Abstract
We describe an isolation method of tumor-infiltrating lymphocytes (TILs) from glioblastoma tumors for the purpose of analysis by flow cytometry. This protocol is unique from many others in that the use of a selective lymphocyte isolation procedure, such as a Ficoll or Percoll gradient, is not used. We find that staining of TILs and analysis by flow cytometry is not affected by the presence of heterogeneous populations, while other selective isolation procedures can significantly decrease lymphocyte yield from already rare populations.
Keywords: Tumor-infiltrating lymphocyte (TIL) isolation, Stomacher, Brain tumor, Flow cytometry
1. Introduction
This chapter describes a relatively rapid isolation of tumor-infiltrating lymphocytes (TILs) from brain tumors, as well as procedures for functional profiling and flow cytometry following TIL isolation. There are many procedures for TIL isolation described in the literature for a variety of solid tumors [1–3], though we have found that the following protocol is best suited to brain tumors, including glioblastoma (GBM). Other TIL isolation procedures include use of Ficoll gradient, magnetic beads, or FACS sorting and result in significant loss of the population of interest. This loss is of particular importance in the setting of brain tumors due to lack of overwhelming T cell infiltration. For this reason, we have omitted these selective steps in our procedure. We find that staining for surface and intracellular markers on T cells by flow cytometry is not affected by the presence of either tumor or brain cells. Although more selective methods may be appropriate if gene expression analysis, proteomic profiling, or other investigations requiring a homogeneous end product are pursued, this protocol works exceedingly well for flow cytometric analyses of TILs.
Briefly, this method entails preparing the materials needed (Subheading 3.1), harvesting the brain tumor (Subheading 3.2), mechanically and enzymatically digesting the tumor (Subheading 3.3), lysing red blood cells (RBCs) and preparing the TILs (Subheading 3.4), stimulating the TILs in the presence of phorbol 12-myristate 13-acetate (PMA)/ionomycin if intracellular cytokine staining is desired (Subheading 3.5), as well as washing and preparing the cells for staining (Subheading 3.6). Additionally, we provide insight regarding acquisition of the samples and gating strategies on a cytometer (Subheading 3.7).
2. Materials
2.1. Equipment
Surgical instruments including 4.5″ scissors and two sets of 4.75″ forceps.
Stomacher® Biomaster 80 (Seward).
Seward Stomacher® Biomaster 80 standard bags (BA6040) and closure bags (BA6040/CLR).
Heat sealer.
0.45 μm syringe filter.
40 μm cell strainer.
2.2. Medium
T Cell Medium (TCM): 500 mL RPMI 1640, 50 mL FBS, 5.5 mL Pen/Strep, 5.5 mL NEAA, 5.5 mL Na-pyruvate, 5.5 mL glutamine, 550 μL β-mercaptoethanol, 550 μL gentamycin.
Digestion Buffer composition (per bag per tumor): 15 mL TCM, 40 μL of 50 mg/mL stock of DNase I grade II from bovine pancreas (Roche Cat#10104159001), 100 μL of 20,000 unit/mL stock Collagenase Type IV powder (Gibco-Life Technologies Cat#17104–019), both prepared in distilled water (DW). To prepare the digestion buffer, add 40 μL of 50 mg/mL of DNase stock dissolved in DW into 15 mL of TCM. Collagenase will need to be passed through a 0.45 μm syringe filter once dissolved in DW. In order to do this, add 900 μL digestion buffer into the Eppendorf containing 100 μL of collagenase at 20,000 units/mL in DW. Aspirate all the contents into a 1 mL syringe, remove the needle, replace with the syringe filter, and plunge the contents into the digestion buffer. Replace the needle onto the syringe and aspirate 1 mL of prepared digestion buffer, and plunge through the syringe filter once more to ensure all collagenase has passed through the filter into the medium.
Stimulation Medium: TCM, 500 nM ionomycin, 50 nM PMA.
FACS Buffer: PBS, 2% fetal bovine serum (FBS).
RBC Lysis buffer: 10× BD Pharm Lyse™ buffer (BD #555899) diluted in DW.
1% w/v Paraformaldehyde (PFA) in FACS buffer.
2.3. Other Reagents
Live/Dead Fixability Stain.
Antibodies for flow cytometry.
3. Methods
3.1. Prepare Materials
Place the Stomacher Biomaster 80 into a room maintained at 37 °C.
