Abstract
Background:
The AJCC/UICC classification is widely used for predicting survival in papillary thyroid cancer (PTC), but has not been evaluated as a predictor of recurrence. The hypothesis of this study was that the eighth edition of the AJCC system can be used in this novel way.
Methods:
All patients in the study underwent surgery for PTC at a high-volume endocrine surgery centre in France between 1985 and 2015. The seventh and eighth editions of the AJCC/UICC staging system for PTC were employed to predict recurrence and disease-specific survival using the Kaplan–Meier and log rank tests.
Results:
Among 4124 patients (79.7 per cent female), median age was 50 (i.q.r. 38–60) years; 3906 patients (94.7 per cent) underwent total thyroidectomy, with lymph node dissection in 2495 (60.5 per cent). The eighth edition placed 91.8, 7.1, 0.4 and 0.7 per cent of patients in stages I–IV respectively. After reclassifying patients from the seventh to the eighth AJCC/UICC edition, the disease was downstaged in 23.8 per cent. Over a median follow-up of 7 years, 260 patients (6.4 per cent) developed recurrent disease, including 5.2 per cent of patients with stage I, 19.6 per cent with stage II, 59 per cent with stage III and 50 per cent with stage IV disease, according to the eighth edition. The eighth edition was a better predictor of recurrence than the seventh edition.
Conclusion:
The eighth edition of the AJCC/UICC staging system appears to be a novel tool for predicting PTC recurrence, which is a meaningful outcome for this indolent disease. The eighth edition can be used to risk-stratify patients, keeping in mind that other molecular and pathological predictive factors must be integrated into the assessment of recurrence risk.
BLURB:
This study evaluated the new AJCC staging system, 8th edition, which was implemented on 1 January 2018, for its ability to predict recurrence of WD thyroid cancer. This is a new application, as AJCC was originally designed for predicting survival. The authors used a single-centre prospective cohort from France, and applied staging to patients who underwent surgery for papillary thyroid cancer from 1985 to 2015.
+A: Introduction
Papillary thyroid cancer (PTC) generally has a favourable course, with a 5-year survival rate exceeding 98 per cent. Growing evidence, however, supports an annual increase in mortality rates overall1, including patients with advanced-stage PTC who develop locally persistent/recurrent disease and/or distant metastases2.
The AJCC/UICC staging system is the most frequently used and a globally recognized system for predicting risk of death. The recently published eighth edition3 was introduced into practice on 1 January 2018. The main changes are an increase in the cut-off point for age at diagnosis (45 years increased to 55 years in the 8th edition), and removal of minor extrathyroidal extension from staging. The eighth edition re-emphasizes gross extrathyroidal extension as an unfavourable prognostic factor and minimizes the significance of minor extension through the thyroid capsule3. Regional lymph node (LN) staging was also amended, such that N1a now includes level VII (upper mediastinal) LN compartments, and pNx and pN0 are used interchangeably. Pontius and colleagues4 recently compared the seventh and eighth editions, and showed that the latter provides a better fit for predicting survival of patients with PTC.
Given that PTC is an indolent disease associated with excellent survival overall, ability to determine the risk of disease recurrence may be a more meaningful outcome for patients and clinicians. Recurrent disease often necessitates remedial surgery, additional administration of radioactive iodine (RAI) and more intensive long-term surveillance. A retrospective review5 of 588 adult patients with thyroid cancer showed that recurrence of PTC could occur over a wide time period, with the majority of recurrences developing within the first 8 years after initial treatment. The development of recurrence prediction tools has been challenging owing to a dearth of adequate recurrent disease data maintained in large national databases. The current reference standard, the American Thyroid Association (ATA) risk stratification system, last updated in 20156, is used widely, and intended to predict recurrence.
The aim of this study was to assess the effectiveness of the eighth edition of the AJCC/UICC staging system in predicting recurrence of disease as well as disease-specific survival (DSS) using a large prospective patient registry.
