Abstract
Background
Transcutaneous electrical nerve stimulation (TENS) is a noninvasive modality which may be used to reduce acute postoperative pain. Intense perioperative pain within the first few days after hip fracture surgery is common and is related to negative consequences such as restricted and delayed ambulation.
Objective
The objective of the present study was to examine the effect of incorporating TENS treatment on pain intensity, and mobility, with standard rehabilitation care during the acute post-operative phase following Gamma-nail surgical fixation of extracapsular hip fractures.
Materials and methods
Forty-one patients were randomly assigned to a supplement of 30 mins of active TENS or sham TENS. The standard rehabilitation care included five daily 30 mins physical therapy treatments beginning 24 hrs after surgery. Outcome measures were: pain intensity at rest, at night and during ambulation (assessed with the Numeric Rating Scale; Functional Ambulation Classification instrument; time to complete five sit-to-stand tests; and two-minute walk test). Data were analyzed with Wilcoxon score rank tests. Significance was set at p≤0.05.
Results
Significantly greater pain reduction during walking was noted in the active TENS group compared to sham TENS group (differences between the fifth and the second days: 2.55±1.37 vs 1.06± 1.11, respectively; p=0.0011). Additionally, advantage of active TENS was demonstrated in greater increase in walking distance on the fifth day and higher level of mobility compared to the sham TENS group. No additional effects of active TENS were noted on pain intensity at rest and at night and on five times sit-to-stand performance.
Conclusion
Addition of TENS to the standard care of elderly patients in the early days following Gamma nail surgical fixation of extracapsular hip fracture is recommended for pain management while walking and functional gait recovery. The effect of TENS on long-term functional outcomes should be explored in future studies.
Trial registration
The trial was registered at the ISRCTN registry: ID ISRCTN32476360.
Keywords: transcutaneous electrical nerve stimulation, TENS, pain, hip fracture, acute postoperative, mobility
Introduction
Hip fractures are the most serious outcome of osteoporosis and the incidence increases exponentially with age.1 Hip fractures may lead to deterioration in activity level and functional capabilities and to a greater risk of mortality.1,2 Rehabilitation of individuals following hip fractures is a global concern due to increased life expectancies worldwide.3
Overall, hip fractures are defined as intracapsular or extracapsular, which are further classified according to the specific location, pattern and stability of the fracture.4 Extracapsular hip fractures are generally surgically fixated using cephalocondylic intramedullary nails or extramedullary implants (e.g. the sliding hip screw).5
Intense perioperative pain after hip fracture is common due to the fracture, inflammatory agents and the surgical procedure (e.g. involvement of soft tissue and sensory nerves).6–8 Undertreated acute postoperative pain is related to longer hospitalization time, restricted and delayed ambulation, prolonged bed rest, reduced compliance with physical therapy and higher immediate postoperative complications.8 Furthermore, uncontrolled, acute postoperative pain also has negative long-term effects, as reflected by poorer outcomes after six months, including higher mortality rate and residual pain, as well as low level of ambulation and less return to living in the community.6
Acute postoperative pain is usually treated by narcotics, which are often accompanied by adverse side effects, such as nausea, vomiting, delirium, constipation and gastrointestinal dysfunction. Generally, physicians wish to limit the use of narcotics, particularly in older individuals, due to the higher incidence of these side-effects in this population.9 Indeed, it is reported that older subjects receive significantly less analgesia post-operatively as compared to younger adults.8 However, poor pain management in older subjects can not only impede rehabilitation, but also may result in other negative consequences such as tachycardia, increased myocardial oxygen demand, cardiac ischemia and higher risk of post-operative delirium.8,10 Accordingly, integrating alternative non-pharmacological, non-invasive analgesia to manage acute post-operative pain is warranted.
One such potential modality is transcutaneous electrical nerve stimulation (TENS), which is a widely used, noninvasive technique that delivers electrical pulses through the skin.11,12 TENS was shown to be an effective supplemental anesthesia to reduce acute postoperative pain and opioid consumption13,14 following thoracic15,16 and abdominal surgery,17 total knee replacement13 and arthroscopic rotator cuff repair.18
To the best of our knowledge, integration of TENS for pain reduction in the context of hip fracture has been evaluated in two studies.19,20 TENS was shown to be an effective analgesic modality for traumatic pain following hip fracture during transfer to a hospital in ambulances staffed by paramedics without physicians.19 A study by Gorodetskyi et al20 focusing on trochanteric fractures of the femur stabilized by a dynamic hip screw (DHS) for non-complex fractures or external fixator for complex fractures demonstrated that a 10-day combined treatment of active TENS and standard rehabilitation, initiated within 24 hrs after surgery, resulted in greater and more rapid pain decline, greater range of hip flexion and less consumption of non-steroidal anti-inflammatory agents as compared to sham TENS and to standard rehabilitation.20
However, evidence regarding the effect of TENS on improving functional outcomes, which is the goal of post-operative rehabilitation treatment, is lacking. In addition, the effect of TENS on extracapsular hip fractures fixed by cephalocondylic intramedullary nails (such as Gamma nail), which involve a significantly smaller surgical incision compared to DHS, has not been explored.
