutation in Fah alters sleep–wake patterns without affecting the function of the SCN.
Behavioral and Metabolic Alterations in swst Mutant Mice Improve with Age.
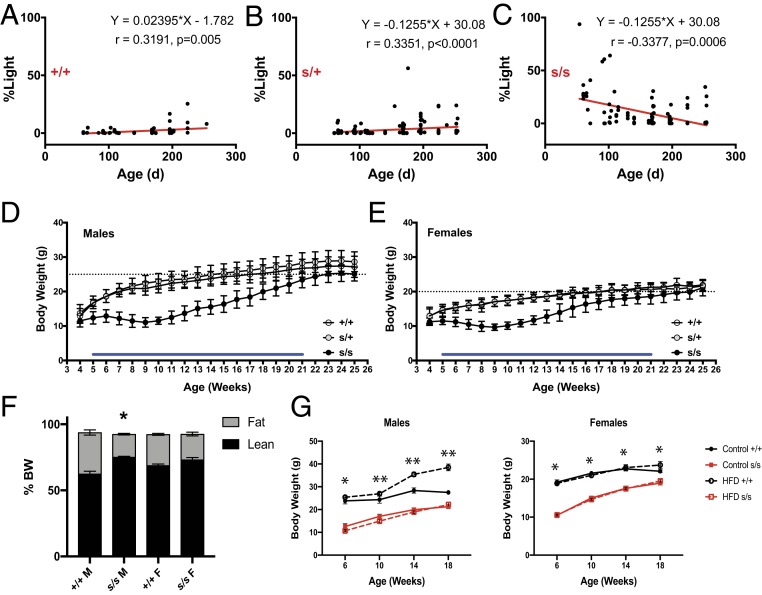
Despite strong genotype–phenotype associations, we found that some older mice did not show a swst phenotype, suggesting an age-dependent effect of the mutation (see SI Appendix, Fig. S2 for representative actograms). When we applied regression analysis to the wheel-running data, total activity was not correlated with age (r = −0.065, P = 0.577 for WT; r = −0.017, P = 0.840 for heterozygous; r = 0.171, P = 0.088 for homozygous mutant). In contrast, the % Light activity was correlated with age in all 3 genotypes. However, this correlation was positive in WT (Fig. 4A; r = 0.3191, P = 0.005) and heterozygous mice (Fig. 4B; r = 0.3351, P < 0.0001) and negative in homozygous mice (Fig. 4C; r = −0.3377, P = 0.0006). The negative correlation in homozygous mutant mice was likely due to the higher light-phase activity levels in younger animals, which were comparable to heterozygous and WT littermates when they were older.
Fig. 4.
Age-dependent phenotypes in swst mice. (A–C) Activity during the light phase was significantly correlated with age. The percentage of light activity (% Light) tended to increase with age in WT (+/+, n = 76) and heterozygous (s/+, n = 147) mice but decrease with age in homozygous mutant (s/s, n = 101) mice. (D and E) Body weights of male (D) and female (E) s/s mice. Blue bars indicate significant differences (P < 0.01) between the s/s mice (n = 22 males, 18 females) and the +/+ (n = 12 males, 16 females) and s/+ (n = 35 males, 30 females) mice. (F) Body compositions of male (n = 8 +/+, 10 s/s) and female (n = 2 +/+, 6 s/s) at 10 to 12 mo of age (*P < 0.0001 as compared to +/+ males). (G) Effects of HFD on body weights in male and female +/+ and s/s mice (n = 10 to 12/group). *P < 0.0001 +/+ different from s/s; **P < 0.0001 control +/+ different from all other groups).
We also found that, starting at 5 wk of age, body weights of homozygous mutant mice were less than that of heterozygous and WT mice in both males (Fig. 4D; P < 0.01) and females (Fig. 4E; P < 0.01). This decrease in body weight was associated with lower body fat in homozygous males, but not in females (Fig. 4F; P < 0.0001). Moreover, when challenged with a high-fat diet (HFD) the homozygous males were resistant to weight gain (Fig. 4G; P < 0.0001). Interestingly, the body weights of homozygous mutant mice gradually caught up with WT and heterozygous mice after 21 wk of age (Fig. 4 D and E). These results suggest that the swst mutation in Fah causes developmental defects that improve with aging.
The swst Mutation Reduces FAH Protein Stability in a Tissue-Specific Manner, Resulting in a Mild, Chronic HT1 Phenotype.