+A: Methods
The study was based on data collected at Pitié Salpêtrière Hospital in Paris, France. Eight endocrine surgeons currently perform approximately 1500 thyroidectomies per year at the centre. All patients who provided informed consent before surgery were included in the institutional database, whose creation was approved by the Comité de Protection des Personnes (institutional review board). Data were collected prospectively. Demographic characteristics included: patient age, sex, BMI, history of other (non-thyroid) cancer diagnosis, and family history of PTC. Operative details included extent of thyroid surgery (lobectomy or total thyroidectomy) and performance of LN dissection. Tumour characteristics, including size, bilaterality, multifocality, extrathyroidal extension, vascular invasion, and pathological tumour staging in accordance with the seventh7 and eighth3 editions of the AJCC/UICC pathological staging system were collected (Table S1, supporting information). For patients who underwent LND, the number of resected LNs was recorded, the number, location (central and lateral) and laterality (ipsilateral, contralateral, bilateral) of LN metastases, and presence of extranodal extension. Use of postoperative RAI was also recorded in the database, along with recurrence of disease and its timing after the index thyroid operation.
+B: Patients
Patients aged at least 15 years who underwent thyroid surgery between 1985 and 2015 for whom final surgical pathology revealed PTC were included in this study. The study was approved by Duke University Hospital, (Durham, North Carolina, USA), with institutional review board exemption.
+B: Operative approach
Patients diagnosed with PTC by preoperative ultrasound-guided fine-needle aspiration cytology or by intraoperative frozen-section analysis underwent total thyroidectomy regardless of tumour size. Therapeutic LND was performed in patients with LN metastases that were confirmed cytologically before or during surgery using frozen-section analysis (N1). Prophylactic LND was done in all patients whose disease was classified as clinically node-negative (N0) before and/or during operation. Prophylactic LND, including central (level VI) and ipsilateral (levels III and IV) lateral LNs is performed routinely at Pitié Salpêtrière Hospital. This is based on studies8–10 demonstrating a lower risk of developing persistent or recurrent disease and facilitates accurate staging of disease, which subsequently informs treatment and follow-up. The authors recognize that prophylactic ipsilateral lateral LN dissection is not a widely accepted procedure, nor is it recommended by the ATA guidelines6. However, this particular cohort lends itself to the present study because it represents a homogeneous group of patients who underwent a standardized surgical procedure from 1985 to 2015, thus eliminating variability in surgical technique and approach. Patients who underwent total thyroidectomy for non-malignant disease, but were then diagnosed with PTC on final pathology, did not undergo LND (Nx). If PTC stage T1b or higher was identified on final pathology after lobectomy, completion thyroidectomy was undertaken in conjunction with either a prophylactic or therapeutic ipsilateral neck dissection, depending on the results of cervical ultrasonography. Patients with incidental T1a tumours (PTC 10 mm or smaller limited to the thyroid) did not undergo completion thyroidectomy.
+B: Radioactive iodine therapy
The RAI dose was determined according to the ATA6 and the French Societies of Nuclear Medicine and Endocrinology11 guidelines. Patients at high risk of recurrence based on ATA guidelines received 100 mCi RAI after thyroid hormone withdrawal for 4 weeks. Patients with intermediate-risk PTC received 30 or 100 mCi RAI, with recombinant human thyroid-stimulating hormone (TSH) before RAI administration. Low-risk PTCs were managed without RAI.
+B: Follow-up and recurrence
All patients followed a standard surveillance schedule, which included physical examination, neck ultrasound imaging, and measurement of serum thyroglobulin (Tg) and Tg autoantibody after stimulation or under suppressive treatment at 6 and 12 months, and then annually thereafter. After 6 years of follow-up, for those who appeared to be disease-free, periodic correspondence with patients or their referring physicians was set to occur every 3 years. No patients were lost to follow-up.
Recurrent disease was determined according to the French Societies of Nuclear Medicine and Endocrinology guidelines11. It was defined by the presence of suspicious LNs in the central or lateral neck, or abnormal tissue in the thyroid bed with positive fine-needle aspiration biopsy (locoregional recurrence). Recurrence was also indicated by the following biochemical evidence of disease: if the stimulated Tg level exceeded 10 ng/ml after thyroid hormone withdrawal or 5 ng/ml after recombinant human TSH administration; progression of basal (unsuppressed) level of Tg and/or basal Tg concentration over 1 ng/ml using the same assay technique; appearance or progressive increase (over 50 per cent) of Tg autoantibodies using the same assay technique; and/or isolated and repeatedly raised serum Tg levels.