The aim of this prospective, double-blinded, randomized trial was to determine whether incorporating TENS treatment during standard rehabilitation care during the acute post-operative phase following Gamma nail surgical fixation of extracapsular hip fracture has a beneficial effect on pain intensity, ambulation and mobility.
Materials And Methods
Patients
The sample included patients admitted to the Orthopedic Department at the Galilee Medical Center, Israel, between December 2014 and December 2015 with extracapsular proximal hip fracture stabilized with Gamma nail. To recruit subjects, the study coordinator (S.A.N) examined the department’s records daily to identify potential participants from among all patients who had undergone surgical fixation of a hip the previous day. Screening was based on an eligibility checklist. Inclusion criteria were: (1) age above 50 years; (2) stable extracapsular fracture proximal to the hip (intertrochanteric or sub-trochanteric fracture) fixed with a Gamma nail; (3) weight-bearing instruction; (4) ability to ambulate independently for at least 10 m with/without assistive device pre-fracture; (5) ability to follow instructions and (6) Mini-mental state score ≥20. Exclusion criteria were: (1) conditions that contraindicate electrical stimulation such as a pacemaker, significant sensory loss in the lower extremities or local wound at the site of the electrode placement; (2) history of cardiovascular, neurological or orthopedic problems with mobility limitation of walking less than 100 meters; (3) prior experience with TENS and (4) infectious or systemic disease that may interfere with the rehabilitation process (such as lupus). Final decision regarding eligibility was determined by the study coordinator and the head of the surgical department (HS), who also provided the weight-bearing instructions. Randomization allocation to active TENS or sham TENS was done using a computer algorithm. The study was approved by the Helsinki Committee of the Galilee Medical Center (number 0110-14-NHR). Prior to participation and following detailed explanation of the study by the study coordinator, participants signed an informed consent.
Procedures
All participants were assessed by a qualified physical therapist (S.A.N.), with clinical experience in the field of orthopedic orthopedics, including the use of objective assessment tools such as functional ambulation classification (FAC). The therapist was blinded to treatment allocation throughout the intervention and assessment period. Demographic data documented by the assessor included age, gender, comorbidity (measured as number of documented diseases and divided into 0–2 diseases or 3 or more) and use of an assistive device for ambulation before the current fracture.
Pain level at rest and during the night was recorded each day during the five-day intervention period. Pain level during ambulation was assessed on days 2–5, as most subjects did not walk on the first postoperative day. Pain intensity was measured by the numeric rating scale (NRS), with 0 representing no pain and 10 representing the most severe pain. The NRS scale is a validated and reliable tool that is frequently used in various acute pathologies including hip surgery.20–22 Pain intensity at night and at rest was assessed by asking the participant, immediately before each physiotherapy session, the degree of pain that he/she had experienced during the previous night and while resting in bed or sitting on a chair during the day. Level of pain during walking was assessed at the end of the training session. In addition, the amount of analgesics medication consumption on days 1–5 was obtained from the nursing stuff reports.
Mobility status was assessed on days 2–5 using the functional ambulation classification (FAC). The FAC is a 6-point categorical assessment tool with scores ranging from 0 (indicating non-functional walking) to 5 (indicating ability to walk independently anywhere). Intermediate scores are determined by the level of assistance and supervision needed. FAC has been validated for day hospital practice23 and for elderly individuals after hip fracture.24
Two physical performance tests were conducted on the fifth day. The first was the five times sit to stand test (5xSTS), which is a valid, reliable measure of functional lower limb muscle strength, which has been used after hip surgery in elderly people.25 The 5xSTS entails measuring with a hand-held stopwatch the time required to rise from a standard height (0.43 m) chair with armrests, five times as fast as possible. No instruction regarding placement of the arms while rising from the chair was given to the participants.26 The second test was the two minutes walk test (2MWT) which is a reliable indicator of gait performance in older adults and can be used after hip fracture, as it is not as fatiguing as the six minutes walk.27 In this test, participants were asked to walk at a self-paced velocity for 2 mins with a rolling walker in accordance with the prescribed protocol following hip surgery.28 Safety was ensured by the assessor who walked behind subjects. The assessor used a digital, hand-held stopwatch and measured the walked distance covered by using a line of tape placed on the floor.27
Intervention
Patients in both groups received standard interdisciplinary postoperative treatment beginning 24 hrs after surgery and were blinded to treatment allocation throughout the study.
