Abstract
Background
In order to address the opioid crisis in North America, many regions have adopted preventative strategies, such as prescription drug monitoring programs (PDMPs). PDMPs aim to increase patient safety by certifying that opioids are prescribed in appropriate quantities. We aimed to synthesize the literature on changes in opioid-related harms and consequences, an important measure of PDMP effectiveness.
Methods
We completed a systematic review. We conducted a narrative synthesis of opioid-related harms and consequences from PDMP implementation. Outcomes were grouped into categories by theme: opioid dependence, opioid-related care outcomes, opioid-related adverse events, and opioid-related legal and crime outcomes.
Results
We included a total of 22 studies (49 PDMPs) in our review. Two studies reported on illicit and problematic use but found no significant associations with PDMP status. Eight studies examined the association between PDMP status and opioid-related care outcomes, of which two found that treatment admissions for prescriptions opioids were lower in states with PDMP programs (p < 0.05). Of the thirteen studies that reported on opioid-related adverse events, two found significant (p < 0.001 and p < 0.05) but conflicting results with one finding a decrease in opioid-related overdose deaths after PDMP implementation and the other an increase. Lastly, two studies found no statistically significant association between PDMP status and opioid-related legal and crime outcomes (crime rates, identification of potential dealers, and diversion).
Conclusion
Our study found limited evidence to support overall associations between PDMPs and reductions in opioid-related consequences. However, this should not detract from the value of PDMPs’ larger role of improving opioid prescribing.
Keywords: Prescription drug monitoring programs, Opioids, Harms, Consequences
Background
The misuse of opioids has reached epidemic levels across North America [1]. The crisis has been perpetuated, in part, by the over and inappropriate prescribing of opioids by health professionals, brought on by improvements in the treatment of chronic pain and pharmaceutical companies’ push to use opioids as a first line therapy [2, 3]. In the United States, 2.4 million people have a severe opioid use disorder (OUD), which involves dependence on opioid analgesic medications, heroin, or both [1, 4]. The increasing use of opioids has led to many consequences such as more frequent incidents of reported opioid misuse, drug diversion, crime, overdoses, and death [5–11].
In order to address the opioid crisis many regions have adopted preventative initiatives, including physician mentoring, continuing medical education on pain management, naloxone kits, and, the focus of the current study, prescription drug monitoring programs (PDMPs) [12–17]. PDMPs facilitate controlled substances like opioids to be prescribed in appropriate quantities, following best practice guidelines, not co-prescribed with potentially harmful substances, and only provided to patients when safe and necessary [14]. This is achieved by monitoring the prescribing practices of healthcare providers and identifying any patterns of drugs received by patients. Most PDMPs give healthcare providers the option to check PDMP data (patient profiles) before prescribing or dispensing opioids to a patient, allowing for more informed decision making. Broadly, PDMPs aim to restrict drug diversion and reduce opioid misuse-related harms [18, 19].
As of 2018, 49 states, the District of Columbia, and two U.S. territories (Guam & Puerto Rico) had implemented a PDMP [20]. A 2009 report estimated that start-up costs of a PDMP in the United States ranged between $450,000 and $1.5 million, with an average annual cost of $500,000 for maintaining a PDMP [21]. Significant resources are directed to these programs on an ongoing basis, and, as such, there is a need to evaluate their effectiveness.
An important measure of PDMP effectiveness is a reduction in opioid-related harms and consequences [22]. A 2018 scoping review addressed the association of PDMPs with fatal and non-fatal overdoses for any drugs [23]. This review found uncertainty in the evidence around an increase or decrease in fatal and nonfatal overdoes after the implementation of PDMPs [23]. However, individual studies on PDMP effectiveness report a broad range of other opioid-related outcomes, including dependence, emergency department (ED) visits, crime, treatment admissions, and illicit opioid use [24–28].
To date, no systematic review has been undertaken to synthesize the evidence on the impact of PDMPs on a range of opioid-related outcomes of interest to health professionals, decision-makers and other knowledge users, including associated harms and consequences. Understanding whether these programs work as intended is a crucial piece of information to combat the current opioid crisis.
Methods
Eligibility criteria
We included full published reports in all languages. Study designs were restricted to those that could draw conclusions on the effectiveness of PDMPs in reducing opioid-related consequences and harms (pre-post studies, controlled before/after, case-control, or cluster RCT designs). Two reviewers independently selected relevant studies from titles and abstracts. Any conflicts at the title and abstract level were discussed between the two reviewers. If consensus could not be achieved after discussion, the study was carried forward to the full-text screening level. Any conflicts at the full-text screening level were resolved by consulting with a third reviewer. We used Covidence software for all study screening [29]. Covidence, an online systematic review management platform, is a key element of Cochrane’s review production toolkit that facilitates study screening and data extraction.
We included studies of any jurisdiction (regional, provincial/state, national) or setting (clinic, hospital, system) where a PDMP had been implemented and where either a within jurisdiction comparison was made (pre- post- PDMP implementation) or a between jurisdiction comparison was made between those with and without a PDMP. We did not restrict studies by geographic region.
Reporting the intervention
We considered the intervention of interest the presence of a PDMP, defined as a program that specifically monitors the outpatient prescription dispensing of opioids (or other drugs) by healthcare providers. To ensure broad scope of our review, we included all types of PDMPs.
Outcomes of interest
We included opioid-related outcomes only in this review. It is important to note that we did not limit these outcomes to those related to prescription opioid use. We also included outcomes related to illicit opioids such as heroin, as the literature suggests that there is the potential for PDMPs to push people who use prescription opioids to illicit sources [6]. We did not include outcomes that addressed non-opioid analgesics, and other controlled substances monitored by PDMPs (e.g. benzodiazepines).
Opioid-related consequences and harms were grouped into categories by theme: opioid dependence (i.e. substance use disorders), opioid-related care outcomes (i.e. hospital visits, treatment program admissions), opioid-related adverse events (i.e. overdose, death), and opioid-related legal and criminal outcomes (i.e. arrests, diversion). Use and consequence outcomes could be linked to all opioids, or to specific types of opioids.