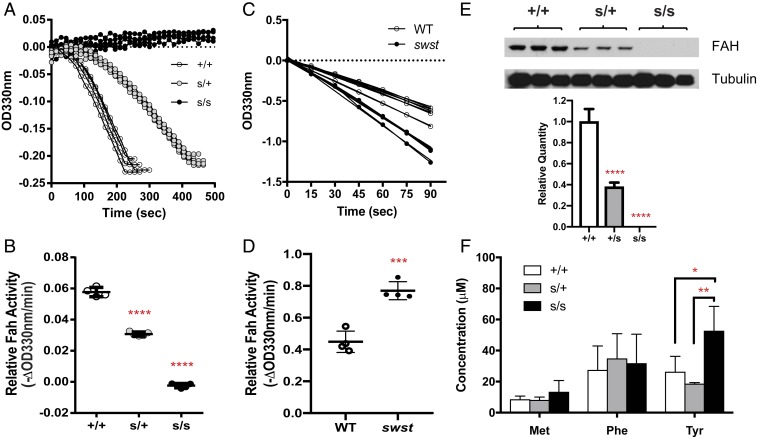
Fah is highly expressed in the liver, and its deficiency causes HT1 due to a lack of enzymatic activity (16). To examine if the swst mutation altered FAH enzymatic activity, we measured FAH activity in liver lysates. Heterozygous livers had FAH activity about half of that in WT livers, while the homozygous livers showed no detectable FAH activity (Fig. 5 A and B; P < 0.0001). However, when we examined FAH activity of purified recombinant proteins expressed in Escherichia coli, we were surprised to find that the swst mutation did not disrupt FAH activity. Rather, the mutant FAH showed slightly higher activity than WT (Fig. 5 C and D; P < 0.001). These results indicate that the lack of FAH activity in homozygous livers might instead be caused by reduced FAH protein levels.
Fig. 5.
The swst mutation causes reduced FAH protein in the liver but does not alter enzymatic activity. (A) Representative spectrometric FAH assay results with liver lysates from homozygous mutant (s/s, n = 5), heterozygous mutant (s/+, n = 5), and WT littermates (+/+, n = 4). (B) Relative enzymatic activity quantified from FAH assays in A. (C) Representative FAH assay results using purified WT and mutant FAH proteins. (D) Relative enzymatic activity quantified from FAH assays in C. Two separate experiments were performed with 2 replicates each (n = 4/group). (E) Representative immunoblots of FAH in liver lysates and relative FAH levels quantified from immunoblotting (n = 3 samples/group, average of 3 separate assays). (F) Plasma concentrations of methionine (Met), phenylalanine (Phe), and tyrosine (Tyr) in s/s (n = 5), s/+ (n = 4), and +/+ (n = 4) mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To test this hypothesis, we next examined FAH protein expression in various tissues from mutant mice and their littermates. Western blots of lysates from mutant and WT livers derived from young (10 d old, Fig. 5E) or adult mice (7 mo old, SI Appendix, Fig. S3A) revealed decreased FAH protein in homozygous livers. In addition to the liver, the mouse kidney also expresses Fah (21, 22). Therefore, we also examined the effects of the swst mutation FAH protein levels in the kidney. Similar to the results in the liver, FAH protein is undetectable in the homozygous kidney, but not in heterozygous or WT kidneys (SI Appendix, Fig. S3B). The behavioral defects in mutant mice prompted us to also examine FAH protein expression in the brain. In contrast to the mutant livers and kidneys, FAH protein is expressed at similar levels in the brains of homozygous, heterozygous, and WT mice (SI Appendix, Fig. S3C). We also established fibroblasts from the ears of the mutant mice and examined FAH protein levels in these cultures. Similar to the brain tissue, fibroblasts derived from homozygous mutants express FAH protein at a level comparable to those fibroblasts derived from heterozygous and WT mice (SI Appendix, Fig. S3D). Taken together, these findings show that the reduced FAH enzymatic activity in swst homozygous mutant livers is due to the loss of FAH protein and that there is a tissue-specific mechanism regulating the expression or degradation of FAH.
Marked elevation of succinylacetone in urine or plasma is considered pathognomonic for HT1 (23–25). Therefore, to assess whether succinylacetone levels are altered in swst mutants, we collected plasma from mutant mice and their littermates. Liquid chromatography followed by mass spectrometry failed to detect succinylacetone in any sample. However, we did detect an elevation of tyrosine in mutant plasma (Fig. 5F; P < 0.05). Interestingly, although HT1 is commonly associated with increased methionine and phenylalanine in addition to tyrosine, the mutant mice did not have altered plasma methionine or phenylalanine (Fig. 5F). These results suggest that the swst mutant may represent a model for a mild form of chronic HT1.
The swst Mutation Enhances Degradation of the FAH Protein.
