Abstract
The dynamic nature of protein posttranslational modification (PTM) allows cells to rapidly respond to changes in their environment, such as nutrition, stress, or signaling. Lysine residues are targets for several types of modifications, including methylation, ubiquitination, and various acylation groups, especially acetylation. Currently, one of the best methods for identification and quantification of protein acetylation is immunoaffinity enrichment in combination with high-resolution mass spectrometry. As we are using a relatively novel and comprehensive mass spectrometric approach, data-independent acquisition (DIA), this protocol provides high-throughput, accurate, and reproducible label-free PTM quantification. Here we describe detailed protocols to process relatively small amounts of mouse liver tissue that integrate isolation of proteins, proteolytic digestion into peptides, immunoaffinity enrichment of acetylated peptides, identification of acetylation sites, and comprehensive quantification of relative abundance changes for thousands of identified lysine acetylation sites.
Keywords: Acetylation, Posttranslational modifications, Mass spectrometry, Data-independent acquisition, Quantification
1. Introduction
Proteomics technology has become the best method to determine changes in relative protein abundances, but also to measure changes in protein posttranslational modifications (PTM). Many unique protein forms with different PTMs, called proteoforms, may exist in parallel, and the quantity of each proteoform is often highly dynamic. Lysine acetylation is one of the most common PTMs among cellular proteins and regulates a variety of physiological processes including enzymatic activity, protein-protein interactions, gene expression, and subcellular localization [1].
Modification of lysine residues by acetylation was first studied over 50 years ago [2, 3]. In recent years, more extensive proteomic acetylation studies revealed previously unappreciated roles for lysine acetylation in the regulation of diverse cellular pathways, and particularly of mitochondrial proteins [4]. Furthermore, it has been demonstrated how drastically the mitochondrial acetylome can be altered, such as through deacetylation by the sirtuin SIRT3 or via nutritional changes [5, 6].
Due to relatively low levels of acetylation modifications and low lysine site occupancies, mass spectrometric identification of acetylation sites is not trivial, and antibody-based enrichment strategies have proven to be highly effective to gain deeper insights into the dynamic acetylome. However, these workflows are challenging due to multiple processing steps that must be highly quantitative and reproducible, and there is a great need for more standardized protocols. Additionally, affinity enrichment protocols often require a high amount of protein lysate input material, which can be a challenge when limited amounts of starting materials are available. For example, the original Cell Signaling Technologies “PTMScan Acetyl-Lysine Motif [Ac-K] Immunoaffinity Bead” protocol recommends the use of 10–20 mg protein lysate as starting material. To overcome these limitations and enable more routine and robust identification and quantification of protein acetylation sites, here we present a detailed protocol describing a high-throughput and quantitative proteomic approach using 1–5 mg protein lysate for acetyl peptide enrichment (also see Note 1). Our method utilizes optimized affinity enrichment in combination with a comprehensive, label-free data-independent acquisition (DIA) mass spectrometric workflow, and several open-source data processing software tools (see Fig. 1). This protocol can accommodate relatively low amounts of starting material and results in the discovery of regulated acetylation sites in proteins via an unbiased acetylomics approach, thus enabling subsequent experiments to decipher the biological significance of site-specific protein acetylation.
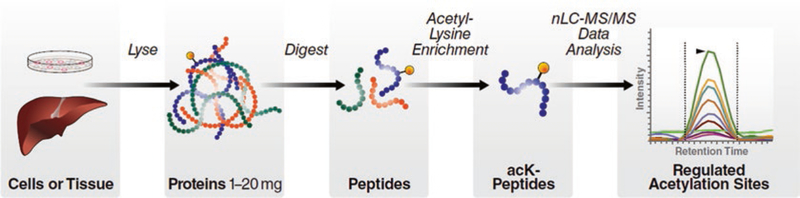
Fig. 1.
Acetyl-lysine immunoaffinity enrichment and label-free quantification workflow
Tissues, such as liver (or similarly, isolated mitochondria or cell lines), are lysed and soluble protein is obtained (1–20 mg), which is subsequently proteolytically digested into tryptic peptides. Acetylated peptides are immunoaffinity enriched using anti-acetyl-lysine antibodies. Enriched peptides are then analyzed by nanoflow liquid chromatography coupled to tandem mass spectrometry (nLC-MS/MS) in data-independent acquisition (DIA) mode. Quantification and analysis is performed using open-source software tools, including Skyline.