Search strategy
We followed a standard systematic review approach, employing a predefined protocol, and structured the report according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [30].
To identify relevant publications, we established a uniform strategy for searching MEDLINE, Embase, CINAHL, PsycInfo, Web of Science, and grey literature including Dissertation and Theses Databases, CADTH, Health Canada, CIHI, and CMA Infobase, following the guidance of a medical librarian [31]. Furthermore, we manually searched reference lists of all included studies, related systematic reviews, and all additional relevant reviews identified in the electronic search. We also contacted authors of key publications and identified relevant conference abstracts, and reviewed personal libraries of the research team [32–35]. We systematically searched terms relevant to PDMPs, matching terms against possible subject headings (e.g. MeSH) and keywords. We performed the search on January 22, 2018 and included all pertinent publications published prior to that date (see Additional file 1).
Risk of bias assessment
We assessed potential risk of bias for each study meeting selection criteria using the Quality of Prognostic Studies (QUIPS) tool. The QUIPS tool assesses risk of bias across six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting [36]. Specifically, we considered low risk of bias for the study sample if the response rate was > = 70% participation, moderate for 60–69.9%, and high for < 60%. Further, we considered whether studies adjusted for the following potential confounders: (a) The characteristics/features of PDMP (i.e. mandatory use), (b) demographic characteristics of sample (either individual, physician or jurisdiction level), and (c) presence of other related interventions in the study period (or other trends in substance use). Studies adjusting for at least two confounders were considered to have a low risk of bias on study confounding, 1 would be moderate, and 0 would be high.
Data extraction, synthesis, and analysis
For all included studies, data extraction was completed by two independent reviewers using pre-tested data extraction forms developed in Covidence [29]. Any discrepancies in data extraction were discussed and the assessment of a third reviewer was sought for resolution. We extracted relevant study details (i.e. authors, year, jurisdiction, study design, sample size), population characteristics (i.e. providers, patients), interventions (i.e. included PDMP characteristics), and data sources (i.e. administrative, survey). Outcomes extracted included any unadjusted and adjusted associations between the presence of a PDMP, or the change in PDMP states and non-PDMP states over time, and each opioid-related harm or consequence outcome, as well as all variables controlled for.
We synthesized our data narratively. If studies had overlapping datasets, years of data, and jurisdictions, the study with most years of data for a dataset and jurisdiction was designated as the primary study in our narrative synthesis and the remaining studies were secondary. If a study presented both unadjusted and adjusted data, both were extracted; however, the adjusted data was included in our primary synthesis.
We used Excel 2016 for data management and Stata 15 for descriptive analyses and calculating pooled estimates [37, 38].
Results
The study selection process for this review is summarized in the PRISMA flow chart presented in Fig. 1. A total of 161 articles were assessed at the full text level, resulting in the inclusion of 22 studies addressing the association of PDMP status with opioid-related consequences or harms. All included studies took place in the United States and 72.7% presented findings among the general population (Table 1). Studies were published between 2006 and 2018 and include data years from 1992 to 2014. Twelve datasets were used, with the Treatment Episode Data Set (TEDS) being the most common, appearing in six publications with treatment admission outcomes. Studies with overlapping data differed in the years of data covered. Opioid-related consequences and harms are described in detail below and separated into the following four categories: illicit and problematic opioid use, opioid-related care outcomes, opioid-related adverse outcomes, and opioid-related legal and criminal outcomes.
Fig. 1.
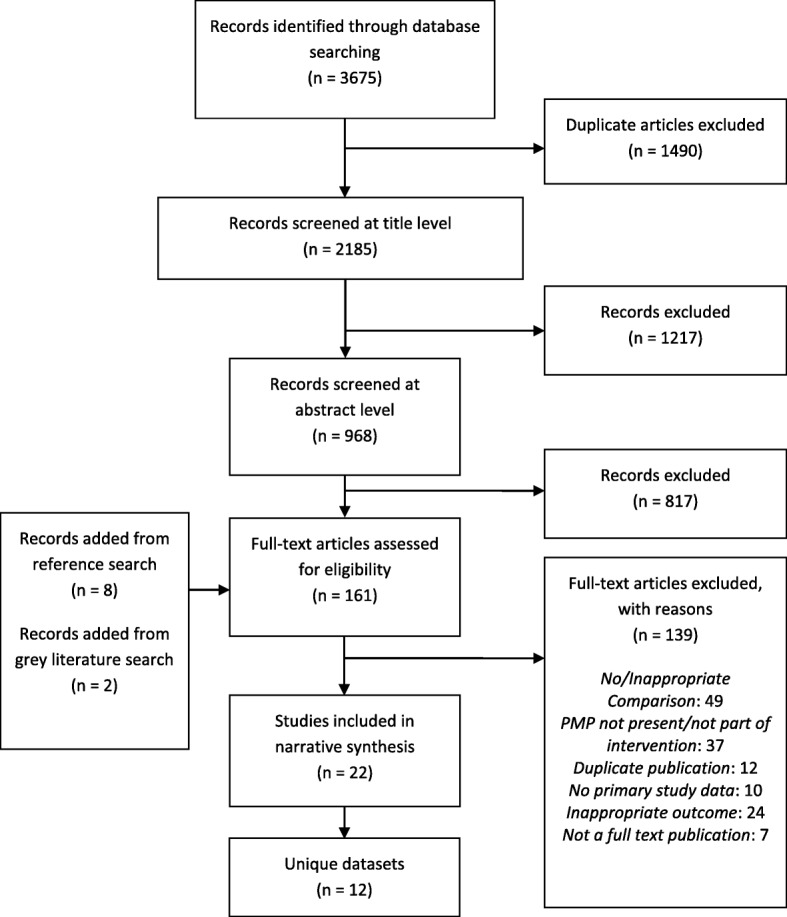
PRISMA flow chart depicting the study selection and inclusion process and results
Table 1.