To examine whether the reduction in FAH protein was due to impaired transcription or mRNA stability, we isolated total RNA from livers and measured pre-mRNA and mature mRNA using qRT-PCR. Neither pre-mRNA nor mature mRNA levels were altered in heterozygous livers, but in homozygous livers both pre-mRNA and mature mRNA levels were reduced (Fig. 6A; P < 0.0001). These findings argue against any deleterious effect of the swst mutation on Fah gene transcription or mRNA stability because otherwise we would expect about half the levels of pre-mRNA or mature mRNA in heterozygous mice compared to WT. Therefore, to further explore the effect of the swst mutation on mRNA stability, we established HEK293T cells stably expressing Myc-tagged mutant and WT FAH proteins. Using primer pairs specifically targeting the plasmid DNA and its derived mRNA, but not the endogenous FAH gene, we found that ectopically expressed FAH mRNA was proportional to the copy numbers of integrated plasmid DNA for both WT and swst mutant cells (Fig. 6B), indicating that neither transcription nor mRNA stability is altered by the swst mutation. Indeed, the mRNA half-life for WT and mutant FAH mRNAs did not differ between the stable cell lines (Fig. 6 C and D). However, as in the mouse liver lysates (Fig. 5E), there were reduced levels of mutant FAH protein in the stable cells (Fig. 6 B and E). We therefore examined the stability of the mutant protein and found that mutant FAH was degraded 5-fold faster than WT FAH, with half-lives of 33.4 and 165.2 h, respectively (Fig. 6F; P < 0.001 after 24 h). These results suggest that faster degradation of mutant FAH protein contributes to its reduced protein expression and enzymatic activity in homozygous swst livers.
Fig. 6.
The swst mutation reduces protein stability but not transcription or mRNA stability. (A) Relative amounts of pre-mRNA and mature mRNA in the livers of WT (+/+, n = 4), heterozygous mutant (+/s, n = 5), and homozygous mutant (s/s, n = 5) mice as measured by RT-qPCR. (B) Relative amounts of protein, mRNA, and DNA construct in HEK293T cells stably expressing WT (293T/FAHWT) or mutant (293T/FAHSwst) FAH vs. untransfected controls (293T, n = 3/group). (C) Representative results of mRNA stability in HEK293T cells stably expressing WT (FAHWT) or mutant (FAHSwst) FAH. Actinomycin D was added at time 0. (D) mRNA half-life calculated from mRNA stability assays (n = 4 per group). (E) Representative immunoblotting for protein stability assay in HEK293T cells stably expressing WT or mutant (SWST) FAH. Time 0 indicates the addition of cyclohexamide. (F) Quantified protein stability from 4 independent assays. *P < 0.05, **P < 0.001, ***P < 0.0001.
Discussion
HT1 is a genetic disease resulting from a deficiency in FAH. To date, more than 150 mutations in the FAH gene are associated with HT1 in humans, including missense and nonsense mutations as well as other variants that cause frameshifts or disrupt splicing (26, 27). In addition, 4 mutations have been described in mouse models (10–12). Several of the known missense mutations in humans decrease FAH enzymatic activity by altering protein folding/trafficking or inducing conformational changes that interfere with substrate binding (28). In this study, we report a missense mutation (swst) that causes an HT1 phenotype in mice, including the absence of FAH enzymatic activity in the liver and an increase in plasma tyrosine. In contrast with the previously reported mouse models that display lethality shortly after birth, these swst mutants survive into adulthood. Therefore, the swst mutation represents a mouse model for the chronic form of HT1, allowing for behavioral and physiological studies throughout the life span without pharmacological intervention. Interestingly, the swst mutation is in a region of the FAH gene (exon 3) deficient in missense mutations in humans. Since the mouse and human genes share 84% sequence identity and 89% amino acid identity, additional targeted mutagenesis of this gene in mice may allow for the discovery of genotype–phenotype correlations as they relate to HT1.
There are several features of the swst mutation that distinguish this model of HT1 from other human/murine mutations in the FAH/Fah gene. First, the swst mutation does not impair enzymatic activity by itself; instead, it causes faster degradation of FAH protein. It is worth noting that the half-lives of the mutant and WT FAH proteins as measured in HEK293T cells (33.4 vs. 165.2 h) do not necessarily represent the true values in mouse livers or kidneys. Nonetheless, faster degradation could potentially diminish FAH to undetectable levels in these tissues, leading to elevated plasma tyrosine. The elevation in tyrosine due to FAH deficiency is thought to be due to reduced inhibition of other steps in the tyrosine catabolic process (11, 29). While we did not assess the function of other enzymes in this pathway, such as tyrosine amino transferase, the swst model could be a useful tool to better understand the complex roles that FAH plays in the regulation of tyrosine catabolism.
A second distinct feature of the swst model is the apparent tissue specificity of its degradation that could be due to the nature of the point mutation, which converts an arginine to a serine. We speculate that such a change could introduce a novel, tissue-specific phosphorylation site, due either to tissue-specific motif recognition or kinase expression (30–32). The liver and kidney have more FAH than any other tissues in mice (21, 22), suggesting that these tissues are major sites of tyrosine catabolism. However, our findings indicate that the presence of functional FAH in other tissues may compensate for the defects in the liver and kidney, resulting in a less severe phenotype than what has been reported previously (10, 12, 16). While we did not assess every possible tissue in detail, our results call for caution when interpreting screening/diagnosing data from succinylacetone assays using plasma samples or from enzymatic assays using tissues or cells other than liver and kidney, as these results may not reflect tissue-specific loss of FAH.
