Abstract
Background
There are meta-analyzes in adults demonstrating the benefits of using gabapentin to improve postoperative pain in orthopedic surgeries. In pediatrics, it has never been studied.
Objectives
The aim of this study was to evaluate the use of gabapentin 10 mg/kg, orally, in postoperative analgesia, hemodynamic stability and its pre/postoperative anxiolytic effect in children subjected to unilateral inferior limb surgery.
Methods
We performed a double-blinded, randomized study. 84 patients in Albert Sabin Children’s Hospital were selected for elective surgery that were divided into 2 groups: gabapentin group, who received gabapentin 1 to 2 hours before the procedure and the control group. Both groups were submitted to the same general anesthesia protocol with 0.125% bupivacaine femoral and sciatic block. Patients received scheduled dipyrone and morphine was used as the rescue analgesic up to 2/2 h. Postoperative pain was assessed using a scale appropriate for age (CRIES, CHIPPS or Wong-Baker face scale). We registered hemodynamic parameters, analgesic consumption and pre/postoperative anxiolytics.
Results
A decrease in pain intensity in the 4th and 8th postoperative hours was observed in gabapentin group, both groups had the same opioid consumption. Children in the gabapentin group had an odds ratio of 25.6 for preoperative sedation and gabapentin promoted reduction of postoperative agitation. During orotracheal intubation the gabapentin group exhibited attenuation of the hemodynamic response.
Conclusions
Gabapentin was superior to placebo in reducing postoperative pain. Children who received gabapentin were more sedated in the operating room, less agitated in the postoperative period and the autonomic response to intubation was reduced.
Keywords: Gabapentin, Pediatric, Postoperative Pain, Orthopedic
1. Background
Analgesia in orthopedics is based on the use of local anesthetics, anti-inflammatories and opioids (1). Peripheral anesthetic block with long-acting local anesthetics has a duration around 8 to 12 hours (2). The search for new adjuvants in the pediatric perioperative period is a constant attempt to improve benefits. The alpha-2-agonists, ketamine and neostigmine were tested for pain reduction, pre and postoperative agitation, postoperative nausea and vomiting (PONV) and anesthetic consumption (3). The search for these benefits in gabapentinoids (gabapentin and pregabalin) is promising in pediatrics, however only gabapentin is available for children of all ages (4).
Gabapentin is an amino acid similar in structure to the neurotransmitter GABA, but it does not interact significantly with this or other neurotransmitters. This promotes a reduction in the synthesis of the neurotransmitter glutamate as well as the reduction of calcium influx through binding to the alpha-2-delta subunit of voltage-dependent calcium channels. Side effects are limited to dizziness, drowsiness, diplopia and ataxia (5). In adults, gabapentin has a relative risk of producing sedation of 1.22 and administered preoperatively seems to attenuate the hemodynamic stress of orotracheal intubation (6, 7).
A review on the use of gabapentin in neuropathic and post-operative pain in pediatrics has demonstrated different doses when used as an adjuvant in the treatment of pain, with doses between 15 and 50 mg/kg/day, compared to the doses for seizure treatment being between 20 and 100 mg/kg/day. In postoperative pain studies in pediatrics surgeries, the single dose most used was between 10 and 20 mg/kg. In adenotonsillectomies, gabapentin reduced postoperative pain, opioid consumption, the incidence of PONV and postoperative agitation. In spinal surgery, there was no significant reduction in pain and opioid consumption (8). Gabapentin may be used in the less than one year old population (9, 10).
2. Objectives
The hypothesis of this study is that the addition of gabapentin as a pre-anesthetic drug would act on the intensity of postoperative pain could improve postoperative period. The main objective was to evaluate the use of oral gabapentin alone in reducing pain intensity and morphine consumption in children undergoing unilateral lower limb surgery and as a secondary objective to evaluate the beneficial effect upon hemodynamic stress of orotracheal intubation and pre/postoperative anxiolytic effect.