Study level characteristics of 22 included studies
| Study ID | Included Jurisdiction with a PMP | Study Population | Study Design | Outcome Groups | Outcome Data Source, Years |
|---|---|---|---|---|---|
| Ali 2017 [24] | All states except MO and DC | general population | Pooled Cross-sectional Logit and generalized linear models | illicit opioid usea, opioid dependencea | National Survey on Drug Use and Health (NSDUH), 2004–2014 |
| Birk 2017 [43] b | All states that established PMPs between 1998 and 2012 | general population, opioid-related deaths | Controlled interrupted time series | opioid-related care outcomes, opioid-related adverse events | Treatment Episode Data Set (TEDS), 1998–2012; National Vital Statistics System (NVSS), 1999–2012 |
| Branham 2018 [27] b | AL, AZ, CO, CT, IN, IA, LA, ME, MN, MS, NV, NM, NC, ND, OH, SC, TN, UT, VT, VA, WV, WY | people who use drugs admitted to treatment | Controlled interrupted time series | opioid-related care outcomesa | TEDS, 1992–2012 |
| Dave 2017 [44] b | AK, AZ, AR, CO, CT, DE, FL, GA, IA, KS, LA, ME, MD, MN, MS, MT, NE, NH, NJ, NM, NC, ND, OH, OR, SC, SD, TN, WA, WI, WY, VT, VA | general population | Controlled interrupted time series | opioid-related care outcomes | TEDS, 2003–2014 |
| Delcher 2015 [50]c | FL | drug-related deaths | Controlled interrupted time series | opioid-related adverse eventsa | Florida Medical Examiners Commission, 2003–2012 |
| Kim 2013 [51] | AL, AZ, CA, CO, CT, IL, IN, LA, ME, MI, MS, NM, NC, ND, OH, OK, SC, TN, VA, WV, NY, HI, ID, OA, RI, TX, MA, NV, UT, WY, KY | general population | Interrupted time series | opioid-related adverse events | CDC Wide-Ranging Online Data for Epidemiologic Research (WONDER); 1999–2008 |
| Kinsell 2015 [40] c | NY, FL | general population | Controlled before and after/ Difference-in-difference approach | opioid-related adverse eventsa, opioid-related care outcomesa | CDC WONDER & Florida Medical Examiner Drug Related Death Data & Florida Agency for Health Care Administration & New York State Inpatient Databases (SID)/ State Emergency Department Databases (SEDD), 2009–2012 |
| Li 2014 [52] | MI, VA, NY, ME, MS, NC, CA, MA, TX, AZ, SC, TN, IL, RI, CO, CT, OH, AL, LA, OK, HI, ID, NM, KY, IN, UT, PA, WV, WY, ND, NV | general population | Controlled interrupted time series | opioid-related adverse events | NVSS, 1999–2008 |
| Mallatt 2017 [55] c |
NSDUH: 49 states (excluding MO) ARCOS: AK, AL, AR, AZ, CA, CO, CT, DE, FL, GA, HI, IA, ID, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MS, MT, NC, ND, NE, NH, NJ, NM, NV, NY, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WI, West VA, WY NIBRS: 26 states |
general population | Repeated cross-sectional (NSDUH); interrupted time series (NIBRS & ARCOS) | opioid-related legal and criminal outcomesa | National Incident-Based Reporting System (NIBRS), 2004–2014 |
| Maughan 2015 [25] c | Detroit, Phoenix, San Francisco, Denver, Chicago, Boston, Miami, Minneapolis, New York City | general population | Generalized Estimate Equations with repeated measures | opioid-related care outcomes | eDrug Abuse Warning Network (DAWN), 2004–2011 |
| McLaughlin 2016 [28] | AL, AZ, CA, CO, CT, IL, IN, IA, LA, ME, MI, MN, MS, NE, NM, NC, ND, OH, OK, SC, TN, VT, VA, WV, AK, NY, HI, ID, PA, RI, TX, MA, NJ, DE, NV, OR, UT, WA, WY, KS, SD, WI, FL, KY, MT | general population | Repeated cross sectional | illicit opioid usea | NSDUH, 2004–2012 |
| Meara 2016 [48] | AL, AZ, CA, CO, CT, IL, IN, IA, LA, ME, MI, MN, MS, NE, NM, NC, ND, OH, OK, SC, TN, VT, VA, WV, AK, NY, HI, ID, PA, RI, TX, MA, NJ, DE, NV, OR, UT, WA, WY, WY, SD, WI, FL, KY, MT | insured population | Cohort; before and after | opioid-related adverse events | Medicare Provider Analysis and Review (MEDPAR), Outpatient, Carrier, and Medicare Beneficiary Summary files, 2006–2012 |
| Meinhofer 2017 [53] | AL, AK, AZ, AR, CO, CT, DE, FL, GA, IA, WY, LA, ME, MD, MN, MS, MT, NE, NJ, NM, NC, ND, OH, OR, SC, SD, TN, VT, VA, WA, WI WY | general population | Interrupted time series | opioid-related adverse eventsa | NVSS, 2000–2013 |
| Nam 2017 [45] c | AL, AZ, CO, CT, IA, LA, ME, MN, MS, NC, ND, NM, OH, SC, TN, VA, VT, WV, WY | general population | Interrupted time series | opioid-related adverse eventsa | CDC WONDER, 1999–2014 |
| Patrick 2016 [47] b | WA, OR, AZ, NM, CO, WY, MT, SD, NE, WY, MN, IA, AR, LA, MS, AL, GA, FL, SC, NC, TN, VA, WV, OH, MD, DE, NJ, CT, VT, ME, AK, ND, WI, KY | general population | Controlled interrupted time series | opioid-related adverse events | CDC WONDER, 1999–2013 |
| Paulozzi 2011 [46] | CA, HI, ID, IL, IN, KY, ME, MA, MI, NV, NM, NY, OK, PA, TX, UT, VA, WV, WY | general population | Controlled interrupted time series | opioid-related adverse events | CDC WONDER, 1999–2005 |
| Pauly 2018 [49] | 48 states (all but MO and PA), and DC | insured population | Interrupted time series | opioid-related adverse events | Truven Health Marketscan claims database, 2004–2014 |
| Radakrishnan 2015 [39] b | All that fall into 1992–2011 | general population | Interrupted time series/ Repeated cross section | opioid-related care outcomesa, opioid-related adverse eventsa | TEDS, 1992–2010; NVSS, 1999–2010 |
| Reifler 2012 [42] c | CT, MA, ME, NY, PA, RI, AZ, CA, CO, HI, ID, NV, UT, WY, IA, IL, IN, MI, ND, OH, AL, KY, MS, NC, OK, SC, TN, TX, VA, WV, WA | general population | Controlled interrupted time series | opioid-related adverse events, opioid-related care outcomesa | Researched Abuse Diversion and Addiction-Related Surveillance System (RADARS), 2003–2009 |