Third, while tyrosine levels were elevated in the swst model, the other metabolites that we measured were not. We also observed a reduction in body weight in our mouse model that improved with age. Presumably, the reduction in body weight is a direct effect of the tyrosinemia, rather than the abnormalities in sleep–wake cycles. Humans and mice with altered sleep tend to weigh more, not less; however, there are other mouse models of disrupted sleep that exhibit a reduced body weight phenotype under certain conditions (33); therefore, we cannot rule out a circadian-metabolic connection in this model. Nonetheless, together these findings suggest a potentially milder form of tyrosinemia that is more phenotypically consistent, perhaps, with tyrosinemia type III, even though that form of tyrosinemia is usually the result of mutations in the HPD (4-hydroxyphenylpyruvic dioxygenase) gene (29, 34, 35). It is unclear why we were unable to detect succinylacetone in our samples, but there are cases of HT1 in the literature with normal or undetectable succinylacetone levels (36, 37). Thus, some FAH mutations may be incompletely penetrant or there may be variable expressivity of phenotypes among mutation carriers. While we did not examine the effects of the swst mutation or NTBC treatment on liver and kidney function or the development of hepatocellular carcinoma (16, 17, 38), 2 of the hallmarks for HT1 patients, those will be interesting questions to address in future studies.
The original purpose of our study was to perform a behavioral screen to find novel genes involved in regulating sleep and circadian behavior; thus, we were surprised to uncover a gene implicated in HT1. The clinical and pathological manifestations in HT1 involve mainly the liver, kidney, and peripheral nerves (7). However, our studies in the swst mutant mice have the potential to open up new research areas, as our results suggest a potential defect in the central nervous system. The swst homozygous mice show disrupted sleep–wake patterns characterized by advanced activity onset and fragmented sleep. These behavioral phenotypes can be rescued with NTBC treatment and also seem to improve with aging. Even though we did not detect the recovery of FAH protein levels in livers of older swst mice at an age (7 mo) when the behavioral and body-weight phenotypes largely disappear, we cannot rule out the possibility that the demand for tyrosine metabolism decreases with aging; thus, the deleterious effects of FAH deficiency may be ameliorated. Although the mechanism is still elusive, circuits downstream from the central circadian clock might be involved, given that the SCN remains intact in terms of period and phase. While these neurobehavioral findings are unusual, there is some indication that HT1 patients suffer from neurological and neuropsychological problems (39), although these studies are complicated by the fact that NTBC treatment may contribute to behavioral and cognitive phenotypes in humans (40). Thus, the swst mouse may allow for more in-depth modeling of the direct effects of tyrosinemia on brain development and behavioral abnormalities.
Conclusions
In summary, we have identified a mutation in the Fah gene that causes a chronic HT1 phenotype in mice through tissue-specific disruption of FAH expression. The mild defects result in a much longer life span for these mutant mice as compared to other HT1 models, which allowed us to reveal not only metabolic anomalies but also potential dysfunction in the central nervous system controlling sleep–wake rhythms.
Methods
Mutagenesis, Behavioral Screening, and Body-Weight Phenotyping.
ENU (Sigma catalog #N3385) was prepared as described previously (41). Six-week-old male C57BL/6J mice were injected with 250 mg per 1 kg of body weight of ENU. After a 6-wk recovery period, the ENU-treated mice were mated with WT C57BL/6J females to generate generation 1 (G1) males. G1 males were mated with WT C57BL/6J females to produce G2 females. Four G2 females were backcrossed to their G1 fathers to produce G3 mice for phenotyping. Five G3 mice from every G2 backcross (20 mice per G1 pedigree) were phenotyped to ensure an 85% probability of detecting a recessive mutation.
Mice (8 to 12 wk of age) were placed in individual running wheel cages and activity was recorded using the ClockLab data collection system (Actimetrics). After at least 2 wk on LD 12:12, some of the mice were put into constant darkness (DD) for an additional 3 wk. Total activity and percentage of activity during the light phase were analyzed using data from the 9th to the 13th day in LD. The free-running period was measured by linear regression analysis of activity onsets from data collected during the DD portion of the assay using ClockLab software (42). To rescue the behavioral phenotypes, NTBC (Nitisinone, Sigma, SML0269) was prepared as described (17) and was given in drinking water (7.5 µg/mL) to heterozygous mating pairs to make sure the pups were treated from the beginning of their lives. The pups were subjected to running wheel cages from 8 to 12 wk of age.
A separate group of mice were weighed weekly from 3 to 28 wk, while another cohort had body composition assessed by NMR at 10 mo of age. An additional group of mice was given either a HFD (D12451, Research Diets) or a control diet (7012, Harlan Teklad) for 3 to 4 wk starting at 6 to 8 wk of age. At the end of the altered diet period, all mice were weighed. The mice were then continued on their same diet and placed on running wheels for an additional 3 to 4 wk to determine if there was a corresponding change in wheel-running behavior and subsequently weighed again. For all experiments, food and water were provided ad libitum. All animal care and experimental procedures were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (43) and approved by the University of Texas Southwestern Institutional Animal Care and Use Committee.