+B: Statistical analysis
The probability of recurrence and DSS was estimated using the Kaplan–Meier method, with the log rank test applied to test for differences in survival among the four staging groups. Time to recurrence was computed from the date of initial surgery. Patients with M1 disease at the time of initial treatment were excluded from all analysis of disease recurrence. Multivariable Cox proportional hazard modelling was used to estimate the effect of stage on thyroid cancer recurrence after adjustment for known co-variables. A two-sided significance level of 0.05 was used for all statistical tests. No adjustments were made for multiple comparisons. Analyses were performed using SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA) and Stata® version 14.2 (StataCorp, College Station, Texas, USA).
+A: Results
A total of 4124 patients met inclusion criteria for the study. The PTC diagnosis was made before (cytology) or during (frozen section) surgery in 2495 patients (60.5 per cent), and on final pathology in 1629 (39.5 per cent). Overall, 79.7 per cent of patients in the cohort were female, and the median age was 50 (i.q.r. 38–60 years); 3.2 per cent had a family history of PTC (Table 1).
Table 1.
Demographic, clinical and pathological characteristics
| No. of patients* (n = 4124) |
|
|---|---|
| Age at diagnosis (years)* | 50 (38–60) |
| Sex ratio (F : M) | 3287:837 |
| BMI (kg/m2)* | 24.4 (21.7–28.1) |
| Family history of PTC | 133(3.2) |
| Other carcinoma | 258(6.3) |
| Type of surgery | |
| Lobectomy | 218(5.3) |
| Total thyroidectomy (1 step) | 3453(83.7) |
| Lobectomy followed by completion thyroidectomy | 453(11.0) |
| LN dissection | |
| None | 1629(39.5) |
| Prophylactic dissection | 2037(49.4) |
| Therapeutic dissection | 458(11.1) |
| Multifocal | 1633(39.6) |
| Bilateral | 987(23.9) |
| Extrathyroidalextension | 1079(26.2) |
| Pathological N category | |
| N0 | 1567(38.0) |
| N1a | 397(9.6) |
| N1b | 531(12.9) |
| Nx | 1629(39.5) |
| Central LNs | |
| Negative | 1771(42.9) |
| Positive | 724(17.6) |
| Not examined | 1629 (39.5) |
| Extranodal extension of positive central LNs (n = 724) | |
| No | 458 (63.3) |
| Yes | 204 (28.2) |
| Not examined | 62 (8.6) |
| Lateral LNs | |
| Negative | 1964(47.6) |
| Positive | 531(12.9) |
| Not examined | 1629 (39.4) |
| Extranodal extension of positive lateral LNs (n = 531) | |
| No | 361 (68.0) |
| Yes | 57 (10.7) |
| Not examined | 113 (21.3) |
| PathologicalM0 category | 4079(98.9) |
With percentages in parentheses unless indicated otherwise.
values are median (i.q.r.). PTC, papillary thyroid cancer; LN, lymph node.
A total of 3906 patients (94.7 per cent) underwent total thyroidectomy or lobectomy followed by completion thyroidectomy, with prophylactic LND also performed in 2037 patients (49.4 per cent) and therapeutic LND in 458 (11.1 per cent). Among the group of patients who underwent any type of LND, 928 (37.2 per cent) had metastatic LNs (Table 1). In the prophylactic LND group, 470 (23.1 per cent) had metastatic LNs on final pathology, including 222 (10.9 per cent) with metastatic LNs in the lateral compartment. A total of 2580 patients in the study (62.6 per cent) received RAI.
