Abstract
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism and ovulatory dysfunction. Women with PCOS have an elevated prevalence of cardiometabolic risk factors that worsen after menopause. Liraglutide (Lira), a glucagon-like peptide-1 receptor agonist, has shown beneficial metabolic effects in small clinic trials in reproductive-age women with PCOS. We have shown that chronic hyperandrogenemia in an experimental model of postmenopausal PCOS is associated with an adverse cardiometabolic profile and upregulation of the intrarenal renin-angiotensin system (RAS). We analyzed the effect of Lira in the cardiometabolic profile, intrarenal RAS, and blood pressure (BP) in postmenopausal PCOS. Four-week-old female Sprague Dawley rats were treated with DHT or placebo for 17 months. Lira administration during the last 3 weeks caused a bigger reduction in food intake, body weight, fat mass, and homeostasis model assessment of insulin resistance index in PCOS than in control rats. Moreover, Lira improved dyslipidemia and elevated leptin levels in PCOS. In contrast, Lira decreased intrarenal expression of RAS components only in the control group. Lira transiently increased heart rate and decreased BP in control rats. However, Lira did not modify BP but increased heart rate in PCOS. The angiotensin-converting-enzyme inhibitor enalapril abolished the BP differences between PCOS and control rats. However, Lira coadministration with enalapril further reduced BP only in control rats. In summary, Lira has beneficial effects for several cardiometabolic risk factors in postmenopausal PCOS. However, hyperandrogenemia blunted the BP-lowering effect of Lira in postmenopausal PCOS. Androgen-induced activation of intrarenal RAS may play a major role mediating increases in BP in postmenopausal PCOS.
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in reproductive-age women, is characterized by hyperandrogenism and ovarian dysfunction (1–5). PCOS is often associated with increased prevalence of metabolic syndrome, hypertension, obesity, type 2 diabetes, and dyslipidemia (6–10). Whether women with PCOS have high cardiovascular disease (CVD) morbidity and mortality is still unclear.
Both reproductive-age and postmenopausal women with PCOS have elevated cardiovascular risk factors (11, 12). The incidence of CVD significantly increases in women after menopause. Whether elevated cardiovascular risk factors throughout the lifespan among women with PCOS lead to increased CVD after menopause remains highly controversial. Epidemiological studies addressing the incidence of CVD in women with PCOS have shown conflicting results. Several reports have shown an increase in CVD in postmenopausal women with PCOS (11, 13–15). On the other hand, adding to the controversy, several studies have reported a lack of increase in CVD in postmenopausal women with PCOS compared with control subjects (12, 16, 17), including a recent large meta-analysis (18). There are multiple plausible explanations for this discrepancy, including biological and methodological ones. Despite the increasing incidence of PCOS in the general population and its socioeconomic importance, there is still a lack of properly designed and controlled large prospective studies on the incidence of CVD in postmenopausal PCOS. Furthermore, the presence of multiple PCOS diagnosis criteria that have overlapped over time complicates long-term follow-up studies. From a biological point of view, the discrepancy between cardiovascular risk factors and CVD in postmenopausal women can be attributed to an overall increase in CVD after menopause and to postulated unknown protective factors that actively decrease cardiovascular events in the aging population with PCOS. In summary, the role of hyperandrogenemia and increased cardiovascular risk factors in CVD in postmenopausal women with PCOS remains unclear, and both basic and clinical research are needed to advance our knowledge about this population.
Glucagon-like peptide-1 (GLP-1) is a gut hormone secreted by L-cells located in the intestine in response to meal ingestion [as reviewed in (19)]. GLP-1 stimulates insulin release in a glucose-dependent manner, decreases glucagon production, and decreases gastric emptying. Clinical trials have shown that liraglutide (Lira), a GLP-1 receptor agonist (GLP-1 RA), causes weight loss and reduces androgen levels in young women with PCOS (20). Interestingly, despite the metabolic beneficial effects of GLP-1 in women with PCOS, systolic and diastolic blood pressure (BP) were not affected and heart rate (HR) increased (21). The mechanisms by which GLP-1 improves some cardiovascular risk factors but did not affect BP in women with PCOS have not been determined.
Women with PCOS often have elevated BP (22), and the renin-angiotensin system (RAS) plays a major role in several forms of hypertension. The liver releases angiotensinogen into the circulation, which is later enzymatically cleaved by renin to angiotensin I. Angiotensin I is then cleaved by angiotensin-converting enzyme (ACE) to produce angiotensin II (Ang II), which binds to the ANG II receptor type 1 (AT1R) to elicit vasoconstriction. Telmisartan, an AT1R blocker, reduces BP in patients with PCOS (23). GLP-1 RAs lower BP in several animal models of hypertension and in normotensive rats (24, 25). Furthermore, GLP-1 can interact with the RAS and lower levels of Ang II [as reviewed in (26)]. Whether Lira would lower BP in postmenopausal PCOS via downregulation of intrarenal RAS remains unknown.
We previously reported that chronic androgen excess in an animal experimental model of postmenopausal PCOS is associated with an adverse cardiometabolic profile and upregulation of intrarenal RAS (27). In the current study we examined the effect of the GLP-1 RA Lira in the cardiometabolic profile and intrarenal RAS expression, as well as the effect of Lira alone or in combination with the RAS inhibitor enalapril on BP in a postmenopausal model of PCOS. We tested the hypothesis that administration of the GLP-1 RA Lira attenuates the cardiometabolic complications triggered by excess androgens in an animal experimental model of postmenopausal PCOS.
Materials and Methods
Experimental model of postmenopausal PCOS
Female Sprague Dawley rats were obtained from Envigo (Indianapolis, IN) at 3 weeks of age. At 4 weeks of age, rats were randomly implanted subcutaneously with continuous-release DHT (7.5 mg/90 days; Innovative Research of America, Sarasota, FL) or placebo pellets on the back of the neck, as previously reported (28). Pellets were replaced every 90 days for the duration of the experimental period. Rats were maintained throughout the study on standard rat chow diet (Teklad 22/5 Rodent Diet; catalog no. 8640; Envigo), housed in temperature-controlled rooms with a constant light/dark cycle (12 h/12 h) and free access to food and water. All experimental protocols were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (29), and reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Anthropometric measurements and body composition
At 17 months of age, a set of postmenopausal PCOS and control rats (n = 5 to 7 per group) were randomly assigned to additionally receive either Lira (0.3 mg/kg body weight) or saline subcutaneously for 21 days. Food intake and body weight were recorded daily. At the end of the experimental period, abdominal circumference (anterior to the forefoot) and body length (nose-anus length) were determined. The measurements were made in anesthetized rats under gas anesthesia (isoflurane). Body mass index (BMI) was calculated as body weight (in grams) divided by the square of body length (in centimeters). Body composition (total body fat mass and total body lean mass) was measured by EchoMRI (4in1-900 model Body Composition Analyzer; EchoMRI, Houston, TX) and replicated three times per rat according to manufacturer’s directions, as previously reported (30). Values and delta change (before and after treatment of each group of rats) are presented in grams.