Identification of the swingshift Gene.
Genetic mapping was carried out using C57BR/sdJ and C57L/J strains as described previously (41). Briefly, homozygous swst mice were outcrossed to C57BR/sdJ and C57L/J for F1 mice, which were then intercrossed to create F2 mice for mapping. Wheel-running behavior of all mapping mice was collected and analyzed as described above. Only presumptive homozygous mice were used for genetic mapping.
Exome sequencing was performed using DNA extracted from the livers of one presumptive homozygous and one WT mouse. Briefly, livers were harvested and quick-frozen in liquid nitrogen, tissue was pulverized using a frozen mortar and pestle, and DNA was extracted and quantified using a NanoDrop spectrophotometer. Only one homozygous mutation in the Fah gene was identified within the mapping locus.
To validate the exome sequencing results, exon 3 of the mouse Fah gene was PCR-amplified from genomic DNA samples of 4 homozygous mutant and 3 WT mice with the following primers: forward primer (5′-cagctctatgccggaggac-3′) and reverse primer (5′-gaggaagttggcctgactgtac-3′). The PCR product was purified using a Qiagen gel extraction kit and sequenced using the above primers. For distribution, swingshift mutant mice have been donated to the Jackson Laboratories (JAX #030622).
swst Genotyping.
Mice were genotyped for the swst mutation using qRT-PCR to detect single-nucleotide polymorphisms. PCR reactions were carried out in 10-μL volumes using 10 to 25 ng genomic DNA with 1× SYBR Green Master Mix (part #4309155, Applied Biosystems, Foster City, CA), 5 pM of forward (WT forward primer: 5′-ctgttttggtcttagacaactctGaa-3′ or swst forward primer: 5′-ctgttttggtcttagacaactctcTg-3′) and 5 pM of reverse primer (5′-ctggcagacagtaagttctgtaagg-3′) in MicroAmp 384-well Optical Reaction Plates (Applied Biosystems). Primer pairs (forward: 5′-GACTTCGACAACCGGCTG-3′; reverse: 5′-CGCTTGTTCTGGCTGATGTC-3′) targeting Heat Shock Protein 70 were used as a normalization control. Thermocycling reaction conditions were as follows: 40 cycles of 95 °C for 15 s and 60 °C for 30 s. Data were collected and analyzed with an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems).
RNA Isolation and qRT-PCR.
Total RNA was isolated from frozen tissues or cell cultures with TRIzol reagent (Invitrogen) and then cleaned up using an RNeasy Plus Mini Kit (Qiagen catalog #74134) after DNase treatment (Ambion). A total of 500 ng of DNase-treated total RNA was reverse-transcribed using Taqman reverse transcription reagent (Roche). The cDNA was diluted 100 times, and the equivalent of 2 ng of total RNA was applied to each qRT-PCR with a total volume of 10 µL. RNA expression was quantified by using SYBR green qRT-PCR analysis. Primer sequences are described in SI Appendix, Table S1.
Cloning and Expression of WT and swst Mutant FAH.
The cDNA collected as described above was used as template to PCR-amplify WT and swst mutant FAH coding sequences. The PCR product was gel-extracted using a Gel Extraction Kit (Qiagen) and cloned into EcoRI-digested pcDNA3.1 (for mammalian cell expression) or pGST-Parallel-1 [for BL21(DE3) expression] vectors using the In-Fusion HD Cloning System (Clontech, catalog #639645), resulting in pcDNA-FAHw (WT) and pcDNA-FAHs (swst mutant) and pGST-FAHw and pGST-FAHs. For mammalian expression, the pcDNA-FAH plasmids were transfected into HEK293T/17 cells (American Type Culture Collection CRL11268) using Lipofectamine 2000 (Thermo Fisher, 11668027). Stable cells were selected in the presence of 500 μg/mL Geneticin (Gibco 11811–031) for 2 wk. For BL21(DE3) expression, DNA was transformed into BL21(DE3) cells, grown in Luria–Bertani medium with 100 μg/mL ampicillin. Cells were induced with 0.4 mM isopropyl-β-d-thiogalactoside at a cell density of 0.6 (OD600), and protein was expressed at 16 °C for 20 h. The expressed proteins were purified with glutathione agarose beads (Sigma #G4510).
Western Blotting.