3. Methods
After approval by the Ethics and Research Committee of the local institution and registration at ClinicalTrials.gov (NCT03005483), a prospective, double-blind, randomized study was carried out at Albert Sabin Children’s Hospital in the city of Fortaleza, Brazil. Inclusion criteria were: healthy children between 3 months and 16 years of age who were subject to unilateral lower limb surgery. For exclusion criteria: presence of cardiac, pulmonary, renal, neurological diseases, allergy to any medication in protocols and refusal of children’s parents their gaurdians and patients themselves. All children’s gaurdians provided written informed consent.
Initially, a total number of 30 patients was calculated for each group (gabapentin group and control group), calculated as proposed by Armitage and Berry to estimate the observation of 90% change in mean difference between the two groups, with an alpha risk of 5% and 80% power (monocaudal). The aim was to obtain 10 patients between the ages of 3 months and 1 year, 10 patients between ≥ 1 and 5 years and 10 patients between 6 and 16 years in each group (9, 10). Due to the surplus of surgeries performed in the higher age range, the following allocation was obtained: control group, 44 patients (10 patients 3 months and 1 year, 11 patients ≥ 1 and 5 years, and 23 patients 6 and 16 years) and gabapentin group, 40 patients (11 patients 3 months and 1 year, 12 patients ≥ 1 and 5 years, and 17 patients 6 and 16 years).
Gabapentin oral solution in a single dose of 10 mg/kg to 600 mg or the placebo were administered 1 to 2 hours before surgery, both syrups had same flavor and features. Our team randomized patients with a drawing software, the pharmacologist was blinded to solutions. For operative and postoperative period, the professionals didn’t know the group they had evaluated. The drug effect before anesthetic induction was evaluated in categories: sedation, dizziness, waking or agitated. Similarly, the postoperative effects were classified in: dizziness, calmness, sedation and agitation. Hemodynamic parameters which were evaluated: systolic pressure (SP), mean pressure (MP), diastolic pressure (DP) and heart rate (HR) at the moments of anesthetic induction, 5 minutes after and every 15 minutes. The anesthesia protocol was the same for both groups, sevoflurane at 6 to 8% flowing by propofol 2.5 mg/kg for orotracheal intubation. Femoral nerve block with anterior branch nerve localization in the inguinal region (volume of 0.7 ml/kg, maximum of 20 mL) and sciatic nerve block in the gluteus following Raj’s technique (volume of 1 mL/kg, maximum of 30 mL) were performed using electrically isolated needles, coupled to a peripheral nerve stimulator (11). The local anesthetic used was 0.125%, levobupivacaine (Cristalia Prod. Quim. Farm Ltda, Brazil). After blockade, cisatracurium 0.1 mg/kg and sevoflurane 2 to 3% were used for anesthesia maintenance. At the end of the surgery, 20 mg/kg dipyrone was given every 6 hours and 50 µg/kg rescue morphine up to 2/2 h was infused if in severe pain.
Pain assessment was performed according to the child’s age range. For patients between 3 months and 1 year CRIES scale (≥ 5 points, we considered moderate pain, analgesic needed, and ≤ 4 points mild pain) was used. The CHIPPS scale (≥ 4 points, severe pain, analgesic needed, and ≤ 3 points mild pain) was used for the age group between ≥ 1 and 5 years and Wong Baker face scale modified to 10 points was used for patients between 6 and 16 years, when the value was ≥ 6 points (moderate pain), rescue opioid was used, and ≤ 5 points mild pain. Scores equal to 0 were no pain. The evaluation times were: 1 hour, 4 hours, 8 hours, 12 hours, 18 hours, and 24 hours after surgery. The time elapsed was recorded from the end of surgery to the first use of morphine in the ward.
We defined 3 types of surgeries based on time, localization and trauma: minor surgeries (duration less than one hour, small incisions and simple biopsies), medium surgeries (median incisions, minor osteotomies, tendinoplasties, surgical time between 1 and 2 hours), and major (osteotomies involving thigh regions, including leg or knee, plaque fixation device, surgical time greater than 2 hours).