| Reisman 2009 [19] | CA, HI, IL, IN, MA, MI, NY, OK, TX, ID, KY, NV, RI, UT | people who use drugs admitted to treatment | Cross-sectional (with interrupted time series) | opioid-related care outcomes | TEDS, 2003 |
| Simeone 2006 [41] | 20 states | general population | Prospective cohort study | opioid-related care outcomes | TEDS, 1997 & 2003 |
| Surratt 2014 [26] c | FL | general population | Interrupted time series | opioid-related legal and criminal outcomes | RADARS, 2009–2012 |
aindicated outcome is available for heroin
b study only includes states with PMPs implemented during the study period
c study only focuses on some PMP states or jurisdictions within a country by choice, due to analytic constrictions, or due to availability of data
AL Alabama, AK Alaska, AZ Arizona, AR Arkansas, CA California, CO Colorado, CT Connecticut, DE Delaware, FL Florida, GA Georgia, HI Hawaii, ID Idaho, IL Illinois, IN Indiana, IA Iowa, KS Kansas, KY Kentucky, LA Louisiana, ME Maine, MD Maryland, MA Massachusetts, MI Michigan, MN Minnesota, MS Mississippi, MO Missouri, MT Montana, NE Nebraska, NV Nevada, NH New Hampshire, NJ New Jersey, NM New Mexico, NY New York, NC North Carolina, ND North Dakota, OH Ohio, OK Oklahoma, OR Oregon, PA Pennsylvania, RI Rhode Island, SC South Carolina, SD South Dakota, TN Tennessee, TX Texas, UT Utah, VT Vermont, VA Virginia, WA Washington, WV West Virginia, WI Wisconsin, WY Wyoming, DC District of Columbia
Illicit and problematic opioid use
Two studies reported an association between PDMP status and heroin use, both using the same dataset and presenting adjusted models [24, 28]. These studies drew on multiple years of cross-sectional survey data, using an interrupted time series analysis that captured data from 36 states from 2004 to 2014 [24]. Neither study found any significant associations between PDMP status and heroin use. One study also examined the association between past-year opioid dependence and PDMP status; no significant association was observed [24]. See Additional file 2: Table S1 for individual study details.
Opioid-related care outcomes
Eight studies examined the association between PDMP status and opioid-related care outcomes [19, 25, 27, 39–44]. One study, using CDC WONDER, SID, and SEDD data sets, reported on inpatient discharges in two jurisdictions with PDMPs from 2009 to 2012 using a difference-in-difference approach and found no change in the rate of discharges related to prescription opioids, and a slight increase in discharges related to heroin (β = 0.014, 90% CI [0.001–0.027]) post PDMP implementation in adjusted models [40]. That same study, along with an interrupted time series study on nine states from 2004 to 2011 using the DAWN data set, found no statistically significant associations when examining emergency department visits for all prescription opioids, Schedule II opioids, and heroin over time when comparing PDMP jurisdictions to non PDMP jurisdictions [25, 40].
Seven studies described opioid-related treatment admissions. Six of the seven used the TEDS dataset, while Reifler et al. used the RADARS dataset [19, 27, 39, 41–44]. Branham et al. covered the most years of data from TEDS; they found no association when examining the association between treatment admissions for heroin and PDMP status [27]. Branham et al. and Reifler et al. found no association between PDMP status and prescription opioid treatment admissions [27, 42]. In Branham et al.’s secondary analysis, they examined each of the states that implemented a PDMP during the study period (1992–2012) separately. They found that 13 out of 22 states saw a significant change in the average heroin admissions post PDMP – 10 states saw more admissions and three saw fewer. Furthermore, 11 US states reported a significant increase in average prescription opioid treatment admissions post-PDMP implementation [27]. See Additional file 2: Table S2 for individual study details.
Opioid-related adverse events
Thirteen studies reported on opioid-related adverse events [39, 40, 42, 43, 45–53]. Of those studies, 10 reported on fatal opioid overdoses, with overlapping datasets in multiple studies [39, 40, 43, 45–47, 50, 51, 53, 54]. Four studies reported on heroin-related overdose deaths, none of which found any association with PDMP status in adjusted models [39, 40, 50, 53].
Six studies reported on overdose deaths related to both prescription and non-prescription opioids from two unique data sources – CDC WONDER and NVSS [39, 43, 45, 47, 50–52]. Both primary studies (with the most years of data available) reported no significant associations between opioid-related deaths and PDMP status [43, 45].
Five studies reported on fatal prescription opioid overdoses using three unique data sources – CDC WONDER, NVSS and state-specific inpatient and emergency databases [40, 45–47, 53]. No significant association between PDMP status and fatal prescription opioid overdose deaths was observed in adjusted models in any of these studies [40, 45, 46, 53].
Two studies reported on the association between specific opioid-related deaths and PDMP status [45, 50]. Nam et al. performed an interrupted time series analysis on data from 19 states that implemented PDMPs from 1999 to 2014 and found no association between methadone-related overdoses and PDMP status over time [45]. Delcher et al. performed a controlled interrupted time series analysis from 2003 to 2012 and observed a significant decline in oxycodone-caused overdoses in Florida post-PDMP implementation (p = 0.0079), but not in non-oxycodone related overdoses [50].