2. Materials
2.1. Tissue Lysis and Tryptic Protein Digestion
Mouse liver tissue (wild-type B57BL).
2 mL Safe-Lock Tubes (Eppendorf).
Lysis buffer: 8 M Urea in 100 mM triethylammonium bicarbonate (TEAB), pH 8.5 containing 1× HALT protease inhibitor cocktail (Pierce), deacetylase inhibitors: 5μM trichostatin A (TSA), 5 mM nicotinamide, and 75 mM sodium chloride (NaCl).
QIAGEN TissueLyser II and stainless steel beads, 5 mm (QIAGEN).
Bioruptor sonicator (Diagenode).
Bicinchoninic acid (BCA) protein assay (Pierce Thermo Scientific).
Reducing Reagent: 1 M dithiothreitol (DTT), freshly prepared in deionized water/MilliPORE (referred to as H2O).
Alkylation Reagent: 200 mM iodoacetamide (IAA), freshly prepared in H2O.
Dilution buffer: 50 mM TEAB in H2O.
Digestion enzyme: Modified sequencing-grade trypsin (Promega).
2.2. Desalting of Proteolytic/Tryptic Peptides After Digestion (Oasis/ HLB)
Oasis HLB (Hydrophilic-Lipophilic-Balanced) 1 cc Vac Cartridge, 30 mg Sorbent per Cartridge, 30 μm particle size (Waters Technologies Corporation).
Extraction manifold, 20-port vacuum manifold (Waters Technologies Corporation).
HPLC MS-grade acetonitrile (ACN) and water (H2O) (Burdick and Jackson).
HPLC MS-grade formic acid (FA) (Sigma-Aldrich).
HLB Solvent A: 0.2% FA in HPLC-MS grade H2O.
HLB Solvent B: 80% ACN/20% of 0.2% FA in HPLC-MS grade H2O.
2.3. Anti-Acetyl Immunoaffinity Enrichment
PTMScan Acetyl-Lysine Motif [Ac-K] Immunoaffinity Beads (Cell Signaling Technologies).
PTMScan Immunoaffinity (IAP) Buffer: 50 mM MOPS, 10 mM Na3PO4, 50 mM NaCl in water at pH 7.2 (Cell Signaling Technologies).
1× Phosphate-buffered saline (PBS): 0.01 M phosphate- buffered saline (0.0027 M KCl, 0.138 M NaCl) at pH 7.4 at 25 °C (Sigma-Aldrich).
Wide-bore 200 μL pipet tips (VWR International).
2.4. Small-Scale Acetyl-Peptide Desalting Prior to MS Analysis
Empore Octadecyl (C18) 47 mm Extraction Disks (3 M).
18-Gauge blunt-tipped needle and plunger.
VWR 200 μL low-binding pipet tips (VWR International).
MulTI SafeSeal Sorenson 0.65 mL microcentrifuge tubes (VWR International).
Snap Cap Low Retention 1.5 and 2 mL graduated microcentrifuge (Eppendorf) tubes (Thermo Scientific).
StageTip Solvent A: 0.2% FA in HPLC-MS grade H2O.
StageTip Solvent B: 0.2% FA in 50% HPLC-MS grade ACN in HPLC-MS H2O.
2.5. Chromatography and Mass Spectrometry: Nanoflow HPLC-MS/ MS
All HPLC-MS/MS buffers are “HPLC-MS grade” (all Burdick and Jackson).
Mobile Phase A: 2% ACN/98% water/0.1% formic acid (v/v/v).
Mobile Phase B: 98% ACN/2% water/0.1% formic acid (v/v/v).
Nanoflow liquid chromatography: Ultra Plus nano-LC 2D HPLC (Eksigent) connected to a cHiPLC system (Eksigent) with a C18 pre-column chip (200 μm × 0.4 mm ChromXP C18-CL chip, 3 μm, 120 Å, SCIEX), and an analytical C18 column chip (75 μm × 15 cm ChromXP C18-CL chip, 3 μm, 120 Å).