Prepare the Stomacher bags: place one standard bag inside a closure bag. Heat-seal the bottom twice (see Note 1).
Place prepared digestion buffer in 37 °C water bath.
Prepare tissue harvest materials: Place 3 mL of digestion buffer into a small petri dish or a well of a 6-well plate. Prepare the surgical instruments and surgical surface. Additionally, prepare appropriate medium and supplies for other tissue harvests (spleens for compensation and/or stimulation positive control, cervical lymph nodes, etc.).
3.2. Harvest the Tumor (See Note 2)
Euthanize the mouse.
Make an incision in the skin from ears to nose.
Stabilize the head and retract the skin with forceps.
Make an incision from the base of the skull to the nose with scissors.
Use a second pair of forceps to peel off the skull on the side of the hemisphere in which the tumor is located.
Use forceps to remove obvious tumor tissue or use scissors to cut through the midline and harvest the entire hemisphere. There are no lymphocytes in naïve brain, so with small tumors or tumors that are harder to see, harvesting the entire hemi-sphere may be appropriate.
Place tissue into the prepared dish with 3 mL of digestion buffer.
3.3. Digest the Tumor
Cut the tumor into small enough pieces that will fit through the tip of a 10 mL serological pipet.
Add 6 mL of digestion medium to the dish, and aspirate all the tumor pieces and medium. Place into the prepared stomacher bag. Make sure you put the suspension into the inner bag, and not between the inner and outer bags.
Rinse the dish or well with 6 mL of remaining digestion buffer, and place into the bag (15 mL total digestion buffer per bag per tumor).
Heat-seal the top of the bag twice. Minimize air pockets.
Place the bag into the Stomacher at 37 °C, turn the machine on, select “Normal” speed and “infinity” time. You can digest up to two bags at a time. Incubate for 20 min.
3.4. Lyse RBCs and Prepare the TILs
Cut the bag open with scissors and pass contents through a 40 μm cell strainer into a 50 mL conical tube. Rinse the bag twice with TCM.
Spin down at 500 × g for 5 min. Aspirate or pour off the supernatant.
Resuspend each tumor in 3 mL of 1× RBC Lysis buffer. Incubate at room temperature for 5 min.
Add 1× PBS to the top of the 50 mL conical and spin down at 500 × g for 5 min.
If stimulating, continue with the following directions. If staining directly, skip to Subheading 3.6.
Resuspend in 5 mL of TCM and count on a hemocytometer. Count only bright, round cells indicative of lymphocytes.
3.5. Stimulate with PMA/Ionomycin (Proceed to Subheading 3.6 If Not Stimulating and Staining for Intracellular Cytokine Secretion)
Resuspend cells at 2 × 106 cells/mL in either plain TCM (unstimulated) or TCM supplemented with 500 nM ionomycin and 50 nM PMA.
Plate 2 mL in a 24-well tissue culture plate.
Place the plate in a 37 °C incubator.
Add 1 μL/mL of brefeldin A after 30 min of incubation (see Note 3).
Incubate for 5.5 more hours.
Harvest cells, washing the well twice with PBS.
3.6. Stain
Wash twice in PBS.
Perform Live/Dead staining at a concentration of 1 × 106 cells/mL in PBS. Stain at room temperature in the dark for 15 min.
Wash once with PBS. Wash again with FACS buffer.
Resuspend in 100 μL of FACS buffer and stain with antibodies of choice on ice. 1 × 106 cells/well is sufficient.
Wash twice with FACS buffer.
Resuspend in 200 μL of 1% PFA in FACS buffer.
3.7. Acquire Samples
When acquiring samples on a flow cytometer, set your initial voltages on unstained splenocytes. Then, acquire an unstained TIL population and ensure >90% of the events are being acquired. It is often difficult to see the lymphocyte population in the TIL sample. The population is easier to identify upon subsequent analysis on FlowJo. For this reason, we set the stopping gate on >1 × 106 cells to ensure that the entire sample is collected and maximize acquisition of lymphocytes (see Notes 4–6).