Pain management protocol in the orthopedics ward is subject-driven. Non-narcotic (non-opioids) analgesics are provided as an initial step following patient’s complaint of pain. Only if no effect is observed in the pain intensity, narcotic analgesics are then prescribed. Physiotherapy was carried out each morning for approximately 30 mins by physical therapists (A.D and M.K.T.), who were familiar with the study protocol and were not involved in patient assessment. Each treatment session included transfer training, balance exercises, lower extremity exercise and ambulation training performed at the end of the session. Prior to walking, four 4 cm2 self-adhesive neuromuscular stimulation electrodes (ValuTrode, Fallbrook, CA, USA) were adhered to the skin on both sides of the surgical incision of all participating subjects. The active or sham electrical stimulation was administered each morning for 30 mins for a total of 5 treatments. On the first treatment day, the TENS (active or sham) was provided after the physical therapy session. On days 2–5, TENS was applied during the ambulation training (10 to 15 mins) and the seated rest period that followed.
A portable, clinical stimulator TENS device was used (GEM-STIM, Gemore Technology Co., Ltd. Taiwan). Active TENS units delivered a bi-phasic symmetric waveform at a continuous frequency of 100 Hz and phase duration of 200 µsec. The intensity was gradually adjusted up to a strong but comfortable l level, as reported by the subject. The sham group were told that not everyone necessarily feels the stimulation. In this group, the device was turned on so that the subject would see a green light. Although the therapist adjusted the intensity, no current was delivered.
Statistical Analysis
Descriptive statistics (mean and standard deviation (SD)) were calculated for participants’ characteristics and outcome measures of each group. Differences between subjects’ characteristics at baseline were examined using chi-squared and Two-Sample t-tests. Due to the sampling distribution, Wilcoxon score rank (Rank sums) was conducted to compare the results of the outcome measures (pain intensity and FAC) between groups at each time period (from the first or second to fifth days) and for the fifth day only for 5XSTS and 2MWT. Signed rank tests were performed to examine the within-group changes. Significance was set at p ≤ 0.05. Power analysis was done after the study as a confirmatory analysis, for the outcome of pain intensity while walking test, which indicated power of >90% for the current sample size. Statistical analyses were performed using JMP Analysis Software (SAS Institute, Inc., Cary, NC, USA).
Results
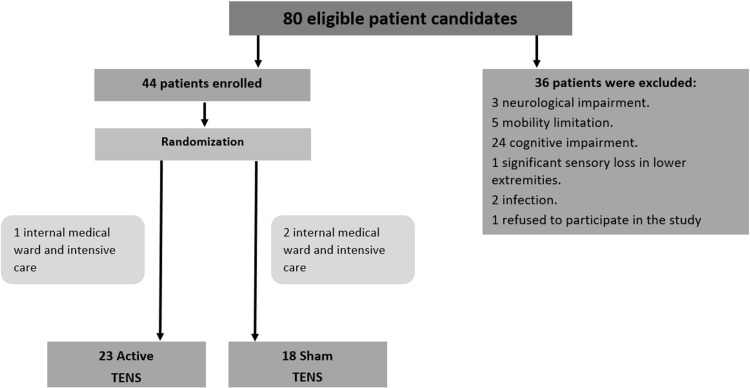
Eighty patients were considered potential candidates for the study and 44 were eligible based on the inclusion/exclusion criteria. These 44 patients (mean age 79.3± 9.2 years) were enrolled and randomly assigned to the active or sham TENS group. Three subjects were transferred 1 or 2 days after surgery (one subject from the active-TENS group and 2 from the sham group) to an Internal Medicine Ward or Intensive Care Unit due to medical complications. Accordingly, the active TENS group included 23 subjects and the sham TENS group included 18 subjects; thus, the data of 41 patients were analyzed. Study flow chart is presented in Figure 1. Summary of the participants’ baseline characteristics is presented in Table 1 and demonstrates no significant differences between groups in all basic variables. Results of outcome measures are presented in Tables 2 and 3. Both groups experienced significant decreases in pain intensity by the fifth day at rest and overnight, as compared to the first and second days. However, no group differences were noted in these measures. In addition, no group differences were found in the non-narcotic and narcotic analgesics consumption during the first four days of the intervention period. On the fifth day, while there was no difference in the narcotic consumption, the average amount of non-narcotic consumption was significantly lower in the active TENS group compared to the sham TENS group.
Figure 1.
Flowchart of the study.
Table 1.