Mass Spectrometer: quadrupole time-of-flight (QqTOF): TripleTOF 6600 system (SCIEX) or other high-resolution mass spectrometry systems.
3. Methods
3.1. Tissue Lysis and Tryptic Protein Digestion
Harvest mouse liver and take ~50 mg tissue (wet weight) and process immediately or freeze at −80 °C.
Chill TissueLyser adapter sets to −20 °C or set on dry ice.
Add frozen tissue and one stainless steel bead to each of the labeled and chilled Safe-Lock tubes (Eppendorf) over dry ice.
Add 400 μL ice-cold lysis buffer containing protease and deacetylase inhibitors to each of the tubes containing tissue and bead.
Vortex briefly to ensure that the lysis buffer volume covers the whole tissue. If the tissue is large and is not fully submerged add more lysis buffer in increments of 50 μL until the tissue is fully covered with buffer.
Place tubes on chilled adapter sets and ensure balancing the tubes among the 2 adapters. Homogenize with TissueLyser II at 30 Hz two times for 3 min at 4 °C.
Remove bead with tweezer. Clean tweezer with deionized water and HPLC-grade methanol and dry in order to prevent cross-contamination.
Spin briefly to collect all volume to the bottom of the tube.
Sonicate on Bioruptor sonicator (Diagenode) for 10 cycles of 30 s ON/30 s OFF at 4 °C at high power.
Centrifuge homogenized tissue lysate for 10 min, at 14,000 × g, at 4 °C.
Transfer supernatant to new 1.5 mL tubes while avoiding any lipid and fat layer above the cleared lysate and any pellet at the bottom of the tube.
Determine protein concentration using the BCA assay.
Remove an aliquot of lysate containing 5 mg of soluble protein according to BCA assay (or more input material if available, e.g., 20 mg, see Note 1), and add DTT to a final concentration of 4.5 mM to reduce disulfide bonds for 30 min at 37 °C with 3.5 × g agitation (Eppendorf ThermoMixer).
Cool reduced protein lysate to room temperature (RT), and add IAA to a final concentration of 10 mM to alkylate free thiols. Allow reaction to proceed at RT in the dark for 30 min.
Dilute reduced and alkylated proteins tenfold with 50 mM TEAB.
Add trypsin to initiate protein digestion (enzyme:protein ratio = 1:50, wt/wt) at 37 °C overnight with 3.5 × g agitation (Eppendorf ThermoMixer).
Quench the digestion by addition of FA to a final concentration of 1% FA.
Remove the lipids and undigested proteins by centrifugation at 1800 × g for 10 min at RT, and desalt the supernatant containing peptides (see Subheading 3.2).
3.2. Desalting Proteolytic/Tryptic Peptides Using Oasis HLB Cartridges
Apply vacuum to the Oasis HLB 1 cc cartridges (30 mg sorbent, max. binding capacity: 5 mg) using the vacuum extraction manifold and condition the cartridges twice with 800 μL of organic HLB Solvent B.
Equilibrate cartridges three times with 800 μL of aqueous HLB Solvent A.
Load the acidified tryptic peptides onto the cartridge (here from 5 mg protein digest).
Wash the bound peptides three times with 800 μL of HLB Solvent A.
Remove the HLB cartridges from the vacuum manifold and place into 1.5 mL microcentrifuge tubes. Elute the peptides by sequential addition of 800 μL followed by 400 μL HLB Solvent B.
Mix the elution with vortexing, and then remove 2.4 μL (1/500th or 10 μg) for independent and parallel protein-level quantification (also see Note 2).
Concentrate/dry the eluted peptides completely using a SpeedVac (see Note 3).
3.3. Anti-Acetyl1 Immunoaffinity Enrichment
For immunoaffinity enrichment [5, 7], resuspend all the dried peptides with 1.4 mL ice-cold IAP buffer and mix by pipetting. Avoid vortexing as this may create bubbles.
Check that the pH of the resuspended peptide solution is between pH 7–8 by pipetting 2 μL on pH paper. If the pH deviates add more IAP buffer (in small increments of 50 μL) or adjust pH otherwise.
Centrifuge the resuspended peptides at 10,000 × g for 5 min at 4 °C. A small pellet may appear, but the majority of peptides will be soluble. Keep peptide solution on ice during preparation of the antibody-bead conjugate (see Note 4).