Two unique studies examined the association between non-fatal overdose and PDMPs [48, 49]. A study examining cohorts of Medicare beneficiaries for each year from 2006 to 2012 in 45 states found no association between PDMP status and the proportion of insurance beneficiaries experiencing non-fatal prescription opioid overdoses in adjusted models [48]. The second study was an interrupted time series of 49 state PDMPs from 2004 to 2014 and found that at baseline (2004), prescription opioid poisoning rates were higher in PDMP states than non PDMP states; however, the rate of prescription opioid poisonings over time decreased more quickly in PDMP states than in non PDMP states (β = − 0.005, 95% CI [− 0.008- -0.003]) [49]. Similarly, a separate study reported on intentional opioid poisonings for five drugs combined (fentanyl, hydromorphone, methadone, morphine, and oxycodone) and observed that, while rates were higher in PDMP states at baseline, rates reduced at a greater rate per quarter for PDMP states compared to non-PDMP states [42]. See Additional file 2: Table S3 for individual study details.
Opioid-related legal and criminal outcomes
Two unique studies reported on three types of opioid-related criminal outcomes: crime rates, identification of potential dealers, and opioid diversion [26, 55]. Employing standardized, adjusted difference in difference models, no association was found between PDMP status and opioid-related crime rates or the identification of potential opioid dealers [55]. As for diversion, an interrupted time series study from 2009 to 2012 found significant reductions in rates of diversion of oxycodone, methadone, and morphine in over time in Florida post- PDMP implementation [26]. Finally, no significant trends were identified for other measured drugs in this study (fentanyl, hydrocodone, hydromorphone, buprenorphine, and tramadol). See Additional file 2: Table S4 for individual study details.
Risk of Bias assessment
A detailed description of risk of bias assessment across the six QUIPs domains by study and overall can be found in Table 2. Overall, study quality was good; a low risk of bias rating was given for 81.8% of studies on study participation, 100.0% on study attrition, 45.5% on PDMP measurement, 54.5% on outcome measurement, 68.3% on study confounding, and 81.8% statistical analysis and reporting. Nine of the included studies were not published in peer-reviewed journals (i.e. working papers, theses) [28, 39–41, 43, 44, 51, 53, 55]. While this does not necessarily indicate poor study quality, it does indicate that the results should be interpreted with caution as these studies have not undergone a rigorous peer review.
Table 2.
Detailed risk of bias and quality assessment using the QUIPs tool
| Study | Study participation | Study attrition | PMP measurement | Outcome measurement | Study confounding | Statistical analysis and reporting |
|---|---|---|---|---|---|---|
| Ali 2017 [24] | moderate | low | moderate | moderate | low | low |
| Birk 2017 [43] | low | low | moderate | low | low | low |
| Branham 2018 [27] | moderate | low | moderate | low | low | low |
| Dave 2017 [44] | low | low | moderate | moderate | low | low |
| Delcher 2015 [50] | low | low | low | low | low | moderate |
| Kim 2013 [ 51] | low | low | moderate | moderate | low | low |
| Kinsell 2015 [ 40] | low | low | low | low | low | moderate |
| Li 2014 [ 52] | low | low | low | low | low | low |
| Maughan 2015 [ 25] | low | low | low | low | low | low |
| Mallatt 2017 [ 55] | low | low | low | low | low | low |
| McLaughlin 2016 [ 28] | moderate | low | moderate | moderate | low | low |
| Meara 2016 [ 48] | low | low | moderate | moderate | low | low |
| Meinhofer 2017 [ 53] | moderate | low | low | low | high | low |
| Nam 2017 [ 45] | low | low | moderate | moderate | low | low |
| Patrick 2016 [ 47] | low | low | moderate | moderate | moderate | low |
| Paulozzi 2011 [ 46] | low | low | moderate | moderate | moderate | low |
| Pauly 2018 [ 49] | low | low | low | low | low | low |
| Radakrishnan 2015 [ 39] | low | low | moderate | moderate | low | low |
| Reifler 2012 [ 42] | low | low | low | low | moderate | low |
| Reisman 2009 [ 19] | low | low | low | low | moderate | moderate |
| Simeone 2006 [ 41] | low | low | moderate | moderate | high | high |
| Surratt 2014 [ 26] | low | low | low | low | moderate | low |
| Overall % low | 81.8% | 100.0% | 45.5% | 54.5% | 68.2% | 81.8% |
| Overall % moderate | 18.2% | 0.0% | 54.5% | 45.5% | 22.7% | 13.6% |
| Overall % high | 0.0% | 0.0% | 0.0% | 0.0% | 9.1% | 4.6% |
Discussion
In this systematic review we sought to identify associations between PDMP status and opioid-related consequences and harms. Twenty-two publications from 12 unique data sets were analysed. Overall, we did not find evidence to indicate that PDMPs were effective in reducing several types of population-level consequences and harms including illicit opioid use, opioid dependence, ED visits, or inpatient discharges. There were, however, very few studies that measured each of these outcomes.
In individual studies, rates of fatal and non-fatal overdoses were higher at baseline in PDMP states, but reductions were observed after PDMP implementation; however, the relationship overall was less clear. Conflicting evidence was found for the association between treatment admissions and PDMP status, with some studies indicating an increase in admissions, and others finding a decrease. An increase in treatment admissions is not necessarily a poor outcome and could be indicative of more people seeking treatment (rather than more people using opioids) due to intervention from PDMP findings, or other arms of opioid related intervention strategies.
While there were no observed effects for the association of PDMPs with harms and consequences related to opioids, PDMPs, if properly operationalized, can be an important piece of a broader opioid strategy. They may work in tandem with other arms of an opioid strategy, rather than functioning as standalone programs. Many studies did not control for the presence and timing of other interventions in their statistical models, which may have masked estimation of the true effect of PDMPs on opioid-related harms. Equally important, in order for PDMPs to function optimally, healthcare providers must use the data whenever they are prescribing an opioid [56]. A recent evidence synthesis by our team found that only 57% of healthcare providers had ever used PDMP data to inform their prescribing decisions (using data from 26 studies), and fewer than 1 in 5 used a PDMP with each prescription. Interventions aimed at increasing PDMP utilization among healthcare providers would impact opioid-related harms and outcomes over time. None of the included studies considered PDMP utilization by healthcare providers when estimating the effect of PDMPs on outcomes.