Mouse tissues were dissected and snap-frozen in liquid nitrogen before storing at −80 °C. Approximately 100 mg of tissue was homogenized using a pellet mixer in 1 mL RIPA buffer [50 mM Tris–Cl, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxylcholate, 0.1% SDS, 1 mM ethylenediaminetetraacetic acid, 10 mM NaF, 1× proteinase inhibitor mixture (Sigma), pH 7.5]. Fibroblast or HEK293T cells cultured on 100-mm dishes were collected by trypsinization and homogenized in 0.2 mL RIPA buffer using pellet mixer. Tissue or cell debris was removed by centrifugation at 14,000 × g for 10 min at 4 °C. Bradford assays (Thermo Scientific 23200) or a Nanodrop were used to determine protein concentrations in the supernatants. After denaturation, the samples were resolved on sodium dodecyl sulfate/polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membrane. Immunoblotting was carried out using the fumarylacetoacetase antibody (C-20, sc-66223, Santa Cruz Biotechnology) that targets the C terminus of FAH, thus recognizing WT and the swst mutant FAH equally well. α-Tubulin antibody (TU-02, sc-8035, Santa Cruz Biotechnology) or β-actin antibody (sc-1616, Santa Cruz Biotechnology) were used as loading controls.
Protein and mRNA Stability Assays.
HEK293T cells stably expressing WT and mutant FAH were seeded onto 12-well plates until they reached confluency. For the mRNA stability assay, actinomycin D (5 µg/mL final concentration) was added to the culture media. At various time points the cells were washed twice with PBS and lysed in 500 µL TRIzol reagent for total RNA isolation and qRT-PCR as described above. For the protein stability assay, cycloheximide (50 µg/mL, final concentration) was added to the media, and the cells were lysed in 150 µL RIPA buffer at different time points. The lysates were then subjected to Western blotting.
Primary Fibroblast Cell Culture.
Primary fibroblasts were isolated from the ears of WT and swst mutant mice. Briefly, minced ear tissue was incubated with collagenase III (1 mg/mL) and trypsin (0.05%). After 10 min of incubation at 37 °C, minced tissue was allowed to adhere onto a 35-mm culture dish. Approximately 5 d after culture in Dulbecco’s Modified Eagle Media (DMEM) (Mediatech) supplemented with 10% FBS, ear fragments were removed, and outgrowing fibroblasts were replated into 100-mm dishes by trypsinization.
FAH Assay.
The enzymatic activity of swst mutant and WT FAH was assessed as described (44), using either purified E. coli expressed proteins or liver homogenates. Briefly, since the FAH substrate FAA is not commercially available, we constructed an FAA-generating system by incubating Homogentisic acid (Sigma, H0751) with homogentisate 1, 2-dioxygenase (Hgd) and glutathione transferase zeta 1 (Gstz1), which were cloned from mouse liver cDNA and expressed and purified from BL21(DE3) cells as described above. The assay buffer contained 50 mM potassium phosphate buffer, pH 7.4, 100 mM KCl, 5 mM ascorbate (omitted for tissue homogenate assay), 50 μM FeSO4, 50 μM glutathione (GSH) reduced, and 200 μM homogentisic acid (HGA). To generate FAA, purified His-HGD (50 μg/mL final) and GST-GSTZ1 (50 μg/mL final) were added to the assay buffer, and the reaction mix was incubated for 1 h at room temperature. One milliliter of the reaction mix was transferred to a cuvette, and the FAH assay started by addition of purified FAH protein or liver homogenate. The enzymatic activity was monitored at OD 330 nm every 15 s for 10 min on a NanoDrop 2000c (Thermo Scientific).
Liquid Chromatography–Mass Spectrometry.
A minimum of 300 μL of whole blood was drawn from tail veins and then centrifuged for 10 min at 10,000 × g to collect plasma. One volume of methanol was added to the plasma to precipitate protein, vortexed vigorously for 15 s, and then spun at 13,000 × g for 5 min in a microcentrifuge to pellet the protein. Supernatants were removed and recentrifuged. Both absolute signal intensity and percentage of recovery as compared to spiked samples were optimized. Formic acid was added to the solution for a final concentration of 0.1%, and internal standard compounds (tyrosine, methionine, phenylalanine, and succinylacetone) were added for a final concentration of 25 to 50 ng/mL. Supernatants were directly analyzed using High Performance Liquid Chromatography (Prominence LC20/SIL-20AC; Shimadzu) coupled to a triple quadruple mass spectrometer (3200 QTRAP; AB SCIEX). Metabolites were separated chromatographically on a C18-based column with polar embedded groups (150 × 2.0 mm at 4 µm; Synergi Fusion; Phenomenex) using a tributylammonium acetate–methanol or formic acid–methanol gradient and subjected to tandem mass spectrometry analysis. The flow rate was set at 0.5 mL/min using the following method: buffer A (10 mM tributylamine adjusted with 15 mM acetic acid to pH 5.0 or 0.1% formic acid) and buffer B (100% methanol or 0.1% formic acid in methanol). Concentrations were as follows: t = 0 min, 0% buffer B; t = 2 min, 0% buffer B; t = 13 min, 100% buffer B; t = 15 min, 100% buffer B; t = 16 min, 0% buffer B, t = 20 min, 0% buffer B; and t = 20.1 min, stop. The best multiple reaction monitoring transitions for each compound were identified through quantitative optimization. Peaks were quantitated and normalized against estimates of the total ion count using Analyst 1.4 software (AB SCIEX).
Organotypic Cultures and Luminescence Recording.