Prepare the PTMScan Acetyl-Lysine antibody beads for peptide affinity enrichment, add 1 mL cold 1× PBS buffer to one tube of antibody-conjugated beads, pipette up and down three times for mixing. The ideal ratio of PTMScan Acetyl-Lysine Motif antibody-conjugated beads to peptide starting material should be 1/4 of a tube of antibody beads for 5 mg of peptides (see Note 1).
Transfer the slurry of antibody-conjugated beads to a new 1.5 mL microcentrifuge tube and centrifuge at 2000 × g for 30 s at RT to prevent beads from sticking to the side of the tube. Remove the PBS buffer by aspiration, leaving some volume to avoid disruption of the beads.
Wash the antibody beads with 1 mL cold 1× PBS and centrifuge at 2000 × g for 30 s at RT. Remove the majority of PBS by aspiration.
Repeat the PBS wash step twice more for a total of four 1 mL 1× PBS washes.
Resuspend the washed beads from one tube of PTMScan Acetyl-Lysine antibody in 440 μL PBS, mix several times by pipetting with wide-bore 200 μL pipette tip, and take out four 100 μL aliquots of bead suspension into 1.5 mL microcentrifuge tubes ensuring the master mixture remained thoroughly mixed. In order to ensure consistent bead quantities in the four 100 μL aliquots, about 40 μL of beads will remain in the original tube (see Note 5). Centrifuge the aliquoted beads at 2000 × g for 30 s at RT. Visually ensure that each tube has a similar quantity of antibody-conjugated beads. Remove all 1× PBS by aspiration using a 0.2 mm gel loading flat pipet tip.
Transfer the resuspended peptides from step 3 directly onto the prepared PTMScan Acetyl-Lysine Motif antibody-conjugated beads.
Incubate the peptides and antibody-conjugated bead mixture(s) at 4 °C overnight on an end-over-end rotator or gentle mixer.
After incubation, centrifuge the peptide/bead mixtures at 2000 × g at 4 °C for 30 s.
Remove the supernatant containing the unbound peptides and save for possible further applications.
Wash the peptide-bound beads with 1 mL cold IAP buffer, mix by inverting the tube 5 times, then centrifuge at 2000 × g, 4 °C for 30 s. Remove the IAP wash solution by aspiration, but leave a small volume to avoid bead disruption.
Repeat the IAP wash step once for a total of two IAP washes.
Wash the peptide-bound beads with 1 mL ice-cold HPLC-MS water, mix by inverting 5 times, then centrifuge at 2000 × g, 4 °C for 30 s. Remove the water wash solution by aspiration, leaving a small volume to avoid bead disruption.
Repeat the water wash twice for a total of three water washes.
After the last water wash, centrifuge once more for 30 s at 2000 × g and 4 °C to collect any remaining volume to the bottom. Aspirate the remaining water with a 0.2 mm gel loading flat pipet tip while avoiding the beads.
Add 55 μL 0.15% TFA in HPLC-MS water to the peptide-bound beads. Incubate at RT for 10 min. Tap the bottom of the tubes to mix intermittently.
Centrifuge the mixture for 30 s at 2000 × g, RT. Remove the eluted peptides with 0.2 mm gel loading flat pipet tip and save in a 0.65 mL microcentrifuge tube.
Add 45 μL 0.15% TFA in HPLC-MS water to the peptide-bound beads. Incubate the mixture at RT for 10 min with intermittent agitation by tapping the bottom of the tubes.
Centrifuge the mixture for 30 s at 2000 × g, RT. Remove the second elution by pressing a 0.2 mm gel loading flat pipet tip to the bottom of the tubes and combine with the first elution.
Centrifuge the eluted peptides at 12,000 × g at RT for 5 min to pellet any beads that may have carried over. Store eluted peptides on ice for immediate desalting.
3.4. Small-Scale Acetyl-Peptide Desalting Prior to LC-MS—C18 StageTips (or ZipTips)
Prepare the C18 StageTips for desalting as described by Rappsilber et al. [8]: assemble a set of three disks (punched out with a 18-gauge needle from an Octadecyl C18 Extraction Disk membrane) in a low-binding 200 μL pipet tip—held together in a 0.65 mL Eppendorf tube with a hole in the bottom allowing for solvent flow upon centrifugation into a 2 mL collection tube.