3.1. Statistical Analysis
Intergroup comparisons at each time were performed using the unpaired t-test (parametric data), or the Mann-Whitney test, associated with non-parametric variables. To compare variables such as age, weight, gender, type of surgery and surgical time between the groups, the chi-square test was used. Regarding the heart rate, systolic blood pressure and diastolic blood pressure, the averages were calculated and analyzed: (a) between the two groups and at each time by the Mann-Whitney test and (b) within each group, according to time by the Friedman test. The level of significance of the analyzes was set at 0.05. For statistical analysis, the SPSS V. 19.0 software was used.
4. Results
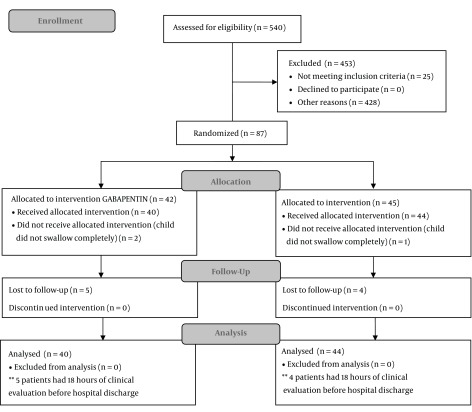
A total of 84 patients were analyzed, see CONSORT flow chart below (Figure 1).
Figure 1. CONSORT Flow Diagram. *In the period from December 2013 to January 2015 the orthopedic service of the hospital operated on approximately 540 patients for unilateral lower limb surgery. Source: prepared by the author.
Demographic data, surgical time and surgical types are summarized in Table 1. The two groups and subgroups were compared for demographic data, in which no significant differences were observed. The predominant age in both groups was 6 to 16 years and the predominant sex was male. The mean weight of the gabapentin group was 23.1 ± 17.8 kg, and the control group was 28.7 ± 20.9 kg. Despite these results, there was no statistically significant difference. The mean surgical time showed no statistical significance between the gabapentin group (51.1 ± 20.0 minutes) and the control group (54.5 ± 25.2 minutes). Orthopedic surgeries were divided into three types: minor, medium and major. The frequency of patients was similar among the subgroups. Minor and medium surgeries accounted for most surgical procedures (81%).
Table 1. Demographic Data, Time and Types of Surgeries. Comparison of all Patients, Gabapentin Group and Controla.
| Baseline Characteristics | All (N = 84) | Gabapentin (N = 40) | Control (N = 44) | P Value |
|---|---|---|---|---|
| Age (months) | 84 ± 64.2 | 62.8 ± 59.2 | 84.8 ± 67.4 | 0.17b |
| Age group | 0.67c | |||
| 3 months - 1 year | 21 (25.0) | 11 (27.5) | 10 (22.7) | |
| ≥ 1 year - 5 years | 23 (27.4) | 12 (30.0) | 11 (25.0) | |
| 6 years - 16 years | 40 (47.6) | 17 (42.5) | 23 (52.3) | |
| Weight (kg) | ||||
| All | 26.0 ± 19.6 | 23.1 ± 17.8 | 28.7 ± 20.9 | 0.17b |
| 3 months - 1 year (n = 21) | 8.5 ± 1.8 | 8.4 ± 1.9 | 8.6 ± 1.8 | 0.94b |
| ≥ 1 year - 5 years (n = 23) | 15.1 ± 5.0 | 13.9 ± 5.0 | 16.5 ± 4.7 | 0.11b |
| 6 years - 16 years (n = 40) | 41.6 ± 17.9 | 39.1 ± 16.6 | 43.4 ± 19.0 | 0.64b |
| Sex | 0.14c | |||
| Male | 53 (63.1) | 22 (55) | 31 (70.5) | |
| Female | 31 (36.9) | 18 (45) | 13 (29.5) | |
| Surgery time (min) | 52.9 ± 22.8 | 51.1 ± 20.0 | 54.5 ± 25.2 | 0.74c |
| Surgery type | 0.59c | |||
| Minor | 32 (38.1) | 17 (42.5) | 15 (34.1) | |
| Medium | 36 (42.9) | 17 (42.5) | 19 (43.2) | |
| Major | 16 (19.0) | 6 (15) | 10 (22.7) |
aValues are expressed as mean ± SD or No. (%).