Baseline Characteristics Of Each Group (Mean ± SD), And Comparison Between Groups (p-Value)
| Characteristics | Sham TENS (n=18) | Active TENS (n=23) | p value |
|---|---|---|---|
| Age, mean ± SD | 78.06±8.45 | 80.26±9.83 | 0.45 |
| Gender, n | 0.12 | ||
| Men | 6 | 3 | |
| Women | 12 | 20 | |
| Operated side, n | 0.09 | ||
| Left | 11 | 8 | |
| Right | 7 | 15 | |
| Comorbidities, n | 0.13 | ||
| 0–2 pathology | 7 | 11 | |
| 3–5 pathology | 11 | 12 | |
| Assistive device pre-fracture, n | 0.17 | ||
| None | 3 | 2 | |
| Cane | 1 | 9 | |
| Walker | 14 | 12 |
Note: Statistical significance p < 0.05.
Table 2.
Pain Intensity Over Time Of The Study Groups (Mean ± SD) (Wilcoxon Signed Rank Test; P Value) And Between Groups (Wilcoxon Scores; Rank Sum; P-Value)
| Outcome measures | Sham TENS (n=18) | p value | Active TENS (n=23) | p value | Between group p value |
|---|---|---|---|---|---|
| Numeric rating scale (NRS) REST | |||||
| Day 1 | 6.12±2.50* | – | 4.17±1.92 | – | 0.02 |
| Day 2 | 5.47±2.43* | – | 3.91±2.02 | – | 0.07 |
| Day 3 | 4.65±2.42* | – | 3.39±1.99 | – | 0.09 |
| Day 4 | 4.47±2.24* | – | 2.83±1.75 | – | 0.02 |
| Day 5 | 4.47±2.48* | – | 2.83±1.97 | – | 0.03 |
| Day 1–Day 5 | 1.65±1.80* | 0.004 | 1.35±1.77 | 0.002 | 0.64 |
| Day 2–Day 5 | 1.00±1.66* | 0.02 | 1.09±1.53 | 0.004 | 0.91 |
| Numeric rating scale (NRS) NIGHT | |||||
| Day 1 | 7.56±1.89 | – | 6.13±3.05 | – | 0.13 |
| Day 2 | 7.39±1.97 | – | 6.04±2.65 | – | 0.08 |
| Day 3 | 6.56±2.53 | – | 5.00±2.71 | – | 0.06 |
| Day 4 | 6.17±2.73 | – | 4.57±2.59 | – | 0.02 |
| Day 5 | 5.94±2.82 | – | 4.17±2.72 | – | 0.03 |
| Day 1–Day 5 | 1.61±2.85 | 0.02 | 1.96±2.70 | 0.002 | 0.60 |
| Day 2–Day 5 | 1.44±2.73 | 0.02 | 1.87±2.01 | <0.0001 | 0.43 |
| Numeric rating scale (NRS) WALK | |||||
| Day 2 | 8.17±1.29 | – | 7.50±1.41** | – | 0.13 |
| Day 3 | 7.83±1.34 | – | 6.18±1.47** | – | 0.002 |
| Day 4 | 7.28±1.23 | – | 5.61±1.64 | – | 0.002 |
| Day 5 | 7.11±1.45 | – | 4.96±1.58 | – | 0.0002 |
| Day 2–Day 5 | 1.06±1.11 | <0.0001 | 2.55±1.37** | <0.0001 | 0.0011 |
Notes: *N=17; **N=22; Statistical significance p < 0.05.
Table 3.
Functional Outcomes By The Two Intervention Groups (Mean ± SD), And Comparison Between Days In Each Group (Wilcoxon Signed Rank Test; P Value) And Between Groups (Wilcoxon Scores; Rank Sum; P-Value)
| Outcome measures | Sham TENS (n=18) | p value | Active TENS (n=23) | p value | Between group p value |
|---|---|---|---|---|---|
| FAC | |||||
| Day 1 | 2.06±0.24 | – | 2.1±0.45* | – | 1.00 |
| Day 2 | 2.17±0.38 | – | 2.35±0.57 | – | 0.3 |
| Day 3 | 2.56±0.62 | – | 2.91±0.67 | – | 0.09 |
| Day 4 | 3.06±0.54 | – | 3.61±0.58 | – | 0.003 |
| Day 5 | 3.22±0.65 | – | 3.78±0.52 | – | 0.002 |
| Day 2–Day 5 | 1.06±0.54 | <0.0001 | 1.43±0.66 | <0.0001 | 0.04 |
| 5XSTS | 40.51±13.87 | – | 38.38±14.40 | – | 0.51 |
| Day 5 | |||||
| 2MWT | 6.83±3.59 | – | 9.36±3.82 | – | 0.02 |
| Day 5 |
Notes: *N=20; Statistical significance P < 0.05.