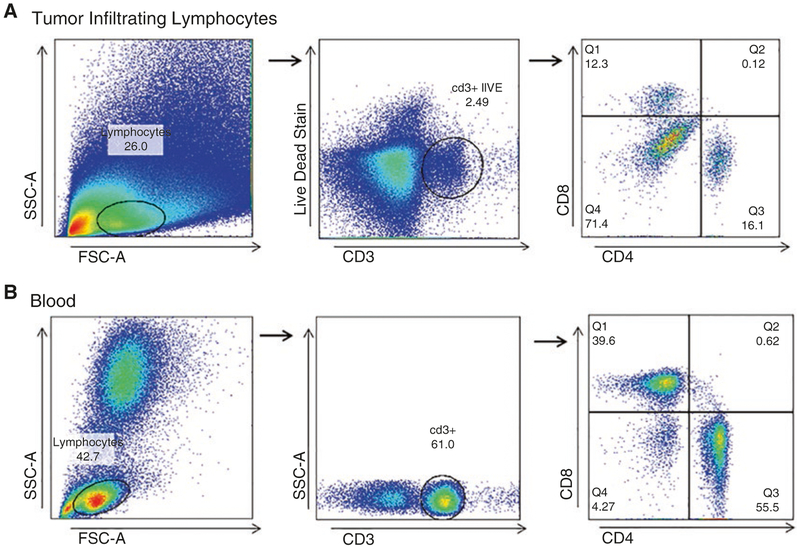
For subsequent analysis on FlowJo, we recommend using the gating strategy depicted in Fig. 1.
Fig. 1.
Gating Strategy for TILs and Blood. (a) Tumor Infiltrating Lymphocytes (TILs) should always be stained at minimum with a Live/Dead discriminator, CD3, CD4, and CD8. With this strategy, the first gate excludes non-lymphocyte populations based on forward and side scatter (FSC and SSC). Following this, CD3+ Live cells are selected. The CD8 by CD4 gate helps distinguish true CD8+ or CD4+ cells and excludes the double negative or positive autofluorescent cells. (b) Blood is gated in a similar manner, with the exception of the Live/Dead stain, which in many cases is not necessary
4. Notes
Leaking Stomacher bag: Ensure that the temperature of the sealer is high enough to appropriately seal the bags together. You should not be able to separate the inner from the outer bag after heat sealing. Additionally, ensure there is as little air as possible remaining between the bottom and top heat seals.
This procedure is also applicable to freshly collected GBM operative specimens.
Brefeldin A arrests protein trafficking through the secretory pathway and therefore allows for staining of intracellular cytokines.
Too few or no lymphocytes: This is a common problem. Some tumors simply do not have many infiltrating lymphocytes, particularly early in the time course. We find that the number of T cells present in tumors increases over time, with the maximal amount of recoverable T cells present when the mouse is moribund. To maximize T cell recovery, we suggest running as much sample as possible through the cytometer.
Antibody staining did not work or is too dim: We do not isolate lymphocytes on a Percoll column because often there are too few TILs from a tumor to begin with, and the additional loss from a Percoll column would leave us with too few cells for further experiments. However, our protocol results in a thick cell pellet that contains tumor cells, brain tissue, and other cell types that pass through the 40 μm filter. Because of this, you may find that you need to increase your antibody concentration from the recommended amount.
The flow cytometer has clogged: To prevent clogs, run the samples on “low” if using tubes or on 1 μL/s if using a high-throughput plate. Acquisition times of 5 min per sample are typical. After each sample, run 30 s of water if using tubes or set the wash volume to >800 μL if using a high-throughput plate. Additionally, watch the machine carefully and if the acquisition rate drops to less than ten events per second, immediately stop the acquisition, prime the machine in either tube or plate mode, and run at least 1 min of water.
References
- 1.Garaud S, Gu-Trantien C, Lodewyckx JN, Boisson A, De Silva P, Buisseret L, Migliori E, Libin M, Naveaux C, Duvillier H, Willard-Gallo K (2014) A simple and rapid protocol to non-enzymatically dissociate fresh human tissues for the analysis of infiltrating lymphocytes. J Vis Exp (94). 10.3791/52392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gigante M, Cox SN, Ranieri E (2014) Negative and positive separation techniques for the isolation of antigen-specific CD8(+) T cells from blood and tumor tissue. Methods Mol Biol 1186:1–11. 10.1007/978-1-4939-1158-5_1 [DOI] [PubMed] [Google Scholar]
- 3.Toh B, Nardin A, Dai X, Keeble J, Chew V, Abastado JP (2013) Detection, enumeration, and characterization of immune cells infiltrating melanoma tumors. Methods Mol Biol 961:261–277. 10.1007/978-1-62703-227-8_17 [DOI] [PubMed] [Google Scholar]