While only two studies received a rating of high risk of bias on any domain, there were some areas of concern including study confounding, PDMP implementation, and outcome measurement. A study was rated high risk of bias on the study confounding domain if there was no evidence of accounting for important confounders (including demographic trends, other opioid-related interventions in the jurisdiction, time trends, and features of PDMPs) in the study design or statistical models. Concerns with bias on PDMP/outcome measurement mainly stemmed from the timing of measurement. Measuring exposure or outcome only on an annual basis raised concerns about potential misclassification of PDMP status for outcomes during that year (i.e. a prescription could have been dispensed before a PDMP was implemented, but still marked as occurring during a year where the state had a PDMP). Studies that accounted for PDMP status more frequently (i.e. monthly or quarterly) raised less concerns about misclassification. As the body of evidence evolves, a systematic review should be performed focusing on features of PDMPs, such as mandatory use, and potential relationships with opioid-related harms and consequences. More primary studies are required for certain outcomes of interest, including hospital visits, crime and illicit opioid use.
The last year of data covered by the studies captured in this review was 2014. We need more recent and robust data as the opioid crisis has drastically evolved since then, with more recent focus on the very potent fentanyl. Finally, all studies included in this review were conducted in the United States. Future research should seek to determine the impact of PDMPs on opioid-related consequences and harms in other countries.
Limitations and strengths of the study
This was a rigorously conducted systematic review that synthesized all studies related to the effectiveness of PDMP status in reducing opioid-related harms and consequences. A thorough evaluation of the literature was executed, and the quality of each included study was reviewed to identify any potential biases. We also considered a broad range of patient safety outcomes such as overdose and hospital admissions.
In terms of limitations, we were unable to perform meta-analyses due to heterogeneity across studies and outcomes. Included studies varied in how they measured associations and employed different units of analysis (i.e. person years, state years, states, etc.), populations (i.e. general, insured, treatment, etc.), covariate adjustments, and most importantly, analytic approach. Many studies included in this review used data from TEDS. States contributing data to TEDS can collect either publically- or privately-funded admissions [57]. This variability limits TEDS in its ability to assess admissions outcomes. Additionally, our search was completed in early 2018 and new studies may have been completed. To address this, a single database scan (Ovid MEDLINE) of the literature for 2018 and 2019 was completed by two reviewers (MW and MA) and identified only one potentially relevant study [58]. This well-designed study provides support for the effectiveness of PDMPs, finding that jurisdictions with online PDMPs observed significant reductions in rates of opioid-related hospitalizations.
The objective of this systematic review was to examine the effect of PDMP implementation (initial and over time) on opioid-related harms and consequences. As such, we did not explore outcomes related to other monitored drugs such as benzodiazepines, which may also be affected by PDMP implementation. Furthermore, although some studies accounted for PDMP features, this review did not focus on differences across types of PDMPs or on the effect of legislative changes to PDMP characteristics in regions with pre-existing PDMPs (e.g. mandatory utilization) given the small number of studies for most outcomes of interest.
Conclusions
Although we did not find evidence to strongly support the overall effectiveness of PDMPs in reducing opioid-related consequences and harms, if operationalized appropriately, they remain a valuable piece of a broader strategy to combat the opioid crisis. The mere presence of PDMPs is a reminder to physicians that they need to be careful when prescribing opioids. PDMPs may not necessarily address the root causes of addiction or guide patients directly to treatment options; however, they can be an important tool for minimizing potential harm and should work in tandem with other opioid preventation programs.
Additional files
Additional file 2: Table S1. Illicit and problematic opioid use. Table S2. Opioid-related care outcomes. Table S3. Opioid-related adverse events. Table S4. Opioid-related legal and crime outcomes.
Acknowledgements
We would like to acknowledge Leah Boulos for her work in developing the search strategy, and guidance in systematic review methods.
Abbreviations
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CDC WONDER
Centers for Disease Control and prevention Wide-ranging ONline Data for Epidemiologic Research
- CIHI
Canadian Institute for Health Information
- CINAHL
Cumulative Index to Nursing & Allied Health Literature
- CMA
Canadian Medical Association
- ED
Emergency Department
- MeSH
Medical Subject Headings
- NVSS
National Vital Statistics System
- OUD
Opioid Use Disorder
- PDMP
Prescription Drug Monitoring Program
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- QUIPS
QUality In Prognosis Studies
- RADARS
Researched Abuse Diversion and Addiction-Related Surveillance system
- RCT
Randomized Controlled Trial
- SEDD
State Emergency Department Databases
- SID
State Inpatient Databases
- TEDS
Treatment Episode Data Set
Authors’ contributions
MA, JH, and MW designed the study. ER and MW conducted the systematic review and analysed the data with help from AR and MA. ER wrote the manuscript, and then MA, JH, and MW critically revised it before all authors read and approved the final version. All authors have read and approved the manuscript.
Funding
This research was funded by the Canadian Institutes of Health Research Operating Grant: Opioid Crisis Knowledge Synthesis/Subvention (#397982). The funder had no role in the design of the study, data collection, data analysis, data interpretation, nor in the writing of the manuscript.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emily Rhodes, Email: erhodes@ohri.ca.
Maria Wilson, Email: maria.wilson@dal.ca.
Alysia Robinson, Email: alysia.robinson@dal.ca.
Jill A. Hayden, Email: jhayden@dal.ca
Mark Asbridge, Email: mark.asbridge@dal.ca.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12913-019-4642-8.