Abbreviations: FAC, functional ambulation classification; 5XSTS, five times sit to stand test; 2MWT, two minutes walk test.
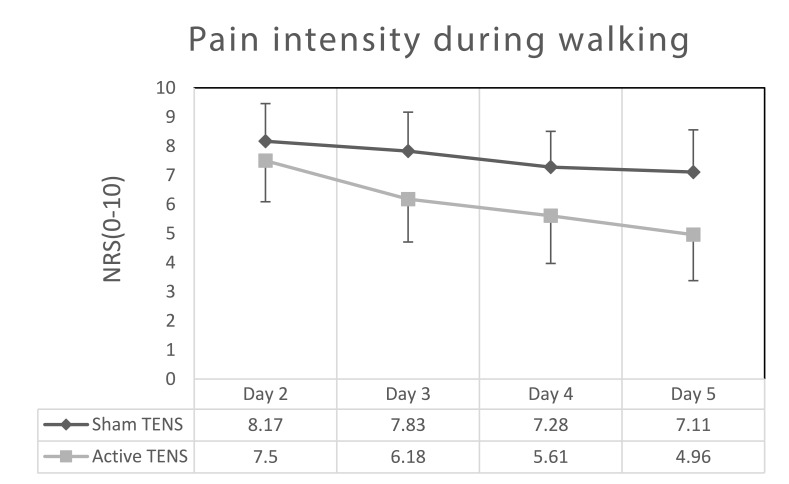
In contrast, a significant difference was observed between groups in terms of pain intensity during walking. Both groups demonstrated a significant decrease in pain intensity over the course of treatments. The difference between day 2 and day 5 was significant (p <0.0001) in both the active and sham treatments (2.55±1.37 and 1.06± 1.11, respectively). However, the difference in the active TENS group was significantly higher (p= 0.0011). Furthermore, mean NRS score for pain during walking was consistently lower for the active TENS group across three-time points (from the third to fifth days) compared to sham TENS (Figure 2).
Figure 2.
Numeric rating scale (NRS) values (mean ± SD) during walking on postoperative days 2 to 5.
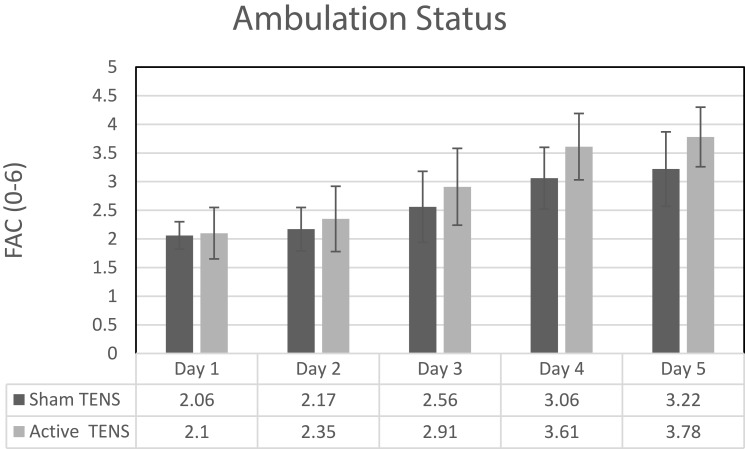
Level of ambulation, as measured by FAC, improved significantly in both groups. However, greater improvement was demonstrated in the active TENS group as compared to the sham TENS (Figure 3). Walking distance in the 2MWT improved more in the active compared to the sham TENS group. However, no difference was observed between groups in the 5xSTS test.
Figure 3.
Functional ambulation classification (FAC) instrument values (mean ± SD) during walking postoperative days 2 to 5.
Discussion
This randomized, controlled study found that adding TENS to the standard care of elderly patients following Gamma nail surgical fixation of extracapsular hip fracture yielded significantly better results as compared to the standard care in terms of pain intensity while walking in the early postoperative period. Although both groups demonstrated reduction in pain intensity while walking on the fifth day compared to the second day, only the change in the active TENS group (2.55) reached the 2 point cut-off considered as minimal clinically important difference (MCID). The change of 1.06 of the sham TENS group did not reach this level.22,29,30
This finding is reinforced by the positive effect of TENS on the walking distance observed by the fifth day following surgery and in the greater improvement in FAC as measured by the difference between the second and fifth days. While both groups demonstrated improvements in their rest and night pain, there was no difference in this variable between groups. Furthermore, the magnitude of the change on the NRS scale (˂2 points), which is not considered clinically significant.22,29,30 Finally, TENS did not affect 5xSTS performance, as measured on the fifth postoperative day.