bMann-Whitney test comparing gabapentin with control.
cχ2 test.
Minor type surgeries (duration less than one hour, small incisions and simple biopsies) corresponded to 38.1% of the surgeries analyzed. Congenital talipes equinovarus (CTEV) correction corresponded to the majority of minor surgeries (81.2%). The medium type of surgical interventions (median incisions, minor osteotomies, tendinoplasties, surgical time between 1 and 2 hours) were the most frequent (42.9%) of the total, 50% of which were CTEV with tendon transposition and osteotomy. Otherwise, major surgeries (osteotomies involving thigh regions, including leg or knee, surgical time greater than 2 hours) corresponded to 19.2%. Other major surgeries involved placement of a limb extension or plaque fixation device.
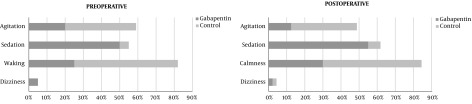
The drug effect before anesthetic induction was observed using four evaluation categories: sedation, dizziness, waking or agitated. The patients who received gabapentin, 50% sedated, only 5% dizzy, 25% remained awake and only 20% were agitated before anesthetic induction. However, in the control group: only 4.5% were sedated, 57% remained awake and 39% agitated. In the analysis of the four categories studied, an χ2 test comparing the two groups was used, and a significant difference was found with P < 0.01. When sedation and dizziness were attributed to the use of gabapentin, and these two categories were added together, 55% of the patients had these manifestations when the drug was administered; while only 4.5% of the patients presented sedation when they used placebo. Figure 2 shows the difference in the preoperative behavior of patients receiving gabapentin. For this study, the odds ratio for pre-anesthetic sedation with gabapentin was 25.6 (5.4 to 120.8) compared to placebo.
Figure 2. Preoperative: comparison of frequencies in percentage of agitation, sedation, waking and dizziness in the two groups in anesthetic induction including all ages. Postoperative: comparison of frequencies in percentage of agitation, sedation, calmness and dizziness in the two groups after one postoperative hour including all age groups. χ2 test, P < 0.01 at induction and P < 0.05 at postoperative.
In the first postoperative hour, the frequency of postoperative agitation common in pediatrics was evaluated. For these postoperative effects, they were classified into 4 categories: dizziness, calmness, sedation and agitation. As we observed in both groups, 2.2% of the children presented with dizziness. It was observed that 55% of patients in the gabapentin group presented with somnolence compared to 6.8% in the control group. During the first postoperative hour, 30% of the children in the gabapentin group were calm and 54.5% of the children in the control group were also calm. In the gabapentin group, 12.5% of the patients were agitated in the first postoperative hour, and 36.3% of the patients in the control group presented with agitation. Using the χ2 test, a significant difference was observed with P < 0.05 for postoperative agitation frequency. A chance ratio of 4 for gabapentin was calculated to reduce the occurrence of agitation in the PO period. Figure 2 shows the differences in the evaluation categories in the immediate PO period.