Leptin, insulin resistance, lipid panel, and DHT measurement
Glucose and insulin levels were measured in blood drops obtained from clipped tails in rats fasted 6 hours (8:00 am to 2:00 pm). Glucose levels were measured with a Contour Next Bayer glucometer and reported in milligrams per deciliter. Blood samples were collected in EDTA tubes, handled at 4°C throughout, and centrifuged at 1300g for 20 minutes. Plasma phases were aliquoted and stored at −80°C. Insulin resistance was assessed via the homeostasis model assessment of insulin resistance (HOMA-IR). Plasma insulin and leptin were measured by commercially available ELISA kits according to the manufacturer’s recommendations [Crystal Chem Inc, Elk Grove Village, IL (31) and R&D Systems Inc, Minneapolis, MN (32), respectively]. Total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured with a VET Axcell Chemistry Analyzer (Alfa Wassermann Diagnostic Technologies, West Caldwell, NJ) and reported in milligrams per deciliter. Plasma DHT was measured at the end of the experimental period with a commercially available DHT RIA kit (33) (DSL9600; Beckman Coulter, Inc., Brea, CA) preceded by an oxidation/extraction procedure, according to the manufacturer’s recommendations, as previously reported (28). DHT is reported in picograms per milliliter.
mRNA expression of intrarenal RAS components
At the end of the experimental protocol, total kidney RNA was extracted, DNAse treated, quantified, and reverse transcribed, as previously reported (30). Gene expression of intrarenal RAS was quantified by quantitative RT-PCR with Sybr-Green I technology, as previously reported (30). PCR product quantification was performed by the relative quantification method and expressed as arbitrary units standardized against β-actin (30). Primers, annealing temperature, and PCR amplicon size have been previously reported (30).
BP and HR measurement
At 16.5 months of age, a different set of PCOS and control rats (n = 6 per group) were implanted with radiotelemetry transmitters (HD-SD10; Data Sciences International, St. Paul, MN) into the abdominal aorta below the renal arteries, as previously described (31). After a 2-week recovery period, all rats received daily vehicle (saline 0.9%) injections subcutaneously for 3 days (baseline period). Then, rats were cotreated with daily Lira (0.3 mg/kg body weight) subcutaneously for 21 days (phase 1). Then, after a 10-day washout period, the ACE inhibitor enalapril (250 mg/L in drinking water) was coadministered to PCOS and control groups for 7 days (phase 2). Finally, a combination of oral enalapril and daily Lira subcutaneous injections was coadministered to PCOS and control groups for eight consecutive days (phase 3). Animals remained treated with DHT or placebo pellets throughout the complete experimental period. Mean, systolic, and diastolic arterial BP and HR were monitored continuously in freely moving conscious animals. Telemetry BP measurements were obtained during a 10-second sampling period (500 Hz), recorded and averaged every 5 minutes, 24 hours per day, as previously reported (30). Light/dark cycle variations (constant light/dark cycle of 12 hours) of systolic and diastolic arterial BP and HR were analyzed throughout the study. Arterial BP is reported in millimeters of mercury and HR as beats per minute.
Statistical analyses
All data are expressed as mean ± SEM. Data were analyzed by Student t test (for two groups) and two- or three-way ANOVA followed by Tukey post hoc tests or linear regression. Time series data were analyzed by two-way ANOVA followed by multiple comparison tests corrected by Benjamini and Hochberg false discovery rate method. Differences were considered statistically significant at P < 0.05. Statistical analyses were performed with GraphPad Prism 8 software package version 8.2 (GraphPad Software Inc., La Jolla, CA).
Results
Effect of Lira on food intake, body weight, and body composition in postmenopausal PCOS
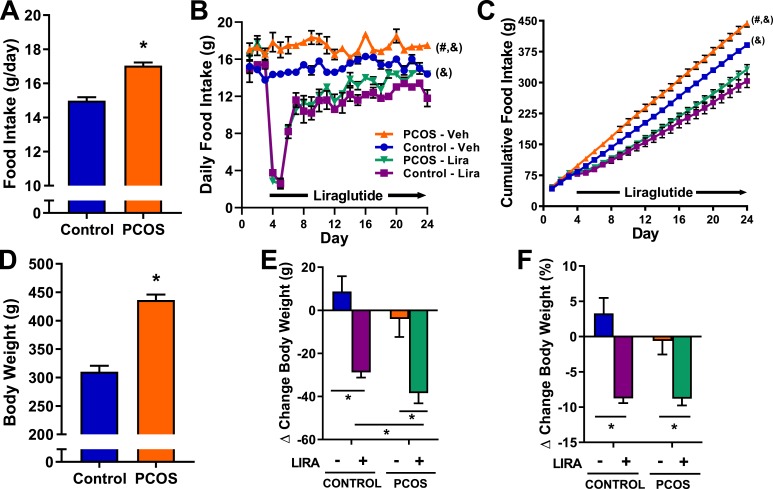
As shown in Fig. 1A, at 17 months of age and before Lira treatment, postmenopausal PCOS rats have higher daily food consumption (17.1 ± 0.2 g vs 14.8 ± 0.2 g, P < 0.05) compared with age-matched control rats. The effects of Lira treatment on daily and cumulative food intake are shown in Fig. 1B and 1C, respectively. Lira abruptly and transiently decreased food intake in both PCOS and control rats for 4 days (Fig. 1B, days 4 to 7). Food intake remained decreased in both PCOS and control animals for the rest of the experimental period (Fig. 1B, days 7 to 24). Although food intake in both Lira-treated groups (PCOS and control) remained lower than in vehicle-treated matched ones, we observed a tachyphylactic effect in Lira treatment in both PCOS and control groups. Tachyphylaxis was analyzed by linear regression on food intake data gathered between days 7 and 24 (Table 1). Lira-treated PCOS and control animals showed a significant positive nonzero slope in food intake over time (days 7 to 24) in contrast to vehicle-treated animals (Table 1). Nevertheless, neither of the Lira-treated groups reached their baseline food intake (before Lira treatment) nor the levels of their vehicle-treated counterparts. (Fig. 1B). Figure 1C shows the cumulative food intake through the experimental period, allowing us to more easily appreciate the treatment effects. The cumulative food intake was significantly elevated in vehicle-treated PCOS compared with vehicle-treated control animals (443.6 ± 8.9 g vs 380.9 ± 6.1 g, P < 0.05). Lira significantly decreased food intake in both PCOS and control animals (332.2 ± 11.3 g vs 443.6 ± 8.9 g, P < 0.05; 303.8 ± 15.7 g vs 380.9 ± 6.1 g, P < 0.05).
Figure 1.
Food intake and body weight in postmenopausal PCOS rats. (A) At 17 months of age and before treatment with Lira, food intake was higher in PCOS rats compared with controls. (B, C) Daily and cumulative food intake were higher in vehicle-treated PCOS rats compared with vehicle-treated controls. Lira treatment caused a significant reduction in daily and cumulative food intake in both PCOS and controls. (D) At 17 months of age and before treatment with Lira, body weight was higher in PCOS rats compared with controls. (E, F) Lira treatment decreased body weight in PCOS and controls, expressed as change in (E) grams and (F) percentage before and after Lira treatment; the absolute amount of body weight lost with Lira was higher in PCOS compared with control rats. Data are expressed as mean ± SEM. Data were analyzed by (A, D) t test, (B, C) two-way ANOVA with repeated measures followed by Tukey post hoc tests, or (E, F) two-way ANOVA followed by Tukey post hoc tests. No significant interactions were observed by two-way ANOVA. *P < 0.05; #P < 0.05 vs vehicle-treated controls; &P < 0.05 vs Lira-treated controls. n = 5 to 7 per group.