These results indicate a difference between the beneficial effects of TENS on pain evoked by movement versus its effect on static pain, which is defined as pain when there is no movement (i.e. at rest and at night). This finding is consistent with previous studies.17,31–35 In a state of the art critical review, Vance et al36 noted that the effects of TENS on pain reduction are related to its effects on hyperalgesia through peripheral and central mechanisms. Reduction in hyperalgesia was found to be correlated with decreased movement-evoked pain, but not with decreased static pain. which is probably influenced by different mechanisms than is the pain with movement.17,33 The longer walking distances achieved by the TENS group compared to the sham TENS group suggest that walking distance following surgical fixation of hip fracture is limited by the pain evoked during this activity. Similar results were reported after abdominal surgery in which standard pharmacological analgesics supplemented with TENS had no effect on pain at rest, but had a positive effect on pain intensity during walking and deep breathing.17 Significantly faster walking speed immediately after total knee arthroplasty was also demonstrated as a result of routine pharmacologic analgesia supplemented with TENS.32
The absence of an effect of TENS on 5xSTS performance is probably because the major contributing factor for the difficulty in individuals following a hip fracture demonstrates that standing up repeatedly is not pain, but rather factors such as functional weakness of the lower limb musculature and postural balance impairments are.37 However, the duration of the 5XSTS in both the standard care group and in the active TENS group (40.51±13.87 s and 38.38±14.40 s, respectively) was higher than the reported 15-s predictive value for detecting elderly subjects at high risk for recurrent falls.38 The clinical implication of these results is that older individuals during hospitalization after hip fracture fixation surgery are at high risk for falls. Consequently, preventive strategies should be implemented.
Previous studies found that the type and degree of tissue damage affected the efficiency of TENS on postoperative pain.39 Accordingly, it seems appropriate to compare the current results with previous studies that examined similar conditions in terms of tissue damage, such as bone fractures and similar surgical fixations. To the best of our knowledge, very few studies focused on the effect of TENS as a noninvasive method of analgesia for reducing acute postoperative pain after bone fracture.20,40,41 While Lee et al40 demonstrated no beneficial effect of active TENS compared to placebo treatment on postoperative pain in patients with Colles fracture, two other studies20,41 examined the effectiveness of TENS post-hip fracture in elderly patients, both reporting significant pain relief compared to sham TENS controls. In one of these two studies, Lang et al19 focused on the application of high frequency (100 Hz) TENS as an analgesic agent in a prehospital setting (during emergency transportation to the hospital) for posttraumatic hip pain. A clinically meaningful reduction in pain intensity was demonstrated in the active TENS group (NRS: 86 ± 12 to 79 ±11 mm) as compared with sham treatment, with no reported adverse effects.14,19
In the second study, Gorodetskyi et al,20 who focused on the effect of noninvasive interactive neurostimulation in patients following hip fracture fixation, found a positive effect in terms of pain intensity, hip flexion range of motion, consumption of nonsteroidal anti-inflammatory drugs and overall rehabilitation. Although the findings of Gorodetskyi et al,20 which as in the present study, addressed the effect of TENS immediately after hip fracture fixation, strongly support our findings, there are several fundamental differences between the two studies. First, and foremost, in addition to measures of pain and passive range of motion, the present study included valid and reliable measures of mobility. As the return to pre-fracture functional performance is the ultimate treatment goal post-fracture, determining that TENS has a positive effect on FAC and walking distance has important clinical implications. Secondly, while both studies examined the effects of TENS, the specific current and stimuli parameters as well as timing and duration of applications were different. Further studies are needed to determine which of these variables are optimal. Finally, while the current study was restricted to one fixation device (the Gamma nail), the study by Gorodetskyi et al20 included both the dynamic condylar screw (DHS) and external fixation. Since TENS was found to decrease hyperalgesia induced in the tissues surrounding the surgical incisions, the degree of tissue damage may contribute to the effectiveness of TENS in reducing pain intensity.42 Accordingly, as different surgical approaches differ in the induced tissue damage, it is important to determine whether the effect of TENS is dependent on the surgical approach employed.
The relatively small sample size is noted as a study limitation to the current study. However, the statistically significant results of this carefully controlled, prospective, double-blinded randomized trial give credence to the implications of this study. Another limitation is lack of follow-up on long-term pain relief and functional outcomes beyond the immediate post-operative period. Yet, the substantial, beneficial results immediately following surgery have important impact on the wellbeing of patients in the early stages after hip fracture repair.
Conclusions
The positive effects of TENS on pain during walking and increased walking distance determined in the present study support the integration of TENS with the standard care of elderly patients following Gamma nail surgical fixation of extracapsular hip fractures. Further research is warranted to explore the effect of TENS on long-term functional outcomes following various types of surgical interventions for hip fracture.