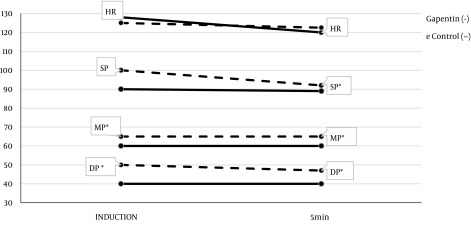
The hemodynamic parameters were evaluated: systolic pressure (SP), mean pressure (MP), diastolic pressure (DP) and heart rate (HR) at the moments of anesthetic induction, 5 minutes after and every 15 minutes until the end of the procedure. The hemodynamics data had statistically significant differences found during anesthetic induction, and in the first 5 minutes of surgery; where patients receiving gabapentin had lower values of measured pressures compared to placebo. However, these pressure values were within the normal range, and are expected in pediatrics. For heart rate, no difference was observed between the groups. Hemodynamic values in the first minutes of surgery are shown in Figure 3.
Figure 3. Hemodynamic curve (systolic, mean and diastolic pressure in mmHg and heart rate in beats per minute-BPM) in the gabapentin and placebo groups at anesthetic induction and in the 5th minute, including all age groups. HR, heart rate; SP, systolic pressure; MP, means pressure; DP, diastolic pressure. * Mann-Whitney test P < 0.05.
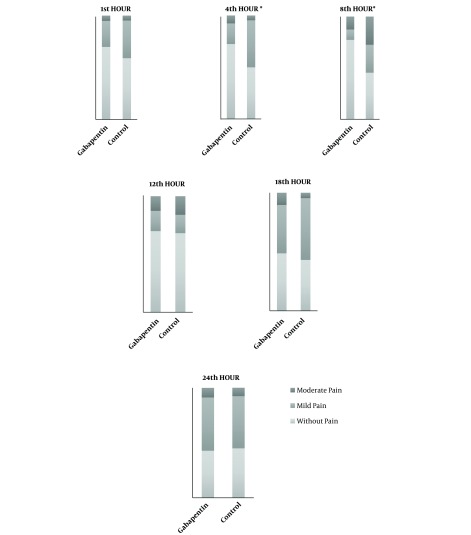
Pain reports were classified according to three categories, without pain, mild pain and moderate pain at different postoperative moments (1st, 4th, 8th, 12th, 18th, and 24th hour) in the two groups. There was a trend of increased pain intensity over time in both groups. In pain intensity, differences were observed with P < 0.05. At the 4th and 8th postoperative hour, there was lower pain intensity in the gabapentin group that doesn’t change tendency of pain period. Overall, during the first 12 hours, approximately 70% of patients reported little or no pain. After 18 hours postoperatively, the incidence of mild and moderate pain increased. Figure 4 shows the frequency of pain at different postoperative times.
Figure 4. Pain category frequency comparison (without pain, mild pain and moderate pain) of the two groups at different PO times including all age groups. * P significant < 0.05.
In general, the two groups had the same times of first administration, number of administrations and frequency of analgesic use. It was observed that the time for the first use of morphine in the gabapentin group had a mean of 7.3 ± 4.6 hours, and in the control group the mean was 7.8 ± 3.6 hours, there were no differences. The mean number of morphine administrations approached one in both groups. The morphine rescue frequency use was 30% when patients received gabapentin and in the control group the morphine rescue frequency was 40.9% with no differences, and daily consumption of morphine was similar, 0.09 mg/kg/day to gabapentin group and 0.08 mg/kg/day in control. When the subgroups were stratified, there were no differences between the gabapentin and control groups either. Patients undergoing major surgery, when they had pain, received a mean of 2.1 morphine administrations. In the same manner, patients subject to surgeries of the medium type, received mean of 1.7 administrations of morphine. While those in the minor type who had pain received only one administration of the opioid. Table 2 shows the need to use rescue morphine and the time of the first administration of the opioid in the different subpopulations of the study.