The swst heterozygous mice were crossed to mPer2Luc knock-in mice to introduce the reporter allele into the swst background. The littermates heterozygous for mPer2Luc were used to monitor the central clock in vitro as previously described (45). Briefly, 1 h before lights off, mice were anesthetized with CO2 and decapitated, and their brains were rapidly removed. Coronal sections of the brain (300-μm thickness) were sliced with a Vibratome in ice-cold Hank’s Balanced Salt Solution (Invitrogen). Slices containing the SCN were examined under a dissection microscope, and the dissected SCNs were cultured on Millicell culture membranes (PICM ORG 50, Millipore) with 1.2 mL DMEM (Invitrogen), supplemented with 10 mM Hepes (pH 7.2), 0.035% NaHCO3, 2% B27 (Invitrogen), 25 units/mL penicillin, 25 μg/mL streptomycin, and 0.1 mM luciferin (Promega) in 35-mm petri dishes. Luminescence signals were recorded using a Lumicycle machine (Actimetrics). Period and phase were calculated using the LumiCycle data-analysis program (Actimetrics).
Statistical Analyses.
All statistics were carried out using Graphpad Prism. For behavioral, body composition, and biochemical data, one-way ANOVA or Student’s t tests were used to identify genotype effects. For body-weight data, repeated measures 1-way ANOVAs were used to identify differences between groups over time. For aging data, linear regression was performed, and r was calculated using Pearson’s correlations. For all statistical tests, significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Guocun Huang, Lisa Thomas, Delali Bassowou, and Izabela Kornblum for technical assistance. This work was funded by the National Institute of Mental Health grants U01 MH61915 and R01 MH078024 (J.S.T.). J.S.T. is an investigator at the Howard Hughes Medical Institute.
Footnotes
The authors declare no competing interest.
Data deposition: Mutant mouse line “swingshift” and the gene Fah have been registered with the Mouse Genome Informatics (MGI) database (http://www.informatics.jax.org/marker/MGI:3611216).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904485116/-/DCSupplemental.
References
- 1.Lindblad B., Lindstedt S., Steen G., On the enzymic defects in hereditary tyrosinemia. Proc. Natl. Acad. Sci. U.S.A. 74, 4641–4645 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grompe M., et al. , A single mutation of the fumarylacetoacetate hydrolase gene in French Canadians with hereditary tyrosinemia type I. N. Engl. J. Med. 331, 353–357 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Labelle Y., Phaneuf D., Leclerc B., Tanguay R. M., Characterization of the human fumarylacetoacetate hydrolase gene and identification of a missense mutation abolishing enzymatic activity. Hum. Mol. Genet. 2, 941–946 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Bliksrud Y. T., Brodtkorb E., Andresen P. A., van den Berg I. E., Kvittingen E. A., Tyrosinaemia type I: De novo mutation in liver tissue suppressing an inborn splicing defect. J. Mol. Med. (Berl.) 83, 406–410 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Sniderman King L., Trahms C., Scott C. R., “Tyrosinemia type I” in GeneReviews(R), Pagon R. A., et al., Eds. (Seattle, WA, 1993). [Google Scholar]
- 6.Kvittingen E. A., Hereditary tyrosinemia type I: An overview. Scand. J. Clin. Lab. Invest. Suppl. 184, 27–34 (1986). [PubMed] [Google Scholar]
- 7.Russo P. A., Mitchell G. A., Tanguay R. M., Tyrosinemia: A review. Pediatr. Dev. Pathol. 4, 212–221 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Tanguay R. M., et al. , Different molecular basis for fumarylacetoacetate hydrolase deficiency in the two clinical forms of hereditary tyrosinemia (type I). Am. J. Hum. Genet. 47, 308–316 (1990). [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K., Tanaka Y., Mitsubuchi H., Endo F., Animal models of tyrosinemia. J. Nutr. 137 , 1556S–1560S; discussion 1573S–1575S (2007). [DOI] [PubMed] [Google Scholar]
- 10.Gluecksohn-Waelsch S., Genetic control of morphogenetic and biochemical differentiation: Lethal albino deletions in the mouse. Cell 16, 225–237 (1979). [DOI] [PubMed] [Google Scholar]
- 11.Grompe M., et al. , Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 7, 2298–2307 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Aponte J. L., et al. , Point mutations in the murine fumarylacetoacetate hydrolase gene: Animal models for the human genetic disorder hereditary tyrosinemia type 1. Proc. Natl. Acad. Sci. U.S.A. 98, 641–645 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon M. M., et al. , Current strategies for mutation detection in phenotype-driven screens utilising next generation sequencing. Mamm. Genome 26, 486–500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldowitz D., et al. , Large-scale mutagenesis of the mouse to understand the genetic bases of nervous system structure and function. Brain Res. Mol. Brain Res. 132, 105–115 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stottmann R. W., Beier D. R., Using ENU mutagenesis for phenotype-driven analysis of the mouse. Methods Enzymol. 477, 329–348 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Grompe M., et al. , Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 10, 453–460 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Al-Dhalimy M., Overturf K., Finegold M., Grompe M., Long-term therapy with NTBC and tyrosine-restricted diet in a murine model of hereditary tyrosinemia type I. Mol. Genet. Metab. 