Acknowledgments
We thank the physical therapy staff and the medical staff of the Department of Orthopedics B, Galilee Medical Center for their full cooperation and support during the execution of the study. We thank Mr. Adeeb Doha and Mrs. Mor Kliger Tendler, two physical therapists, for their help in data collection. We also thank all our patients who were willing to participate in this placebo-controlled blinded study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
TENS, transcutaneous electrical nerve stimulation; DHS, dynamic hip screw; FAC, functional ambulation classification; NRS, numeric rating scale; 5XSTS, five times sit to stand test; 2MWT, two minute walk test; SD, standard deviation.
Ethics Approval
The study was approved by the Helsinki Committee of the Galilee Medical Center (number 0110-14-NHR).
Availability Of Data And Materials
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
Author Contributions
All authors contributed towards study conception and design, interpretation and collection of data, drafting and critically revising the paper, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. In addition, SAN was the study coordinator, HS provided the weight-bearing instructions and was responsible for final decision regarding eligibility and MEG was responsible for data analysis and the preliminary writing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16(1):158. doi: 10.1186/s12877-016-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings SR, Melton LJ III. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9 [DOI] [PubMed] [Google Scholar]
- 3.Boulton C, Bunning T, Cromwell D, et al; Royal College of Physicians. National Hip Fracture Database (NHFD) aAnnuall report 2016. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-hip-fracture-database-annual-report-2016. Accessed September 17, 2019. [Google Scholar]
- 4.Uzoigwe CE, Burnand HGF, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726–729. doi: 10.1016/j.injury.2012.08.025 [DOI] [PubMed] [Google Scholar]
- 5.Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Lib. 2010;9. [Google Scholar]
- 6.Feldt KS, Oh HL. Pain and hip fracture outcomes for older adults. Orthop Nurs. 2000;19(6):35–44. [DOI] [PubMed] [Google Scholar]
- 7.Hallström I, Elander G, Rooke L. Pain and nutrition as experienced by patients with hip fracture. J Clin Nurs. 2000;9(4):639–646. [DOI] [PubMed] [Google Scholar]
- 8.Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303–311. doi: 10.1016/s0304-3959(02)00458-x [DOI] [PubMed] [Google Scholar]
- 9.Pasero CL, McCaffery M. Overcoming obstacles to pain assessment in elders. AJN Am J Nurs. 1997;97(9):20. [PubMed] [Google Scholar]
- 10.Nishizawa Y, Hata T, Takemasa I, et al. Clinical benefits of single-incision laparoscopic surgery for postoperative delirium in elderly colon cancer patients. Surg Endosc. 2018;32(3):1434–1440. [DOI] [PubMed] [Google Scholar]
- 11.Breit R, Van der Wall H. Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty. J Arthroplasty. 2004;19(1):45–48. doi: 10.1016/s0883-5403(03)00458-3 [DOI] [PubMed] [Google Scholar]
- 12.Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013;93(10):1397–1402. doi: 10.2522/ptj.20120281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Song Y. Transcutaneous electrical nerve stimulation for postoperative pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine. 2017;96(37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson PM, Fouche PF, Thomas RE, Bendall JC. Transcutaneous electrical nerve stimulation for relieving acute pain in the prehospital setting: a systematic review and meta-analysis of randomized-controlled trials. Eur J Emerg Med. 2014;21(1):10–17. doi: 10.1097/MEJ.0b013e328363c9c1 [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Peng W, Tian X, et al. Effects of transcutaneous electrical acupoint stimulation at different frequencies on perioperative anesthetic dosage, recovery, complications, and prognosis in video-assisted thoracic surgical lobectomy: a randomized, double-blinded, placebo-controlled trial. J Anesth. 2017;31(1):58–65. doi: 10.1007/s00540-015-2057-1 [DOI] [PubMed] [Google Scholar]
- 16.Sbruzzi G, Silveira SA, Silva DV, Coronel CC, Plentz RDM. Transcutaneous electrical nerve stimulation after thoracic surgery: systematic review and meta-analysis of 11 randomized trials. Braz J Cardiovasc Surg. 2012;27(1):75–87. doi: 10.5935/1678-9741.20120012 [DOI] [PubMed] [Google Scholar]
- 17.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4(8):455–464. [DOI] [PubMed] [Google Scholar]