Table 2. Use of Morphine in Percentage and Time in Hours of the 1st Administration. Comparison of the Gabapentin Group with the Placebo Group, and Subgroups of Age Groups and Surgical Types.
| Gabapentin | Control | P1a | P2b | |||||
|---|---|---|---|---|---|---|---|---|
| No. | % Use | Hourc | No. | % Use | Hourc | |||
| All | 40 | 30 | 7.3 ± 4.6 | 44 | 40.9 | 7.8 ± 3.6 | 0.29 | 0.85 |
| Minor type | 17 | 11.7 | 4.50 ± 4.9 | 15 | 26.6 | 6.6 ± 4.5 | 0.28 | 1 |
| Medium type | 17 | 41.1 | 6.7 ± 4.5 | 19 | 42.1 | 9.3 ± 1.4 | 0.95 | 0.39 |
| Major type | 6 | 50 | 10.6 ± 4.2 | 10 | 60 | 6.6 ± 4.6 | 0.69 | 0.26 |
aχ2 test of the percentage of morphine use.
bMann-Whitney test for the time of 1st administration of morphine.
cValues are expressed as mean ± SD.
5. Discussion
The present study pioneered the use of gabapentin in pediatric orthopedic surgery working specifically with unilateral lower limb surgeries. The studies in adults in this subject are already well defined, compared to pediatrics without a predilection for the best dose (12). Patients who used gabapentin reported lower pain intensity, but there was no difference in morphine consumption, perhaps due to the concomitance of the nerve block. The duration of bupivacaine block in the lower limb is around 8 hours (13). A mean of two administrations of morphine was used. This finding was similar to that of an Australian study on postoperative pain in pediatric lower limb fractures, where 106 patients with postoperative femoral or tibial fractures were evaluated. The analgesia scheme was based on anti-inflammatory, weak and strong opioids (14). There have been other studies with gabapentin in the pediatric population using a single dose with satisfactory response (15).
One of the side effects of gabapentin that could be beneficial for anesthesia is drowsiness. In adult population studies, the occurrence of this effect is between 15.2 and 20% (5). In the current study, 50% of gabapentin patients were drowsy on anesthetic induction, compared to 95.4% who were agitated or awake in the control group. Only in one clinical study, has the use of gabapentin as an anxiolytic in children with neurological deficit and with gastrointestinal irritation been described (16).
In this study, a high index of drowsy patients in the 1st postoperative hour was found during the immediate postoperative period among the patients who received gabapentin. Gabapentin promoted reduction in the occurrence of postoperative agitation. In a recent meta-analysis in adults, the relative risk of gabapentin sedation in the postoperative period was calculated as 1.3 for sedation (17). There is only one study with children submitted to adenotonsillectomy observing their postoperative agitation using gabapentin 15 mg/kg; after which a reduction in agitation after general anesthesia with sevoflurane was observed. Sevoflurane is related with a higher incidence of agitation (18). In the present study, children who received gabapentin did indeed appear to be more sedated than the control group in the postoperative period, yet there was no delay in awakening. This finding suggests that gabapentin could be beneficial as a pre-anesthetic medication and in reducing agitation on waking up. Alpha-2-agonists are superior to midazolam as a pediatric pre-anesthetic medication and in reducing agitation (3). Although midazolam is the most prescribed drug, a negative impact on patients’ postoperative cognition can be observed (19). In a meta-analysis study, oral clonidine (2 and 5 µg/kg) were compared to oral midazolam 0.5 mg/kg where both a lower incidence of agitation and prolonged awakening time were observed (20). In another study, clonidine prevented postoperative agitation in pediatric patients, but prolonged awakeness and delayed discharge (21).
In anesthetic induction and orotracheal intubation, no hemodynamic instability or tendencies of hypotension and bradycardia were observed. The gabapentin group had better intubation stress attenuation than the control. The reduction in arterial pressures in the gabapentin group was at the lower normal limit for the age and there was no impairment in systemic perfusion. The use of gabapentin had a positive influence on hemodynamic parameters, during anesthetic induction and intubation.