Table 1.
Tachyphylaxic Analysis of Lira on Daily Food Intake by Linear Regression
| Slope (95% CI) | F Statistic | P Value | |
|---|---|---|---|
| Control-vehicle | 0.0184 (−0.018–0.055) | F(1, 88) = 1.02 | 0.316 |
| Control-Lira | 0.159 (0.085–0.233) | F(1, 88) = 18.3 | <0.0001 |
| PCOS-vehicle | −0.034 (−0.080–0.012) | F(1, 106) = 2.10 | 0.150 |
| PCOS-Lira | 0.249 (0.197–0.302) | F(1, 124) = 89.0 | <0.0001 |
Daily food intake from experimental days 7–24 (days 4–21 after Lira treatment initiation) were analyzed by linear regression. Statistically significant values are in bold type.
At 17 months of age and before Lira treatment, PCOS rats were 40.6% heavier than controls (436.3 ± 9.7 g vs 310.1 ± 10.5 g, P < 0.05) (Fig. 1D). Lira treatment significantly decreased body weight in PCOS and control rats. As shown in Fig. 1E, the amount of body weight lost with Lira was bigger in PCOS compared with control rats (−38.4 ± 4.7 g vs −28.8 ± 2.4 g, P < 0.05), although the percentage of body weight loss was similar in both groups (Fig. 1F).
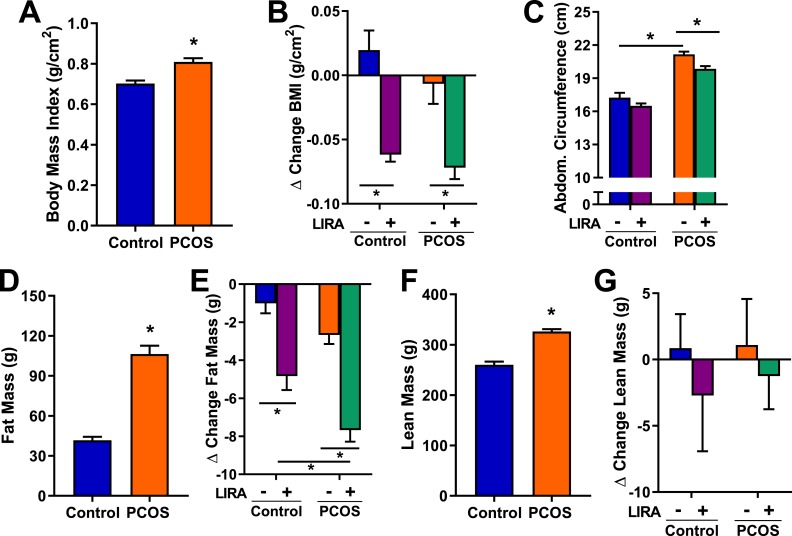
As shown in Fig. 2A, at 17 months of age and before Lira treatment, PCOS rats had a higher BMI compared with controls (0.81 ± 0.02 g/cm2 vs 0.7 ± 0.07 g/cm2, P < 0.05). Lira treatment decreased BMI by ∼8% in PCOS and control rats (Fig. 2B). The abdominal circumference was bigger in PCOS rats compared with controls at baseline (21.2 ± 0.2 g vs 17.2 ± 0.4 g, P < 0.05). Lira treatment significantly decreased the abdominal circumference in PCOS rats (Fig. 2C). At 17 months of age and before Lira treatment, body mass composition analysis by EchoMRI showed higher fat mass in PCOS rats compared with controls (Fig. 2D). Lira caused a greater fat mass reduction in PCOS (−7.7 ± 0.6 g vs −4.8 ± 0.7 g, P < 0.05) compared with controls (Fig. 2E). Lean mass by EchoMRI was higher in PCOS rats compared with controls and was unchanged by Lira treatment (Fig. 2F and 2G).
Figure 2.
Anthropometric measurements and body composition in postmenopausal PCOS rats. (A) At 17 months of age and before treatment with Lira, PCOS rats had higher BMI compared with controls. (B) Lira decreased BMI, expressed as change before and after Lira treatment, in both PCOS and control rats. (C) Abdominal circumference was greater in PCOS; after Lira treatment, a decrease in abdominal circumference was observed only in PCOS rats. (D, F) At 17 months of age and before treatment with Lira, PCOS rats had greater (D) fat and (F) lean mass compared with controls. (E) Lira treatment caused a bigger decrease in fat mass, expressed as change before and after Lira treatment, in PCOS compared with controls. (G) Lira treatment did not modify lean mass, expressed as change before and after Lira treatment, in any group. Data are expressed as mean ± SEM. Data were analyzed by (A, D, F) t test or (B, C, E, G) two-way ANOVA followed by Tukey post hoc tests. No significant interactions were observed by two-way ANOVA. *P < 0.05. n = 5 to 7 per group.
Effect of Lira on leptin, insulin resistance, and lipids in postmenopausal PCOS
At 17 months of age and before Lira treatment, fasting insulin levels and HOMA-IR were higher in PCOS rats compared with controls (Fig. 3A and 3B). After Lira treatment, a significant decrease in HOMA-IR was observed only in PCOS rats (−4.5 ± 0.6 vs −1.8 ± 0.9, P < 0.05), whereas no changes were observed in controls (Fig. 3C). Lira significantly decreased leptin levels in PCOS rats (2.3 ± 0.4 ng/mL vs 4.1 ± 0.5 ng/mL, P < 0.05), whereas no effect was observed in controls (Fig. 3D). PCOS rats had significantly higher total cholesterol, LDL cholesterol, and triglycerides levels than controls (Fig. 3E, 3F, and 3H). HDL cholesterol showed a nonsignificant tendency to increase (Fig. 3G). Lira lowered total cholesterol (177.8 ± 17.6 mg/dL vs 123.1 ± 13.9 mg/dL, P < 0.05), LDL cholesterol (23.4 ± 3.1 mg/dL vs 16 ± 1.8 mg/dL, P < 0.05), HDL cholesterol (47.6 ± 2.1 mg/dL vs 33.7 ± 2.9 mg/dL, P < 0.05), and triglycerides (205.6 ± 29.6 mg/dL vs 116.1 ± 16.2 mg/dL, P < 0.05) in PCOS rats without significant changes in control rats (Fig. 3E–3H).
Figure 3.
Metabolic parameters, lipid panel, and DHT levels in postmenopausal PCOS rats. (A, B) At 17 months of age and before treatment with Lira, (A) fasting insulin levels and (B) HOMA-IR were higher in PCOS rats compared with controls. (C) Lira treatment decreased HOMA-IR, expressed as change before and after Lira treatment, in PCOS; however, no significant changes were observed in control rats. (D–H) Lira treatment decreased (D) leptin, (E) total cholesterol, (F) LDL cholesterol, (G) HDL cholesterol, and (H) triglycerides in PCOS; however, no significant changes were observed in control rats. (I) PCOS rats had higher DHT levels that were unaffected by Lira treatment. Data are expressed as mean ± SEM. Data were analyzed by (A, B) t test or (C–H) two-way ANOVA followed by Tukey post hoc tests. No significant interactions were observed by two-way ANOVA. *P < 0.05. n = 5 to 7 per group.