- 18.Mahure SA, Rokito AS, Kwon YW. Transcutaneous electrical nerve stimulation for postoperative pain relief after arthroscopic rotator cuff repair: a prospective double-blinded randomized trial. J Shoulder Elbow Surg. 2017;26(9):1508–1513. doi: 10.1016/j.jse.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 19.Lang T, Barker R, Steinlechner B, et al. TENS relieves acute posttraumatic hip pain during emergency transport. J Trauma Acute Care Surg. 2007;62(1):184–188. doi: 10.1097/01.ta.0000197176.75598.fc [DOI] [PubMed] [Google Scholar]
- 20.Gorodetskyi I, Gorodnichenko A, Tursin P, Reshetnyak V, Uskov O. Non-invasive interactive neurostimulation in the post-operative recovery of patients with a trochanteric fracture of the femur. Bone Joint J. 2007;89(11):1488–1494. [DOI] [PubMed] [Google Scholar]
- 21.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x [DOI] [PubMed] [Google Scholar]
- 22.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (vas pain), numeric rating scale for pain (nrs pain), McGill pain questionnaire (mpq), short‐form McGill pain questionnaire (sf‐mpq), chronic pain grade scale (cpgs), short form‐36 bodily pain scale (sf‐36 bps), and measure of intermittent and constant osteoarthritis pain (icoap). Arthritis Care Res (Hoboken). 2011;63(S11):S240–S252. [DOI] [PubMed] [Google Scholar]
- 23.Martin B, Cameron M. Evaluation of walking speed and functional ambulation categories in geriatric day hospital patients. Clin Rehabil. 1996;10(1):44–46. doi: 10.1177/026921559601000109 [DOI] [Google Scholar]
- 24.Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023–1031. doi: 10.1093/aje/kwg081 [DOI] [PubMed] [Google Scholar]
- 25.Eichler S, Rabe S, Salzwedel A, et al. Effectiveness of an interactive telerehabilitation system with home-based exercise training in patients after total hip or knee replacement: study protocol for a multicenter, superiority, no-blinded randomized controlled trial. Trials. 2017;18(1):438. doi: 10.1186/s13063-017-2173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills. 2006;103(1):215–222. doi: 10.2466/pms.103.1.215-222 [DOI] [PubMed] [Google Scholar]
- 27.Connelly D, Thomas B, Cliffe S, Perry W, Smith R. Clinical utility of the 2-minute walk test for older adults living in long-term care. Physiother Can. 2009;61(2):78–87. doi: 10.3138/physio.61.2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen MT, Kehlet H. The basic mobility status upon acute hospital discharge is an independent risk factor for mortality up to 5 years after hip fracture surgery: survival rates of 444 pre-fracture ambulatory patients evaluated with the cumulated ambulation score. Acta Orthop. 2018;89(1):47–52. doi: 10.1080/17453674.2017.1382038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. doi: 10.1016/j.ejpain.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33. doi: 10.1136/ard.2004.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vance CGT, Rakel BA, Blodgett NP, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92(7):898–910. doi: 10.2522/ptj.20110183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakel BA, Zimmerman MB, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo-controlled trial. Pain®. 2014;155(12):2599–2611. doi: 10.1016/j.pain.2014.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeSantana JM, Sluka KA, Lauretti GR. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: a randomized controlled trial. Clin J Pain. 2009;25(1):12–19. doi: 10.1097/AJP.0b013e31817d1070 [DOI] [PubMed] [Google Scholar]
- 34.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10(6):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain®. 2013;154(11):2554–2562. doi: 10.1016/j.pain.2013.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4(3):197–209. doi: 10.2217/pmt.14.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenkman M, Hughes MA, Samsa G, Studenski S. The relative importance of strength and balance in chair rise by functionally impaired older individuals. J Am Geriatr Soc. 1996;44(12):1441–1446. doi: 10.1111/j.1532-5415.1996.tb04068.x [DOI] [PubMed] [Google Scholar]
- 38.Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community‐living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575–1577. doi: 10.1111/j.1532-5415.2008.01777.x [DOI] [PubMed] [Google Scholar]
- 39.Woolf CJ. Obituary: Patrick D. Wall (1925–2001). Nature. 2001;413(6854):378. doi: 10.1038/35096680 [DOI] [PubMed] [Google Scholar]
- 40.Lee C-H, Lee T-Y, Her J-S, Liao W-L, Hsieh C-L. Single-blinded, randomized preliminary study evaluating the effect of transcutaneous electrical nerve stimulation on postoperative pain in patients with colles’ fracture. J Altern Complement Med. 2015;21(12):754–758. doi: 10.1089/acm.2015.0119 [DOI] [PubMed] [Google Scholar]
- 41.Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A. 2002;57(8):M539–M543. doi: 10.1093/gerona/57.8.M539 [DOI] [PubMed] [Google Scholar]
- 42.Gopalkrishnan P, Sluka K. TENS reduces primary hyperalgesia and dorsal horn neuron sensitization in rats. Soc Neurosci Abstr. 1998;24:893. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.