There is still a lack of studies on the hemodynamic stability promoted by gabapentin in pediatric anesthesia. In adults, gabapentin demonstrated attenuation of anesthetic induction stress and orotracheal intubation. Gabapentin had lower scores when compared to clonidine and fentanyl. These two drugs had pressure measures and heart rate values tending towards hypotension and bradycardia with negative influences. The mechanism of action being suggested for cardiovascular changes mediated by calcium channel blockade and reduction of plasma catecholamines is still unclear (22). In another study, different doses of gabapentin, used to attenuate hemodynamic changes during orotracheal intubation were compared with a reduced response (23, 24).
5.1. Conclusions
We conclude that gabapentin in a single dose associated with a peripheral blockade plus bupivacaine 0.125% in children subjected to unilateral lower limb surgery is superior to placebo results. Gabapentin attenuated hemodynamic response to orotracheal intubation. Gabapentin promotes pre-anesthetic and postoperative sedation, preventing agitation in the immediate postoperative period.
5.2. Limitations
The present this study presents some limitations, sample size is limited, a large age group in the study stands out, and a great variety of surgeries were performed, despite the equalization of pain descriptors and surgeries occurring in a single limb.
Footnotes
Authors' Contribution: Washington Aspilicueta Pinto Filho and Lara de Holanda Juca Silveira complied the data. Josenilia Alves Gomes and Claudia Regina Fernandes analyzed the data. Mariana Lima Vale supervised the research. Washington Aspilicueta Pinto Filho wrote the manuscript.
Conflict of Interests: The authors declare that they have no conflict of interests.
Ethical Approval: This article received approval by the Ethics and Research Committee of the local institution (Protocol Certificate of Presentation for Ethical Appreciation- 05074712.4.0000.5042 in August 18, 2012, Albert Sabin Children’s Hospital Ethics Committee, Fortaleza, Brazil).
Funding/Support: There was no funding for this work.
Patient Consent: NCT03005483.
Contributor Information
Washington Aspilicueta Pinto Filho, Email: washfilho@hotmail.com.
Lara de Holanda Juca Silveira, Email: lara.juca@gmail.com.
Mariana Lima Vale, Email: marianavale@yahoo.com.
Claudia Regina Fernandes, Email: clauregifer@gmail.com.
Josenilia Alves Gomes, Email: gomes.josenilia@gmail.com.
References
- 1.Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth. 2001;13(7):524–39. doi: 10.1016/s0952-8180(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 2.Bosenberg A. Benefits of regional anesthesia in children. Paediatr Anaesth. 2012;22(1):10–8. doi: 10.1111/j.1460-9592.2011.03691.x. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt AP, Valinetti EA, Bandeira D, Bertacchi MF, Simoes CM, Auler JO Jr. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17(7):667–74. doi: 10.1111/j.1460-9592.2006.02185.x. [DOI] [PubMed] [Google Scholar]
- 4.Chung AM, Eiland LS. Use of second-generation antiepileptic drugs in the pediatric population. Paediatr Drugs. 2008;10(4):217–54. doi: 10.2165/00148581-200810040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rose MA, Kam PC. Gabapentin: Pharmacology and its use in pain management. Anaesthesia. 2002;57(5):451–62. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 6.Grant MC, Lee H, Page AJ, Hobson D, Wick E, Wu CL. The effect of preoperative gabapentin on postoperative nausea and vomiting: A meta-analysis. Anesth Analg. 2016;122(4):976–85. doi: 10.1213/ANE.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 7.Marashi SM, Saeedinia SM, Sadeghi M, Movafegh A, Marashi S. Attenuation of hemodynamic responses to intubation by gabapentin in coronary artery bypass surgery: A randomized clinical trial. Acta Med Iran. 2015;53(12):733–7. [PubMed] [Google Scholar]