Condition the StageTip with 100 μL of 100% ACN by passing the supernatant through the assembly by centrifugation at 3000 × g for 1 min. Wash with 100 μL of Stage Tip Solvent B by centrifugation at 3000 × g for 1 min (see Note 6).
Equilibrate the StageTip with 100 μL of Stage Tip Solvent A by centrifugation at 3000 × g for 1.5 min. Repeat this step for a total of two equilibration washes.
Load the acidified immunoaffinity peptide elution from Subheading 3.3, step 22 onto the StageTip and centrifuge at 3000 × g for 1.5 min.
Wash the peptides bound to the StageTip with 100 μL of Solvent A by centrifugation at 3000 × g for 1.5 min. Repeat this step for a total of two washes.
Elute the peptides with 50 μL of Stage Tip Solvent B into a new Eppendorf tube, and centrifuge at 3000 × g for 3 min to ensure all elution volume passes through. Subsequently, dry the peptide eluate completely in vacuo.
Resuspend the peptides in an appropriate volume of mobile phase A of your LC-MS system, e.g., 7 μL of 2% ACN/98% water/0.1% formic acid (v/v/v), add a retention time standard, for example, 0.1 μL of indexed retention time standard (iRT from Biognosys or other standards). Vortex the peptide solution for 10 min at 4 °C, and then centrifuge for 2 min at 12,000 × g and 4 °C. Transfer the supernatant to an autosampler vial for nano LC-MS/MS (see Note 7).
3.5. Nanoflow LC-MS/MS Analysis
Samples are analyzed by reverse-phase HPLC-ESI-MS/MS using the Eksigent Ultra Plus nano-LC 2D HPLC system combined with a cHiPLC System, directly connected to a quadrupole time-of-flight SCIEX TripleTOF 6600 mass spectrometer (SCIEX). Typically, mass resolution for precursor ion scans is ~45,000 (TripleTOF 6600), and fragment ion resolution is ~15,000 (“high sensitivity” product ion scan mode, see Note 8). After injection, peptide mixtures are transferred onto a C18 pre-column chip and washed at 2 μL/min for 10 min with the Mobile Phase A (loading solvent). Subsequently, peptides are transferred to the analytical column ChromXP C18-Cl chip and eluted at a flow rate of 300 nL/ min typically with a 2–3 h gradient using aqueous and acetonitrile solvent buffers (Mobile Phases A and B).
Data-dependent acquisitions (DDA). For spectral library building, initial data-dependent acquisitions (DDA) are carried out to obtain MS/MS spectra for the 30 most abundant precursor ions (100 ms per MS/MS) following each survey MS1 scan (250 ms), yielding a total cycle time of 3.3 s.
Data-independent acquisitions (DIA). For label-free relative quantification, all study samples are analyzed by data-independent acquisitions (DIA), using a 64 variable window SWATH acquisition strategy [9, 10]. Briefly, instead of the Q1 quadrupole transmitting a narrow mass range through to the collision cell, windows of variable width (5–90 m/z) are passed in incremental steps over the full mass range (m/z 400–1250). The cycle time of 3.2 s includes a 250 ms precursor ion scan followed by 45 ms accumulation time for each of the 64 DIA- SWATH segments.
3.6. Traditional MS Workflow A: Identification and Quantification of Acetylation Sites Using DDA & DIA
Mass spectrometric data from data-dependent acquisitions (DDA) is analyzed with the database search engine ProteinPilot 5.0 (SCIEX) using parameters such as trypsin digestion, cysteine alkylation set to iodoacetamide, lysine acetylation, and species Mus musculus; false discovery rates of 1% are used (see Note 9).
Using the database search engine results generated above, MS/MS spectral libraries are generated in Skyline daily v 4.1.1 [11], an open-source data processing workspace for quantitative proteomics; DIA raw data files are imported into Skyline and both MS1 precursor ion scans as well as MS2 fragment ion scans are extracted for all acetylated peptides present in the spectral libraries [12]. In Skyline, typically 6–10 MS2 fragment ions are extracted per acetylated peptide based on ranking from the corresponding MS/MS spectra in the spectral libraries, and fragment peak areas are summed per peptide.