DHT plasma levels in postmenopausal PCOS
At the end of the experimental protocol, plasma DHT levels were higher in PCOS rats than in controls (277.3 ± 53.3 pg/mL vs 20.2 ± 3.1 pg/mL, P < 0.05). Lira treatment did not affect plasma DHT levels in PCOS or control rats (Fig. 3I).
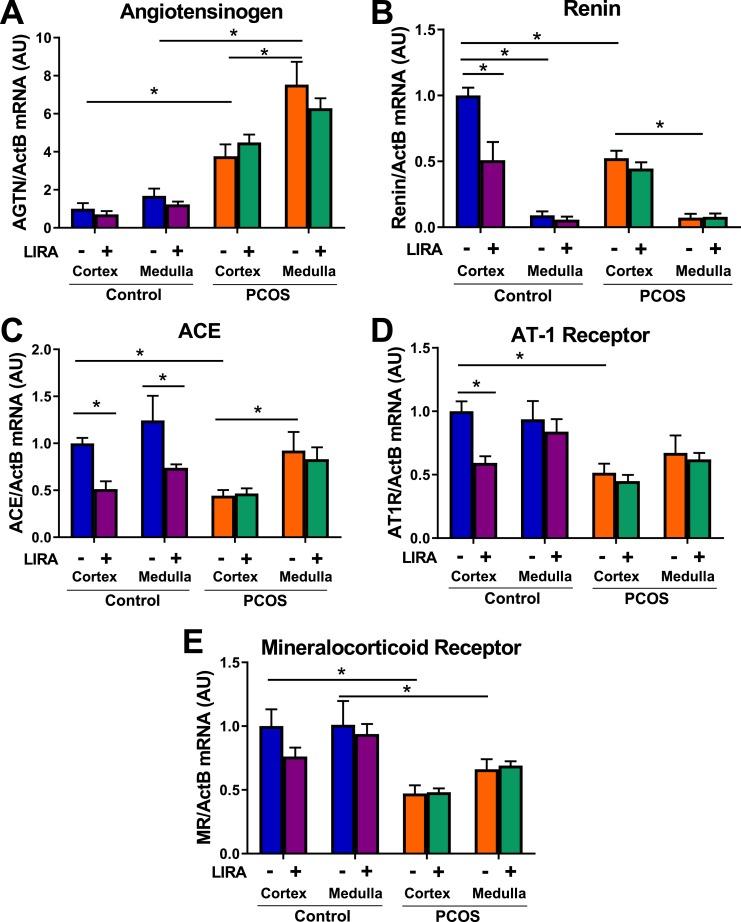
Effect of Lira on intrarenal mRNA expression of RAS in postmenopausal PCOS
Renal cortical and medullary mRNA expressions of angiotensinogen were approximately four and six times higher, respectively, in PCOS rats compared with control rats. Lira did not affect angiotensinogen mRNA expression in either experimental group (Fig. 4A). Renin and AT1R mRNA expression were significantly higher in controls compared with PCOS rats in the kidney cortex. Lira lowered mRNA cortical expression of renin and AT1R in control rats but not in PCOS rats (Fig. 4B and 4D). Lira lowered renal ACE mRNA expression in the cortex and medulla of control rats but had no effect in PCOS rats (Fig. 4C). Renal cortical and medullary mineralocorticoid receptor mRNA expression was higher in control rats than in PCOS rats but was not affected by Lira in either experimental group (Fig. 4E).
Figure 4.
Expression of intrarenal RAS components in postmenopausal PCOS rats. (A) Renal cortical and medullar mRNA expression of angiotensinogen were four and six times higher, respectively, in PCOS compared with control rats. Lira did not affect renal angiotensinogen mRNA expression. (B) Renal expression of renin was higher in the cortex compared with medulla in both groups. Renal cortical expression of renin was significantly higher in controls than in PCOS rats. Lira lowered renal cortical mRNA expression of renin in control rats but not in PCOS rats. (C) Renal cortical ACE mRNA expression was significantly decreased in PCOS rats; in contrast, no differences were observed in the renal medulla. Lira lowered renal ACE mRNA expression in the cortex and medulla of control rats and had no effect in PCOS. (D) Renal cortical expression of AT1R was significantly higher in controls than in PCOS rats. Lira lowered renal cortical mRNA expression of renin in control rats but not in PCOS rats. (E) Renal cortical and medullar mRNA expressions of mineralocorticoid receptor were higher in control rats. Lira did not affect renal mineralocorticoid mRNA expression. *P < 0.05. Data are expressed as mean ± SEM. Data were analyzed by three-way ANOVA followed by Tukey post hoc tests. Significant interaction was observed by three-way ANOVA only for renin, whereas it was not significant for all other analyzed genes. *P < 0.05. n = 5 to 7 per group.
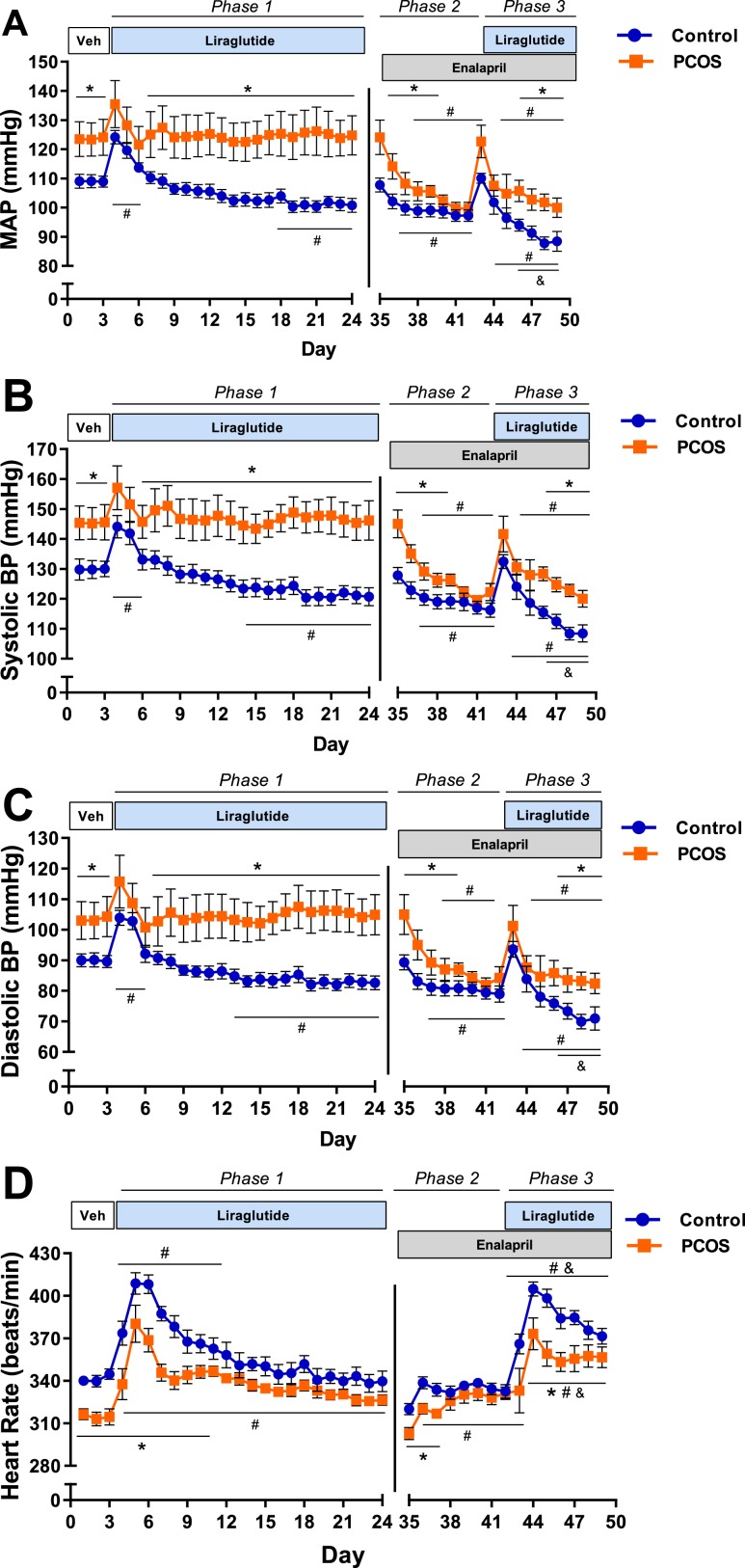
Effect of Lira and ACE inhibitor on BP and HR in postmenopausal PCOS
As shown in Fig. 5A, at baseline, mean arterial pressure (MAP) was significantly higher in PCOS rats than in controls (124 ± 3 mm Hg vs 109 ± 1 mm Hg, P < 0.05). Lira treatment caused a significant increase in MAP the first 2 days in both PCOS and control rats. Chronic Lira administration (21 days) decreased MAP in control rats (108 ± 1 mm Hg vs 101 ± 1 mm Hg, P < 0.05) and had no effect on MAP in PCOS (123 ± 2 mm Hg vs 126 ± 3 mm Hg, P = 0.56, phase 1). Enalapril administration abolished differences in BP between both groups (99 ± 2 mm Hg vs 97 ± 2 mm Hg, P = 0.42, phase 2). Lira and enalapril coadministration further decreased BP in the control group (100 ± 2 mm Hg vs 87 ± 2 mm Hg, P < 0.05); however, it did not affect PCOS rats (phase 3). A similar pattern of BP regulation was observed in both systolic and diastolic BP (Fig. 5B and 5C).