- 8.Buck M. Gabapentin use in postoperative and neuropathic pain in children. Pediatr Pharmacother. 2016;22(2) [Google Scholar]
- 9.Nascimento Junior P, Modolo NS, Rodrigues Junior GR. [Postoperative analgesia in children less than 1 year of age: a retrospective analysis.]. Rev Bras Anestesiol. 2002;52(6):739–46. [PubMed] [Google Scholar]
- 10.Armitage P, Berry G. The planning of statistical investigations. Stat Meth Med Res. 1988;7(7):817–8. [Google Scholar]
- 11.Enneking FK, Chan V, Greger J, Hadzic A, Lang SA, Horlocker TT. Lower-extremity peripheral nerve blockade: Essentials of our current understanding. Reg Anesth Pain Med. 2005;30(1):4–35. doi: 10.1016/j.rapm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhai L, Song Z, Liu K. The effect of gabapentin on acute postoperative pain in patients undergoing total knee arthroplasty: A meta-analysis. Medicine (Baltimore). 2016;95(20):e3673. doi: 10.1097/MD.0000000000003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cucchiaro G, Ganesh A. The effects of clonidine on postoperative analgesia after peripheral nerve blockade in children. Anesth Analg. 2007;104(3):532–7. doi: 10.1213/01.ane.0000253548.97479.b8. [DOI] [PubMed] [Google Scholar]
- 14.Shrestha-Ranjit JM, Manias E. Pain assessment and management practices in children following surgery of the lower limb. J Clin Nurs. 2010;19(1-2):118–28. doi: 10.1111/j.1365-2702.2009.03068.x. [DOI] [PubMed] [Google Scholar]
- 15.Amani S, Abedinzadeh MR. Effects of oral gabapentin, local bupivacaine and intravenous pethidine on post tonsillectomy pain. Iran J Otorhinolaryngol. 2015;27(82):343–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Hauer JM, Wical BS, Charnas L. Gabapentin successfully manages chronic unexplained irritability in children with severe neurologic impairment. Pediatrics. 2007;119(2):e519–22. doi: 10.1542/peds.2006-1609. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam S, Lau CS, Chamberlain RS. Use of preoperative gabapentin significantly reduces postoperative opioid consumption: A meta-analysis. J Pain Res. 2016;9:631–40. doi: 10.2147/JPR.S112626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salman AE, Camkiran A, Oguz S, Donmez A. Gabapentin premedication for postoperative analgesia and emergence agitation after sevoflurane anesthesia in pediatric patients. Agri. 2013;25(4):163–8. doi: 10.5505/agri.2013.98852. [DOI] [PubMed] [Google Scholar]
- 19.Millar K, Asbury AJ, Bowman AW, Hosey MT, Martin K, Musiello T, et al. A randomised placebo-controlled trial of the effects of midazolam premedication on children's postoperative cognition. Anaesthesia. 2007;62(9):923–30. doi: 10.1111/j.1365-2044.2007.05148.x. [DOI] [PubMed] [Google Scholar]
- 20.Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies. Acta Anaesthesiol Scand. 2010;54(4):397–402. doi: 10.1111/j.1399-6576.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 21.Malviya S, Voepel-Lewis T, Ramamurthi RJ, Burke C, Tait AR. Clonidine for the prevention of emergence agitation in young children: Efficacy and recovery profile. Paediatr Anaesth. 2006;16(5):554–9. doi: 10.1111/j.1460-9592.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 22.Doleman B, Sherwin M, Lund JN, Williams JP. Gabapentin for the hemodynamic response to intubation: Systematic review and meta-analysis. Can J Anaesth. 2016;63(9):1042–58. doi: 10.1007/s12630-016-0668-0. [DOI] [PubMed] [Google Scholar]
- 23.Ali AA, Elnakera AM, Samir A. Effect of two different doses of gabapentin on the intraocular pressure and hemodynamic stress responses to laryngoscopy and tracheal intubation. ISRN Anesthesiol. 2013;2013:1–5. doi: 10.1155/2013/698205. [DOI] [Google Scholar]
- 24.Imani F, Rahimzadeh P. Gabapentinoids: Gabapentin and pregabalin for postoperative pain management. Anesth Pain Med. 2012;2(2):52–3. doi: 10.5812/aapm.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]