Relative quantification of acetylation levels comparing different conditions, for example, knockout versus wild-type strains, can be performed directly in Skyline using integrated statistical algorithms. Statistical assessment of peak selection can be done within Skyline using mProphet [13], which was adjusted to specifics of DIA data. Alternatively, the corresponding extracted acetylation site peak areas can be exported and subjected to other open-source programs, such as mapDIA [14] which is specialized for processing and statistical analysis of quantitative proteomics data from DIA-MS (see Note 8).
3.7. Alternative MS Workflow B: Identification and Quantification of Acetylation Sites Using Exclusively DIA with PIQED Software [15]
In addition to the presented traditional protocol describing MS data acquisition (see Subheading 3.6), an alternative workflow (see Note 10) is presented that does not require DDA but only DIA, which can be advantageous when working with very low amounts of starting material or many replicates.
Data from DIA mass spectrometry only, without spectral libraries generated by DDA (as described under Subheading 3.6), can alternatively be analyzed using the automated pipeline PIQED and a corresponding java interface in which spectral libraries are generated directly from DIA data files that are subsequently also used for quantification [15]. Briefly, the raw data is converted to mzXML file formats, pseudo-MS/MS spectra are generated using DIA-Umpire [16], and acetylated peptides are identified by database search with MS-GF+ [17], Comet [18], and X! Tandem (thegpm.org). Subsequently, files are automatically imported into Skyline [12] and peak area results and outputs from acetylated peptides are submitted to mapDIA [14] for statistical analysis as part of the PIQED pipeline.
3.8. Anticipated Results
Depending on experimental design we typically identify and quantify 1000–2000 acetylation sites from an enrichment of 5 mg of protein lysate. See Fig. 2.
Numbers of acetylation sites vary per protein, ranging mostly between 1–20 detected sites per protein.
Workflow reproducibility is assessed between replicates, and we typically measure coefficients of variations CV <20%.
The affinity workflow using the PTMScan Acetyl-Lysine Motif [Ac-K] Immunoaffinity Beads to enrich for acetylated peptides typically yields high acetylation enrichment, with 50–70% acetylated over total peptides detected.
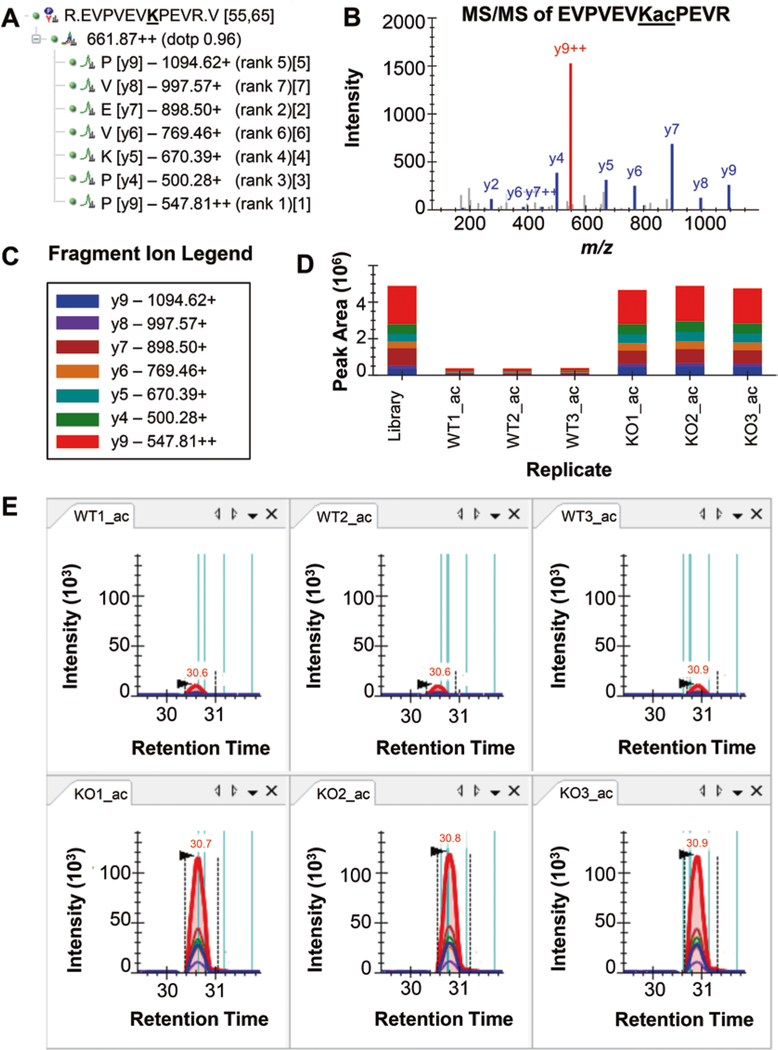
Fig. 2.