Figure 5.
Measurements of BP and HR in postmenopausal PCOS rats. (A, B, C) Female Sprague Dawley rats were treated with DHT or placebo pellets for 16.5 months, implanted with telemetry probes, and allowed to recover for 2 weeks. Animals were injected daily with saline (Veh), and BP was recorded for 3 days. Then, animals were treated daily with Lira and BP recorded for 21 days (phase 1). Animals were subjected to a 10-day washout period and then treated with enalapril, and BP was recorded for 7 days (phase 2). Then, animals were cotreated with enalapril and Lira, and BP was recorded for 7 days (phase 3). DHT treatment was maintained throughout the experimental period. (A) Mean, (B) systolic, and (C) diastolic arterial BP were higher in PCOS rats than in controls at baseline. Lira treatment decreased BP in control rats; however, it did not lower BP in PCOS rats (phase 1). Enalapril treatment abolished the BP differences between PCOS rats and controls (phase 2). The combination of enalapril and Lira further decreased BP in controls but not in PCOS rats (phase 3). (D) HR was significantly lower in PCOS rats at baseline. Lira caused a significant increase in HR in controls over 8 days. From day 9 until the end of the treatment, HR returned to the baseline level (phase 1). In contrast, Lira caused a significant increase in HR in PCOS rats compared with baseline; this increase in HR remained throughout the treatment period (phase 1). Enalapril treatment increased HR in both groups and abolished the differences between them (phase 2). Although Lira and enalapril coadministration further increased HR in PCOS and control groups, the increase in HR was greater in controls than in PCOS rats (phase 3). Data are expressed as mean ± SEM. Data were analyzed by two-way ANOVA followed by multiple-comparison tests corrected by Benjamini and Hochberg false discovery rate method. Significant interactions (treatment and time) were observed in all cases by two-way ANOVA. *P < 0.05 vs controls; #P < 0.05 vs baseline, same treatment; &P < 0.05 vs enalapril, same treatment. n = 6 per group.
As shown in Fig. 5D, HR was significantly lower in PCOS rats at baseline (315 ± 3 beats/min vs 342 ± 2 beats/min, P < 0.05) compared with controls. Lira caused a significant increase in HR in controls during the first 8 days of treatment; however, at day 9, HR returned to the baseline levels until the end of the treatment (phase 1). In contrast, in PCOS rats, Lira caused a significant increase in HR that remained throughout the phase 1 treatment period (326 ± 2 beats/min vs 315 ± 3 beats/min, P < 0.05). At the end of 21 consecutive days of Lira treatment (phase 1), no differences in HR were observed between PCOS rats and controls. Enalapril increased HR in both groups and abolished the difference between them (phase 2). Lira and enalapril coadministration further increased HR in PCOS and controls; however, the increase in HR was greater in controls than in with PCOS rats (phase 3).
Whole hearts (1.49 ± 0.05 g vs 1.07 ± 0.07 g, P < 0.05) and left ventricles (1.01 ± 0.09 g vs 0.75 ± 0.05 g, P < 0.05) were heavier in PCOS rats compared with controls. Lira treatment did not affect the heart or the left ventricle weight in any group (data not show).
Light/dark cycle variations of BP and HR in postmenopausal PCOS
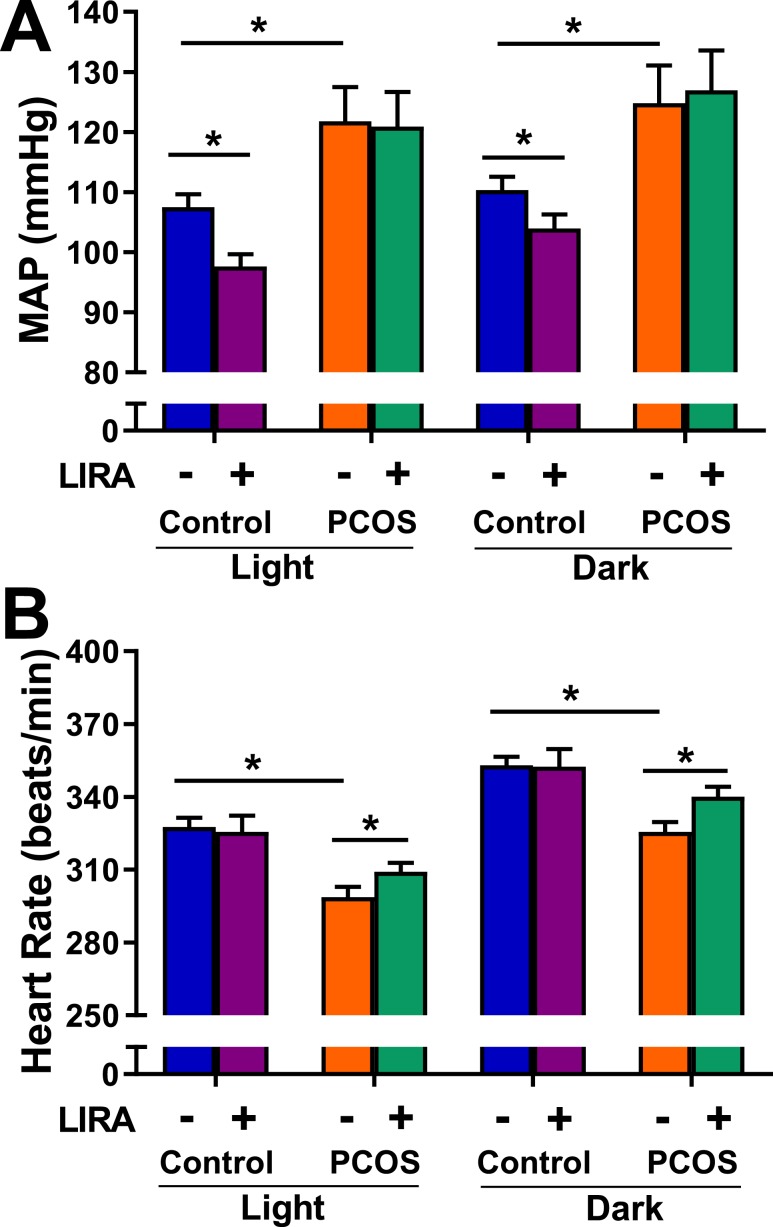
At baseline, MAP was higher in PCOS rats in light and dark cycles compared with controls (Fig. 6A). Lira treatment lowered MAP in control rats during light and dark cycles; however, no significant changes in MAP were observed in PCOS animals (Fig. 6A). Baseline HR was higher in light and dark cycles in controls compared with PCOS rats (Fig. 6B). Lira treatment significantly increased HR during both light and dark cycle only in PCOS (Fig. 6B). The decrease in MAP observed in controls was due to similar level of decrease in both systolic and diastolic BP (data not shown).