75, 38–45 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Takahashi J. S., Pinto L. H., Vitaterna M. H., Forward and reverse genetic approaches to behavior in the mouse. Science 264, 1724–1733 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holme E., Lindstedt S., Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J. Inherit. Metab. Dis. 21, 507–517 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Lindstedt S., Holme E., Lock E. A., Hjalmarson O., Strandvik B., Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340, 813–817 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Lattin J. E., et al. , Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res. 4, 5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C., Jin X., Tsueng G., Afrasiabi C., Su A. I., BioGPS: Building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 44, D313–D316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott C. R., The genetic tyrosinemias. Am. J. Med. Genet. C. Semin. Med. Genet. 142C, 121–126 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Grenier A., Lescault A., Laberge C., Gagné R., Mamer O., Detection of succinylacetone and the use of its measurement in mass screening for hereditary tyrosinemia. Clin. Chim. Acta 123, 93–99 (1982). [DOI] [PubMed] [Google Scholar]
- 25.la Marca G., et al. , The inclusion of succinylacetone as marker for tyrosinemia type I in expanded newborn screening programs. Rapid Commun. Mass Spectrom. 22, 812–818 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Harrison S. M., et al. , Using ClinVar as a resource to support variant interpretation. Curr. Protoc. Hum. Genet. 89, 8.16.1–18.16.23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angileri F., et al. , Geographical and ethnic distribution of mutations of the fumarylacetoacetate hydrolase gene in hereditary tyrosinemia type 1. JIMD Rep. 19, 43–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergeron A., D’Astous M., Timm D. E., Tanguay R. M., Structural and functional analysis of missense mutations in fumarylacetoacetate hydrolase, the gene deficient in hereditary tyrosinemia type 1. J. Biol. Chem. 276, 15225–15231 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Chinsky J. M., et al. , Diagnosis and treatment of tyrosinemia type I: A US and Canadian consensus group review and recommendations. Genet. Med. 19, 1380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huttlin E. L., et al. , A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karabulut N. P., Frishman D., Sequence- and structure-based analysis of tissue-specific phosphorylation sites. PLoS One 11, e0157896 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundby A., et al. , Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat. Commun. 3, 876 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L., Summa K. C., Olker C., Vitaterna M. H., Turek F. W., Altered body weight regulation in CK1ε null and tau mutant mice on regular chow and high fat diets. Genet. Res. Int. 2016, 4973242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endo F., Katoh H., Yamamoto S., Matsuda I., A murine model for type III tyrosinemia: Lack of immunologically detectable 4-hydroxyphenylpyruvic acid dioxygenase enzyme protein in a novel mouse strain with hypertyrosinemia. Am. J. Hum. Genet. 48, 704–709 (1991). [PMC free article] [PubMed] [Google Scholar]
- 35.Endo F., et al. , Four-hydroxyphenylpyruvic acid oxidase deficiency with normal fumarylacetoacetase: A new variant form of hereditary hypertyrosinemia. Pediatr. Res. 17, 92–96 (1983). [DOI] [PubMed] [Google Scholar]
- 36.Blackburn P. R., et al. , Silent tyrosinemia type I without elevated tyrosine or succinylacetone associated with liver cirrhosis and hepatocellular carcinoma. Hum. Mutat. 37, 1097–1105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassiman D., Zeevaert R., Holme E., Kvittingen E. A., Jaeken J., A novel mutation causing mild, atypical fumarylacetoacetase deficiency (Tyrosinemia type I): A case report. Orphanet J. Rare Dis. 4, 28 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angileri F., et al. , Molecular changes associated with chronic liver damage and neoplastic lesions in a murine model of hereditary tyrosinemia type 1. Biochim. Biophys. Acta 1852, 2603–2617 (2015). [DOI] [PubMed] [Google Scholar]
- 39.van Ginkel W. G., et al. , Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J. Rare Dis. 11, 87 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ginkel W. G., Jahja R., Huijbregts S. C. J., van Spronsen F. J., Neurological and neuropsychological problems in tyrosinemia type I patients. Adv. Exp. Med. Biol. 959, 111–122 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Siepka S. M., Takahashi J. S., Forward genetic screens to identify circadian rhythm mutants in mice. Methods Enzymol. 393, 219–229 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siepka S. M., Takahashi J. S., Methods to record circadian rhythm wheel running activity in mice. Methods Enzymol. 393, 230–239 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 44.Knox W. E., “Enzymes involved in conversion of tyrosine to acetoacetate: A. l-tyrosine-oxiding system of liver” in Methods in Enzymology (Academic Press, 1955), vol. 2, pp. 287–300. [Google Scholar]
- 45.Yoo S. H., et al. , PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 101, 5339–5346 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.