Example of anticipated results from quantification of acetylation comparing conditions for the peptide sequence EVPVEVK(ac)PEVR. (a) Skyline target tree showing the precursor ion mass, fragment ion masses, and fragment ion intensity rankings. (b) Annotated mass spectrum showing matched fragment ions with the most abundant y9++ ion highlighted in red. (c) Fragment ion color key and legend for panels (d and e). (d) Fragment ion peak areas among three replicates of wild-type (WT) and knockout (KO) computed by Skyline. (e) Extracted ion chromatograms (XICs) of the fragment ions for this peptide from DIA data showing excellent peak shape, retention time reproducibility, and altered intensity between WT and KO
4. Notes
From whole liver tissue lysate, we recommend a minimum of 5 mg protein input for the acetyl-lysine affinity enrichments. Alternatively, 1 mg of protein from isolated liver mitochondria results in equivalent acetyl-peptide identification efficiency. When possible, more input protein is desirable (up to about 4x the quantities described here: 20 mg of protein from whole liver tissue or 4 mg of protein from isolated liver mitochondria). Subsequently, the amount of antibody should be scaled up accordingly to maintain a high percentage of acetyl-peptide enrichment (ideally 50–70% or higher). The ideal ratio of PTMScan Acetyl-Lysine Motif antibody-conjugated beads to peptide starting material should be ¼ of a tube of antibody beads for 5 mg of peptides from whole tissue (or 1 tube of antibody beads for 20 mg of peptides from whole tissue).
To decipher which changes are truly based on changes of the acetylation profile and not changes in protein abundance, we normalize acetyl-peptide areas by dividing them by their corresponding protein-level areas measured in parallel. This can also be automated using the PIQED workflow described in Subheading 3.7. In Subheading 3.2, step 6 a small aliquot of digested protein lysate was set aside to assess protein-level quantity changes.
To enable easy and consistent peptide resuspension after drying, it is important to freeze the solution quickly and keep it frozen throughout the drying process. The result should be a fluffy white/yellow powder. If an oily film is formed, the drying process was not performed optimally.
All steps during peptide and antibody-bead conjugate preparation should be done on ice and centrifugation should be performed at 4 °C.
When generating aliquots of the antibody-bead conjugate, it’s important to ensure approximately the same number of beads per aliquot.
Ensure the StageTips (packed with C18 disks) remain wetted with liquid throughout all steps until after elution. Check the StageTips frequently during the centrifugation steps and adjust the centrifugation time accordingly.
Even though there may be no visible material at the bottom after centrifugation, handle samples delicately post-spin without much agitation to avoid any potential small particulate from getting resuspended.
Other high-resolution mass spectrometric systems from other instrument vendors will equally be able to perform these labelfree, high-resolution workflows.
To potentially increase the number of identified acetylated peptides, additional database search engines can be used after conversion of the raw data to mzXML format using msConvert from ProteoWizard [19].
We provide two options of data collection and analysis that differ in how the spectral library of PTM identifications is produced. Workflow A utilizes the traditional strategy where PTM identifications are produced from data-dependent acquisition (DDA), and those peptides are quantified with additional DIA. Workflow A is preferred when sample number is low and sample quantity is not limited. Workflow B describes a newer strategy where identification and quantification are done entirely with DIA using the PIQED software [15], which is preferred when projects have many replicates or sample quantity is limited.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R24 DK085610 E.V. and R01 DK090242, Goetzman/B.S.). We acknowledge support from the NIH shared instrumentation grant for the TripleTOF system at the Buck Institute (1S10 0D016281, Gibson). J.G.M. was supported by a National Institutes of Health grant (T32 AG000266). We thank Dr. Davalyn Powell for editing the manuscript.