Figure 6.
Light/dark cycle variations of BP and HR in postmenopausal PCOS rats. (A) MAP was higher in PCOS during light and dark cycles compared with control. Lira lowered MAP in controls during light and dark cycles; however, no changes were observed in PCOS. (B) HR was higher in control rats during light and dark cycles. Lira increased HR during both light and dark cycles only in PCOS. Data are expressed as mean ± SEM. Data were analyzed by three-way ANOVA followed by Tukey post hoc tests. No significant interactions were observed by three-way ANOVA. *P < 0.05. n = 6 per group.
Discussion
Our study shows that chronic administration of the GLP-1 RA Lira results in a significant improvement of several cardiometabolic risk factors such as insulin resistance, obesity, dyslipidemia, and leptin levels in an experimental model of postmenopausal PCOS. Interestingly, the well-known BP-lowering effect of GLP-1 RAs was observed only in control rats, whereas a significant increase in HR was observed only in PCOS. Additionally, our data support the key role played by androgens in mediating upregulation of intrarenal RAS and increasing BP after menopause in PCOS.
PCOS in reproductive-age women is characterized by ovarian dysfunction, hyperandrogenism, and increased prevalence of cardiometabolic risk factors. Data from epidemiological studies analyzing the PCOS cardiometabolic complications in the postmenopausal years are limited and conflicting. Some studies suggest that insulin resistance, myocardial infarction, angina, and carotid intima media thickness worsen with age in postmenopausal women with PCOS (34–36). Conversely, other studies have not shown similar findings. In a small cohort of postmenopausal women (25 women), the clinical features of PCOS were not associated with coronary artery disease or mortality despite a trend toward more frequent angiographic evidence of multivessel coronary artery disease (17). Larger prospective studies with women with established PCOS who transition to a postmenopausal state are needed to clarify whether the postmenopausal period carries additional risk for CVD in PCOS. The present findings confirmed our previous data that chronic hyperandrogenemia in an experimental model of postmenopausal PCOS leads to a significant increase in body weight, adiposity, insulin resistance, BP, and upregulation of intrarenal RAS. In perimenopausal women, high free androgen index is strongly associated with cardiovascular risk factors in racially diverse women (37). Furthermore, we have recently shown that the deleterious effects of androgens are long lasting and persist even after the hyperandrogenemia is normalized (30). The current study highlights the cardinal role of androgens in mediating the adverse cardiometabolic profile in PCOS and worsening years after menopause.
Lira is a GLP-1 RA that is approved by the US Food and Drug Administration to treat type 2 diabetes mellitus (T2DM) and promote weight loss in obesity. More than 80% of women with PCOS in the United States are obese or overweight. Furthermore, women with PCOS have a difficult time losing weight, as reported in a recent international cohort study (38). Our study shows that GLP-1 RAs result in a ∼9% weight reduction in PCOS, which is associated with marked improvement in insulin resistance and dyslipidemia.
Dyslipidemia is a common cardiovascular risk factor in women with PCOS (39). However, marked variations has been reported in the type and prevalence of dyslipidemia in different populations. For example, women with PCOS in the United States are more obese and have more severe insulin resistance and a worse lipid profile (lower serum HDL cholesterol and higher serum triglycerides) than Italian women with PCOS (40). Our data show that the animal experimental model of postmenopausal PCOS used in this study is characterized by elevated LDL cholesterol and triglycerides and that Lira treatment abolished such increases. On the other hand, HDL cholesterol was not low in our experimental model, but Lira treatment decreased HDL cholesterol in PCOS animals to control levels. Hyperandrogenemia is associated with low HDL cholesterol levels in women with PCOS (41); however, some studies report that DHT administration does not modify HDL levels in men [as reviewed in (42)]. Compared with other lipoproteins, HDL is highly heterogeneous in size, charge, and composition, varying throughout its stages of maturation from small to large HDL particles. It is noteworthy that in rodents most cholesterol is carried by HDL, whereas in humans the main carrier is LDL (43, 44). Our data agree with those of Mannerås et al. (44), who reported no changes in HDL cholesterol in the young rats from the same PCOS experimental model. It remains unclear whether DHT administration alters the HDL composition or whether these variations are based on species-specific differences.
The beneficial effects of GLP-1 RAs may be due to the low GLP-1 plasma levels in women with PCOS (46). Several studies have shown the beneficial effects of GLP-1 RAs in body weight, endothelial dysfunction, inflammation, hyperandrogenemia, and fertility in reproductive-age women with PCOS [(47–49), reviewed in(50)]. Our data suggest that the metabolic beneficial effects of GLP-1 RAs could be observed in the postmenopausal years as well.
A high prevalence of hypertension across multiple ethnic groups has been reported in PCOS (8, 9, 13, 51–56). GLP-1 RAs reduce BP in patients with T2DM or obesity without T2DM (57). GLP-1 RAs also reduce BP in normotensive rats (24), several male animal models of hypertension such as salt-sensitive mice (25), Ang II–induced hypertension (25), and reproductive-age PCOS rats (58). Our data show that Lira administration decreased BP in controls; however, Lira did not lower BP in postmenopausal PCOS. Interestingly, similar findings were reported in women with PCOS, where exenatide, a GLP-1 RA, did not lower BP (47). Our data suggest that the mechanisms by which GLP-1 RAs reduce BP in the control rats may be related to a reduction in the components of the intrarenal RAS. These findings are in line with the known effect of androgens mediating RAS activation, as shown in other animal models (59). Our data also suggest that postmenopausal status may change the BP response to GLP-1 RAs, because it has been shown recently that administration of Lira to reproductive-age PCOS rodents resulted in lower BP (58).