References
- 1.Jones CM, Campopiano M, Baldwin G, Mccance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55–e64. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhalla IA, Persaud N, Juurlink DN. Facing up to the prescription opioid crisis. BMJ. 2011;343:1–5. doi: 10.1136/bmj.d5142. [DOI] [PubMed] [Google Scholar]
- 3.Rummans TA, Burton MC, Dawson NL. How good intentions contributed to bad. Mayo Clin Proc. 2018;93(3):344–350. doi: 10.1016/j.mayocp.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 4.National Drugs Technical Report: Estimated world requirements for 2013: statistics for 2011 [internet]. 2012. Available from: https://www.incb.org/incb/en/narcotic-drugs/Technical_Reports/2012/narcotic-drugs-technical-report_2012.html
- 5.Brat GA, Agniel D, Beam A, Yorkgitis B, Bicket M, Homer M, et al. Postsurgical prescriptions for opioid naive patients and a ssociation with overdose and misuse : retrospective cohort study. BMJ. 2018;360:1–9. doi: 10.1136/bmj.j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imtiaz S, Shield KD, Fischer B, Rehm J. Harms of prescription opioid use in the United States. Subst Abuse Treat Prev Policy. 2014;9(43):1–5. doi: 10.1186/1747-597X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnert A, Valenstein M, Bair MJ, Ganoczy D, Mccarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 8.Dunn K, Saunders K, Rutter C, Al E. Overdose and prescribed opioids: associations among chronic non-cancer pain patients, Ann Intern Med. 2010;152(2):85–92 Available from: 10.1059/0003-4819-152-2-201001190-00006.
- 9.Borwein A, Kephart G, Whelan E, Asbridge M. Prescribing Practices Amid the OxyContin Crisis: Examining the Effect of Print Media Coverage on Opioid Prescribing Among Physicians. J Pain. 2013;14(12):1686–93.e1. doi: 10.1016/j.jpain.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Fischer B, Rehm J. Deaths related to the use of prescription opioids. CMAJ. 2009;181(12):881–882. doi: 10.1503/cmaj.091791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD. Economic costs of nonmedical use of Prescription opioids. Clin J Pain. 2011;27(3):194–202. doi: 10.1097/AJP.0b013e3181ff04ca. [DOI] [PubMed] [Google Scholar]
- 12.Prescription SB. Canadian Centre on Substance Abuse. 2015. Monitoring Programs in Canada: Best Practice and Program [Internet] pp. 209–210. [Google Scholar]
- 13.Furlan AD, Frcpc PM, Pellerin D, Shaw K, Bsp DS, Wilson G, et al. Overview of four prescription monitoring / review programs in Canada. Pain Res Manag. 2014;19(2):102–106. doi: 10.1155/2014/634171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandeis University . Technical assistance guide: history of Prescription drug monitoring programs [internet] 2018. [Google Scholar]
- 15.Egan JE, Casadonte P, Gartenmann T, Martin J, Mccance-katz EF, Netherland J, et al. The physician clinical support system-buprenorphine ( PCSS-B ): a novel project to expand / improve buprenorphine treatment. J Gen Intern Med. 2010;25(9):936–941. doi: 10.1007/s11606-010-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devonshire E, Nicholas MK. Continuing education in pain management : using a competency framework to guide professional development. Pain reports. 2018;3(5):1–7. doi: 10.1097/PR9.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggertson L. Take-home naloxone kits preventing overdose deaths. CMAJ. 2014;186(1) Available from: 10.1503/cmaj.109-4663. [DOI] [PMC free article] [PubMed]
- 18.Ashburn MA. The evolution of prescription drug monitoring programs. Pharmacoepidemiol Drug Saf. 2016;25:852–853. Available from: 10.1002/pds.4036. [DOI] [PubMed]
- 19.Reisman RM, Shenoy PJ, Atherly AJ, Flowers CR. Prescription opioid usage and Abuse relationships : an evaluation of state Prescription drug monitoring program efficacy. Subst Abus Res Treat. 2009;3:41–51. doi: 10.4137/sart.s2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.State Profiles [Internet]. 2018. Available from: https://www.pdmpassist.org/content/state-profiles
- 21.Maryland Department of Health. Maryland Advisory Council on Prescription Drug Monitoring Legislative Report. Baltimore, MD; 2009.
- 22.Finley EP, Garcia A, Rosen K, Mcgeary D, Pugh MJ, Potter JS. Evaluating the impact of prescription drug monitoring program implementation : a scoping review. BMC Health Serv Res. 2017;17(420):1–8. doi: 10.1186/s12913-017-2354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink DS, Schleimer JP, Sarvet A, Grover KK, Delcher C, Castillo-carniglia A, et al. Association between Prescription drug monitoring programs and nonfatal and fatal drug overdoses a systematic review. Ann Intern Med. 2018;168(11):783–790. doi: 10.7326/M17-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali MM, Dowd WN, Classen T, Mutter R, Novak SP. Addictive Behaviors Prescription drug monitoring programs , nonmedical use of prescription drugs , and heroin use : Evidence from the National Survey of Drug Use and Health. Addict Behav. Elsevier Ltd; 2017;69:65–77. Available from: 10.1016/j.addbeh.2017.01.011 [DOI] [PubMed]
- 25.Maughan BC, Bachhuber MA, Mitra N, Starrels JL. Prescription monitoring programs and emergency department visits involving opioids , 2004–2011. Drug Alcohol Depend, Available from: 10.1016/j.drugalcdep.2015.09.024, Elsevier Ireland Ltd. 2015;156(January):282–8. [DOI] [PMC free article] [PubMed]
- 26.Surratt HL, Grady CO, Kurtz SP, Stivers Y, Cicero TJ, Dart RC, et al. Reductions in prescription opioid diversion following recent legislative interventions in Florida. Pharmacoepidemiol Drug Saf. 2014;23:314–320. doi: 10.1002/pds.3553. [DOI] [PubMed] [Google Scholar]
- 27.Branham DK. Time-series analysis of the impact of Prescription drug monitoring programs on heroin treatment admissions time-series analysis of the impact of Prescription drug monitoring programs on heroin treatment admissions. Subst Use Misuse. 2018;53(4):694–701. doi: 10.1080/10826084.2017.1363232. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin S. Empirical Essays to inform marijuana legalization and Prescription opioid Abuse policy [internet] 2016. [Google Scholar]