References
- 1.Glozak MA, Sengupta N, Zhang X, Seto E (2005) Acetylation and deacetylation of nonhistone proteins. Gene 363:15–23 [DOI] [PubMed] [Google Scholar]
- 2.Verdin E, Ott M (2015) 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol 16:258–264 [DOI] [PubMed] [Google Scholar]
- 3.Carrico C, Meyer JG, He W, Gibson BW, Verdin E (2018) The mitochondrial acylome emerges: proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab 27:497–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23:607–618 [DOI] [PubMed] [Google Scholar]
- 5.Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW (2013) Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci U S A 110:6601–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49:186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svinkina T, Gu H, Silva JC, Mertins P, Qiao J, Fereshetian S, Jaffe JD, Kuhn E, Udeshi ND, Carr SA (2015) Deep, quantitative coverage of the lysine acetylome using novel anti-acetyllysine antibodies and an optimized proteomic workflow. Mol Cell Proteomics 14:2429–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rappsilber J, Ishihama Y, Mann M (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75:663–670 [DOI] [PubMed] [Google Scholar]
- 9.Schilling B, Gibson BW, Hunter CL (2017) Generation of high-quality SWATH(R) acquisition data for label-free quantitative proteomics studies using TripleTOF(R) mass spectrometers. Methods Mol Biol 1550:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins BC, Hunter CL, Liu Y, Schilling B, Rosenberger G, Bader SL, Chan DW, Gibson BW, Gingras AC, Held JM, Hirayama–Kurogi M, Hou G, Krisp C, Larsen B, Lin L, Liu S, Molloy MP, Moritz RL, Ohtsuki S, Schlapbach R, Selevsek N, Thomas SN, Tzeng SC, Zhang H, Aebersold R (2017) Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH–mass spectrometry. Nat Commun 8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26:966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rardin MJ, Schilling B, Cheng LY, MacLean BX, Sorensen DJ, Sahu AK, MacCoss MJ, Vitek O, Gibson BW (2015) Ms1 peptide ion intensity chromatograms in MS2 (SWATH) data independent acquisitions. Improving post acquisition analysis of proteomic experiments. Mol Cell Proteomics 14:2405–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiter L, Rinner O, Picotti P, Huttenhain R, Beck M, Brusniak MY, Hengartner MO, Aebersold R (2011) mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat Methods 8:430–435 [DOI] [PubMed] [Google Scholar]
- 14.Teo G, Kim S, Tsou CC, Collins B, Gingras AC, Nesvizhskii AI, Choi H (2015) mapDIA: preprocessing and statistical analysis of quantitative proteomics data from data independent acquisition mass spectrometry. J Proteome 129:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer JG, Mukkamalla S, Steen H, Nesvizhskii AI, Gibson BW, Schilling B (2017) PIQED: automated identification and quantification of protein modifications from DIA-MS data. Nat Methods 14:646–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsou CC, Avtonomov D, Larsen B, Tucholska M, Choi H, Gingras AC, Nesvizhskii AI (2015) DIA-Umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat Methods 12:258–264, 257 p following 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Pevzner PA (2014) MS-GF+ makes progress towards a universal database search tool for proteomics. Nat Commun 5:5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eng JK, Jahan TA, Hoopmann MR (2013) Comet: an open-source MS/MS sequence database search tool. Proteomics 13:22–24 [DOI] [PubMed] [Google Scholar]
- 19.Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, Hoff K, Kessner D, Tasman N, Shulman N, Frewen B, Baker TA, Brusniak MY, Paulse C, Creasy D, Flashner L, Kani K, Moulding C, Seymour SL, Nuwaysir LM, Lefebvre B, Kuhlmann F, Roark J, Rainer P, Detlev S, Hemenway T, Huhmer A, Langridge J, Connolly B, Chadick T, Holly K, Eckels J, Deutsch EW, Moritz RL, Katz JE, Agus DB, MacCoss M, Tabb DL, Mallick P (2012) A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30:918–920 [DOI] [PMC free article] [PubMed] [Google Scholar]