The RAS plays a major role in several forms of hypertension. Telmisartan, an AT1R blocker, reduces BP in patients with PCOS (23). Our data show that the increases in BP after menopause are mediated by activation of the intrarenal RAS. We showed that RAS blockage with enalapril decreased BP in both groups; however, GLP-1 RAs were able to further decrease BP only in controls but not in postmenopausal PCOS. GLP-1 RAs can inhibit sodium reabsorption in the proximal tubule [as reviewed in (60)]. In contrast, androgens can directly stimulate sodium reabsorption in the proximal tubule (61), which may explain, at least partially, why BP remained significantly elevated in postmenopausal PCOS rats.
Our data show that Lira treatment increases HR (during both dark and light cycle phases) in postmenopausal PCOS. GLP-1 receptors are expressed in the central nervous system, kidney, and heart (62). Human and rodent studies have shown that Lira increases HR (63, 64). This effect had been attributed to activation of autonomic sympathetic preganglionic brainstem neurons in normoglycemic rats (65) and modification of HR through attenuation of parasympathetic tone (66). Therefore, the chronotropic effect of GLP1 RAs may be exacerbated by the presence of androgens. In the rat brain, GLP-1 can activate neurons innervating sympathetic preganglionic neurons in the paraventricular hypothalamus, the arcuate nucleus, and the lateral hypothalamic area [as reviewed in (67)]. Elevated androgens have been previously shown to increase gene expression of pro-opiomelanocortin, the precursor for the MC4R ligand α-MSH, in monkeys and rats (66). Activation of brain MC4R by acute intracerebroventricular injections of pharmacological agonists increases renal sympathetic nervous system activity and HR (68). Maranon et al. (69) recently reported that α/β adrenergic blockade and renal denervation normalize MAP in the young PCOS model, demonstrating that the sympathetic nervous system contributes to the increase in MAP in this experimental model. Thus, central sympathetic activation by androgens could be an indirect mechanism by which HR increases during GLP-1 RA treatment of postmenopausal rats with PCOS. This hypothesis must be tested in the future.
Therapeutic options to treat obesity and its cardiometabolic complications in reproductive-age or postmenopausal women with PCOS are limited. In this study, Lira showed a promising pharmacotherapeutic profile ameliorating most of the cardiometabolic complications in postmenopausal PCOS. However, hyperandrogenemia blunted the BP-lowering effect of GLP-1 RAs independently of the intrarenal RAS. Additional studies are needed to elucidate the cardiovascular actions of GLP-1 RAs in the presence of hyperandrogenemia.
Acknowledgments
We thank the University of Mississippi Medical Center Telemetry, Analytical and Assay, and Histology and Imaging Cores for outstanding service. Research reported in this publication was supported by the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Support: This work was supported by National Institutes of Health (NIH) Grants P20GM-121334 (to L.L.Y.C. and D.G.R.) and R21DK-113500 (to D.G.R.). Mississippi Center of Excellence in Perinatal Research Telemetry, Analytical and Assay, and Histology and Imaging Cores are supported by NIH Grant P20GM-121334. Cores are also supported by NIH Grants P20GM104357 and P01HL51971.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- ACE
angiotensin-converting enzyme
- Ang II
angiotensin II
- AT1R
angiotensin II receptor type 1
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- GLP-1
glucagon-like peptide-1
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment of insulin resistance
- HR
heart rate
- LDL
low-density lipoprotein
- Lira
liraglutide
- MAP
mean arterial pressure
- PCOS
polycystic ovary syndrome
- RAS
renin-angiotensin system
- T2DM
type 2 diabetes mellitus
References and Notes
- 1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. [DOI] [PubMed] [Google Scholar]
- 2. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):16057. [DOI] [PubMed] [Google Scholar]
- 4. McCartney CR, Marshall JC. Clinical practice. Polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. [DOI] [PubMed] [Google Scholar]
- 6. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038–2049. [DOI] [PubMed] [Google Scholar]
- 7. Aziz M, Sidelmann JJ, Faber J, Wissing ML, Naver KV, Mikkelsen AL, Nilas L, Skouby SO. Polycystic ovary syndrome: cardiovascular risk factors according to specific phenotypes. Acta Obstet Gynecol Scand. 2015;94(10):1082–1089. [DOI] [PubMed] [Google Scholar]
- 8. Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1357–1363. [DOI] [PubMed] [Google Scholar]
- 9. Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001;16(3):556–560. [DOI] [PubMed] [Google Scholar]
- 10. Dokras A. Cardiovascular disease risk factors in polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):39–44. [DOI] [PubMed] [Google Scholar]
- 11. Krentz AJ, von Mühlen D, Barrett-Connor E. Searching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular disease. Menopause. 2007;14(2):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meun C, Franco OH, Dhana K, Jaspers L, Muka T, Louwers Y, Ikram MA, Fauser BCJM, Kavousi M, Laven JSE. High androgens in postmenopausal women and the risk for atherosclerosis and cardiovascular disease: The Rotterdam Study. J Clin Endocrinol Metab. 2018;103(4):1622–1630. [DOI] [PubMed] [Google Scholar]
- 13. Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf). 2000;52(5):595–600. [DOI] [PubMed] [Google Scholar]
- 14. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. [DOI] [PubMed] [Google Scholar]
- 15. Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, Blackledge H, Khunti K, Howlett TA. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol (Oxf). 2013;78(6):926–934. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt J, Landin-Wilhelmsen K, Brännström M, Dahlgren E. Cardiovascular disease and risk factors in PCOS women of postmenopausal age: a 21-year controlled follow-up study. J Clin Endocrinol Metab. 2011;96(12):3794–3803. [DOI] [PubMed] [Google Scholar]
- 17. Merz CN, Shaw LJ, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-DeHoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Cardiovascular disease and 10-year mortality in postmenopausal women with clinical features of polycystic ovary syndrome. J Womens Health (Larchmt). 2016;25(9):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol. 2019;6:1–12. [DOI] [PubMed] [Google Scholar]
- 19. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niafar M, Pourafkari L, Porhomayon J, Nader N. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Arch Gynecol Obstet. 2016;293(3):509–515. [DOI] [PubMed] [Google Scholar]
- 21. Frøssing S, Nylander M, Kistorp C, Skouby SO, Faber J. Effect of liraglutide on atrial natriuretic peptide, adrenomedullin, and copeptin in PCOS. Endocr Connect. 2018;7(1):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luque-Ramírez M, Martí D, Fernández-Durán E, Alpañés M, Álvarez-Blasco F, Escobar-Morreale HF. Office blood pressure, ambulatory blood pressure monitoring, and echocardiographic abnormalities in women with polycystic ovary syndrome: role of obesity and androgen excess. Hypertension. 2014;63(3):624–629. [DOI] [PubMed] [Google Scholar]
- 23. Jensterle M, Janez A, Vrtovec B, Meden-Vrtovec H, Pfeifer M, Prezelj J, Kocjan T. Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with polycystic ovary syndrome: case series. Croat Med J. 2007;48(6):864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laugero KD, Stonehouse AH, Guss S, Landry J, Vu C, Parkes DG. Exenatide improves hypertension in a rat model of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7(4):327–334. [DOI] [PubMed] [Google Scholar]
- 25. Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380(1):44–49. [DOI] [PubMed] [Google Scholar]
- 26. Skov J, Persson F, Frøkiær J, Christiansen JS. Tissue renin-angiotensin systems: a unifying hypothesis of metabolic disease. Front Endocrinol (Lausanne). 2014;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalmasso C, Maranon R, Patil C, Bui E, Moulana M, Zhang H, Smith A, Yanes Cardozo LL, Reckelhoff JF. Cardiometabolic effects of chronic hyperandrogenemia in a new model of postmenopausal polycystic ovary syndrome. Endocrinology. 2016;157(7):2920–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med. 2011;8(2):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed Washington, DC: National Academy Press; 2011. [Google Scholar]
- 30. Torres Fernandez ED, Adams KV, Syed M, Maranon RO, Romero DG, Yanes Cardozo LL. Long-lasting androgen-induced cardiometabolic effects in polycystic ovary syndrome. J Endocr Soc. 2018;2(8):949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.RRID:AB_2732074, https://scicrunch.org/resolver/AB_2732074.