- 29.Veritas Health Innovation . Covidence Systematic Review Software. 2017. [Google Scholar]
- 30.PRISMA . PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses [Internet] 2015. [Google Scholar]
- 31.Higgins J, Green S. Cochrane handbook for systematic reviews for interventions. The Cochra. West Sussex, England: John Wiley & Sons Ltd.; 2008. [Google Scholar]
- 32.Fleming M, Barner J, Bamgbade Mortality rate comparison between states with and without prescription drug monitoring programs and association federal costs [Abstract] Value Health. 2010;13(3):A93. doi: 10.1016/S1098-3015(10)72444-X. [DOI] [Google Scholar]
- 33.Menchine M, Georgis M, Lam C, Seabury S. Do state prescription drug monitoring programs reduce opioid consumption? [abstract] Ann Emerg Med. 2016;68(4):S79. [Google Scholar]
- 34.Miller D, Miller L, Franger S. Reduction of opioid and illicit drug abuse after initiation of state electronic prescription monitoring program [abstract] Pain Med. 2009;10(1):275–276. doi: 10.1111/j.1526-4637.2008.00492.x. [DOI] [Google Scholar]
- 35.Painter J. Effect of prescription monitoring programs (PMP’s) on opioid overdose admission [abstract] Value Health. 2011;14(3):A197–A198. doi: 10.1016/j.jval.2011.02.1089. [DOI] [Google Scholar]
- 36.Hayden JA, Van Der Windt DA, Cartwright JL, Co P. Research and reporting methods annals of internal medicine Assessing Bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 37.Microsoft . Excel. 2016. [Google Scholar]
- 38.StataCorp . Stata 15. 2017. [Google Scholar]
- 39.Radakrishnan S, ESSAYS IN. THE ECONOMICS OF RISKY HEALTH BEHAVIORS [Internet]: Cornell University; 2015. Available from: http://search.proquest.com/docview/1661461833/
- 40.Kinsell H, ASSESSING THE, EFFECTS OF. FLORIDA ’ S ANTI -PILL MILL LAW ON PRESCRIPTION DRUG RELATED HEALTH OUTCOMES [Internet]: University of Florida; 2015. Available from: http://search.proquest.com/docview/1727613694/
- 41.Simeone R, Holland L. An Evaluation of Prescription Drug Monitoring Programs. 2006. [Google Scholar]
- 42.Reifler LM, Droz D, Bailey JE, Schnoll SH, Fant R, Dart RC, et al. State trends in opioid Abuse / misuse ? Pain Med. 2012;13:434–442. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 43.Birk E, Waddell G. The mitigating role of Prescription drug monitoring programs in the Abuse of Prescription drugs. IZA Inst Labor Econ. 2017;10990:1–42. [Google Scholar]
- 44.Dave DM, Grecu AM, Saffer H. MANDATORY ACCESS PRESCRIPTION DRUG MONITORING PROGRAMS AND PRESCRIPTION DRUG ABUSE [Internet] Cambridge: MA; 2017. [PubMed] [Google Scholar]
- 45.Nam YH, Shea DG, Shi Y, Moran JR. State Prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care. 2017;23(5):297–303. [PubMed] [Google Scholar]
- 46.Paulozzi LJ, Kilbourne EM, Desai HA, Sci MM, Church F, Hamilton BA. Prescription drug monitoring programs and death rates from drug overdose. Pain. 2011;12:747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 47.Patrick SW, Fry CE, JT F, Buntin MB. Implementation of Prescription drug monitoring programs associated with reductions in opioid- related death rates. Health Aff. 2016;35(7):1324–1332. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley AJ, et al. State legal restrictions and Prescription- opioid use among disabled adults. N Engl J Med. 2016;375:44–53. doi: 10.1056/NEJMsa1514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauly NJ, Slavova S, Delcher C, Freeman PR, Talbert J. Features of prescription drug monitoring programs associated with reduced rates of prescription opioid-related poisonings. Drug Alcohol Depend. 2018;184:26–32. Available from: 10.1016/j.drugalcdep.2017.12.002. Elsevier [DOI] [PMC free article] [PubMed]
- 50.Delcher C, Wagenaar AC, Goldberger BA, Cook RL, Maldonado-molina MM. Abrupt decline in oxycodone-caused mortality after implementation of Florida’s Prescription drug monitoring program. Drug Alcohol Depend. 2015;150:63–68. doi: 10.1016/j.drugalcdep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Kim M. THE IMPACT OF PRESCRIPTION DRUG MONITORING PROGRAMS ON OPIOID-RELATED POISONING DEATHS [Internet]: Johns Hopkins University; 2013. Available from: http://search.proquest.com/docview/1432503368/
- 52.Li G, Brady JE, Lang BH, Giglio J, Wunsch H, Dimaggio C. Prescription drug monitoring and drug overdose mortality. Inj Epidemiol. 2014;1:1–8. doi: 10.1186/2197-1714-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meinhofer A. Prescription drug monitoring programs : the role of asymmetric information on drug availability and Abuse. 2017. pp. 1–48. [Google Scholar]
- 54.Lin DH, Lucas E, Murimi IB, Jackson K, Baier M, Frattaroli S, et al. Physician attitudes and experiences with Maryland ’ s prescription drug monitoring program ( PDMP ) Addiction. 2016;112:311–319. doi: 10.1111/add.13620. [DOI] [PubMed] [Google Scholar]
- 55.Mallatt J. The Effect of Prescription Drug Monitoring Programs on Opioid Prescriptions and Heroin Crime Rates. 2017. [Google Scholar]
- 56.Asbridge M, Hayden J, Campbell S, Wilson M, Macdougall P. Utilization and effectiveness of Prescription monitoring programs : a systematic review (CIHR Report) Halifax: In Press; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Substance Abuse & Mental Health Data Archive. Treatment Episode Data Set: Admissions (TEDS-A) [Internet]. Available from: https://www.datafiles.samhsa.gov/study-series/treatment-episode-data-set-admissions-teds-nid13518
- 58.Castillo-Carniglia A, Ponicki WR, Gaidus A, Gruenewald PJ, Marshall BD, Fink DS, Martins SS, Rivera-Aguirre A, Wintemute GJ, Cerdá M. Prescription drug monitoring programs and opioid overdoses: exploring sources of heterogeneity. Epidemiology. 2019;30(2):212–220. doi: 10.1097/EDE.0000000000000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Table S1. Illicit and problematic opioid use. Table S2. Opioid-related care outcomes. Table S3. Opioid-related adverse events. Table S4. Opioid-related legal and crime outcomes.
Data Availability Statement
Not applicable.