- 32.RRID:AB_2732075, https://scicrunch.org/resolver/AB_2732075.
- 33.RRID:AB_2732078, https://scicrunch.org/resolver/AB_2732078.
- 34. Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90(6):3236–3242. [DOI] [PubMed] [Google Scholar]
- 35. Alalami H, Sathyapalan T, Atkin SL. Cardiovascular profile of pharmacological agents used for the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2018;10:2042018818805674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, Kuller LH. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20(11):2414–2421. [DOI] [PubMed] [Google Scholar]
- 37. Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torréns JI; SWAN Investigators. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111(10):1242–1249. [DOI] [PubMed] [Google Scholar]
- 38. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(2):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073–1079.e1071-1011. [DOI] [PubMed] [Google Scholar]
- 40. Carmina E, Legro RS, Stamets K, Lowell J, Lobo RA. Difference in body weight between American and Italian women with polycystic ovary syndrome: influence of the diet. Hum Reprod. 2003;18(11):2289–2293. [DOI] [PubMed] [Google Scholar]
- 41. Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18(7):280–285. [DOI] [PubMed] [Google Scholar]
- 42. Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA. Dihydrotestosterone: biochemistry, physiology, and clinical implications of elevated blood levels. Endocr Rev. 2017;38(3):220–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordon SM, Li H, Zhu X, Shah AS, Lu LJ, Davidson WS. A comparison of the mouse and human lipoproteome: suitability of the mouse model for studies of human lipoproteins. J Proteome Res. 2015;14(6):2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin W, Carballo-Jane E, McLaren DG, Mendoza VH, Gagen K, Geoghagen NS, McNamara LA, Gorski JN, Eiermann GJ, Petrov A, Wolff M, Tong X, Wilsie LC, Akiyama TE, Chen J, Thankappan A, Xue J, Ping X, Andrews G, Wickham LA, Gai CL, Trinh T, Kulick AA, Donnelly MJ, Voronin GO, Rosa R, Cumiskey AM, Bekkari K, Mitnaul LJ, Puig O, Chen F, Raubertas R, Wong PH, Hansen BC, Koblan KS, Roddy TP, Hubbard BK, Strack AM. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. 2012;53(1):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. [DOI] [PubMed] [Google Scholar]
- 46. Vejrazkova D, Lischkova O, Vankova M, Stanicka S, Vrbikova J, Lukasova P, Vcelak J, Vacinova G, Bendlova B. Distinct response of fat and gastrointestinal tissue to glucose in gestational diabetes mellitus and polycystic ovary syndrome. Physiol Res. 2017;66(2):283–292. [DOI] [PubMed] [Google Scholar]
- 47. Dawson AJ, Sathyapalan T, Vince R, Coady AM, Ajjan RA, Kilpatrick ES, Atkin SL. The effect of exenatide on cardiovascular risk markers in women with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2019;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang FF, Wu Y, Zhu YH, Ding T, Batterham RL, Qu F, Hardiman PJ. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta-analysis. Obes Rev. 2018;19(10):1424–1445. [DOI] [PubMed] [Google Scholar]
- 49. Salamun V, Jensterle M, Janez A, Vrtacnik Bokal E. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol. 2018;179(1):1–11. [DOI] [PubMed] [Google Scholar]
- 50. Lamos EM, Malek R, Davis SN. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2017;10(4):401–408. [DOI] [PubMed] [Google Scholar]
- 51. Holte J, Gennarelli G, Berne C, Bergh T, Lithell H. Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Hum Reprod. 1996;11(1):23–28. [DOI] [PubMed] [Google Scholar]
- 52. Vrbíková J, Cífková R, Jirkovská A, Lánská V, Platilová H, Zamrazil V, Stárka L. Cardiovascular risk factors in young Czech females with polycystic ovary syndrome. Hum Reprod. 2003;18(5):980–984. [DOI] [PubMed] [Google Scholar]
- 53. Chang AY, Oshiro J, Ayers C, Auchus RJ. Influence of race/ethnicity on cardiovascular risk factors in polycystic ovary syndrome, the Dallas Heart Study. Clin Endocrinol (Oxf). 2016;85(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morgan CL, Jenkins-Jones S, Currie CJ, Rees DA. Evaluation of adverse outcome in young women with polycystic ovary syndrome versus matched, reference controls: a retrospective, observational study. J Clin Endocrinol Metab. 2012;97(9):3251–3260. [DOI] [PubMed] [Google Scholar]
- 55. Pinola P, Puukka K, Piltonen TT, Puurunen J, Vanky E, Sundström-Poromaa I, Stener-Victorin E, Lindén Hirschberg A, Ravn P, Skovsager Andersen M, Glintborg D, Mellembakken JR, Ruokonen A, Tapanainen JS, Morin-Papunen LC. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril. 2017;107(3):788–795.e2. [DOI] [PubMed] [Google Scholar]
- 56. Marchesan LB, Spritzer PM. ACC/AHA 2017 definition of high blood pressure: implications for women with polycystic ovary syndrome. Fertil Steril. 2019;111(3):579–587.e1. [DOI] [PubMed] [Google Scholar]
- 57. Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344(jan10 2):d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoang V, Bi J, Mohankumar SM, Vyas AK. Liraglutide improves hypertension and metabolic perturbation in a rat model of polycystic ovarian syndrome. PLoS One. 2015;10(5):e0126119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296(4):F771–F779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thomas MC. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43(suppl 1):2s20–22s27. [DOI] [PubMed] [Google Scholar]
- 61. Quigley R. Androgens stimulate proximal tubule transport. Gend Med. 2008;5(suppl A):S114–120. [DOI] [PubMed] [Google Scholar]
- 62. Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. [DOI] [PubMed] [Google Scholar]
- 63. Mikhail N. Cardiovascular effects of liraglutide. Curr Hypertens Rev. 2019;15(1):64–69. [DOI] [PubMed] [Google Scholar]
- 64. Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, Mighiu AS, Zhang H, Ludwig A, Seeley RJ, Heximer SP, Drucker DJ. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol Metab. 2017;6(11):1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Griffioen KJ, Wan R, Okun E, Wang X, Lovett-Barr MR, Li Y, Mughal MR, Mendelowitz D, Mattson MP. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011;89(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr Opin Pharmacol. 2013;13(6):964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. da Silva AA, do Carmo JM, Wang Z, Hall JE. Melanocortin-4 receptors and sympathetic nervous system activation in hypertension. Curr Hypertens Rep. 2019;21(6):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regul Integr Comp Physiol. 2015;308(8):R708–R713. [DOI] [PMC free article] [PubMed] [Google Scholar]