+B: Staging in the seventh and eighth editions of the AJCC/UICC classifications
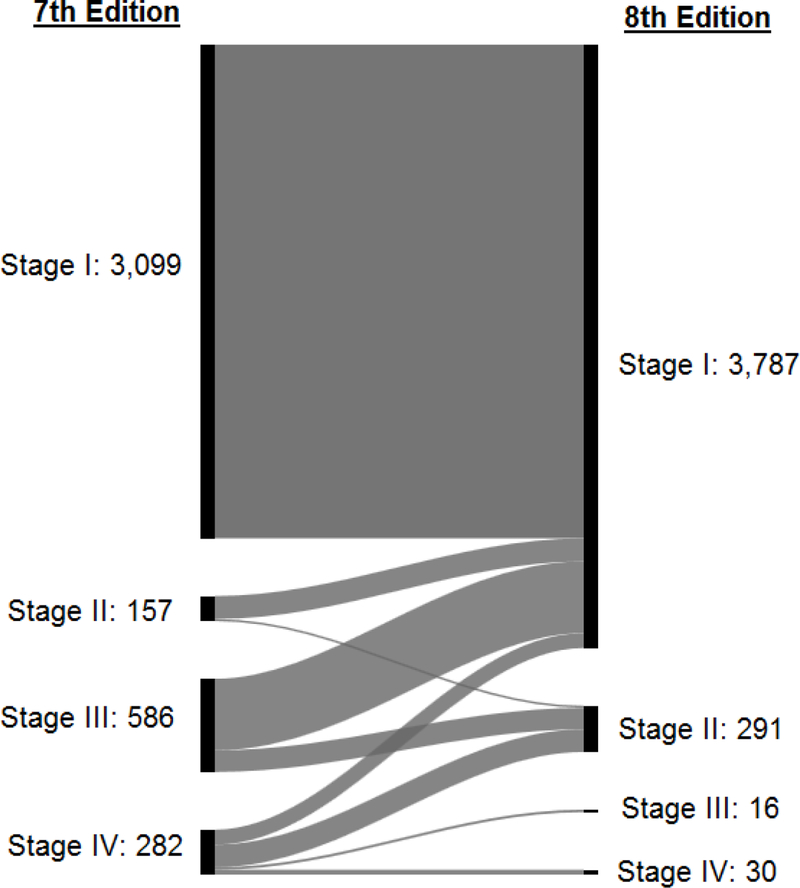
Some 38.4 per cent of the patients were considered younger (aged less than 45 years) according to the AJCC seventh edition, compared with 61.6 per cent who were deemed to be younger (aged less than 55 years) according to the eighth edition (Table S2, supporting information). After reclassifying patients from the seventh to the eighth edition, tumours were downstaged in 23.8 per cent, whereas 76.2 per cent had the same stage in both the seventh and eighth editions (Table S3, supporting information). When minimal extrathyroidal extension was removed from staging, pT3 category included intrathyroidal tumours larger than 4 cm and tumour of any size with gross extrathyroidal extension; 779 (67.7 per cent) of pT3 tumours in the seventh edition were reclassified as pT1 and 196 (17.0 per cent) as pT2 in the eighth edition. All patients classified as having stage 1 disease in the seventh edition retained this classification in the eighth edition. In 144 patients in stage II (91.7 per cent), 450 in stage III (76.8 per cent) and 94 in stage IV (33.3 per cent) in the seventh edition, the disease was downstaged to stage I according to the eighth edition (Fig. 1).
Fig. 1.
Alluvial flow diagram representing the restaging of patients in the French cohort from the 7th to the 8th edition of AJCC/UICC TNM staging system.
+B: Disease recurrence
Median follow-up was 84 (i.q.r. 12–360) months. Some 260 of 4079 patients (6.4 per cent) without M1 disease at the time of initial treatment experienced recurrence, with a median time to recurrence of 16 (range 3–324) months; 168 patients (64.6 per cent) developed recurrent disease in the first 2 years after surgery. Analysis according to LND showed that 113 of 2009 patients (5.6 per cent) experienced a recurrence after prophylactic LND and 116 of 441 (26.3 per cent) after therapeutic LND; 31 of 1629 who did not undergo LND (Nx) developed recurrence (1.9 per cent). These included 4.6 per cent in stage I, 6.3 per cent in stage II, 6.6 per cent in stage III and 27.9 per cent in stage IV, according to the seventh edition of the AJCC/UICC staging system. According to the eighth edition, 5.2 per cent experienced recurrence in stage I, 19.6 per cent in stage II, 59 per cent in stage III and 50 per cent in stage IV. Of 70 patients with recurrent disease who were classified in stage IV according to the seventh edition, only two retained this classification in the eighth edition; 13 per cent were downstaged to stage III, 54 per cent to stage II and 30 per cent to stage I.
Given the long study period and potential for changing practice in RAI administration, the analysis was divided into two time intervals (1985–2000 and 2001–2015) to examine the effect of this potential bias. The mean rate of RAI use decreased from 86.9 to 59.2 per cent between these periods (Table S4, supporting information), and probability of disease recurrence decreased for all stages (Table S5, supporting information).
+B: Unadjusted and adjusted recurrence analyses
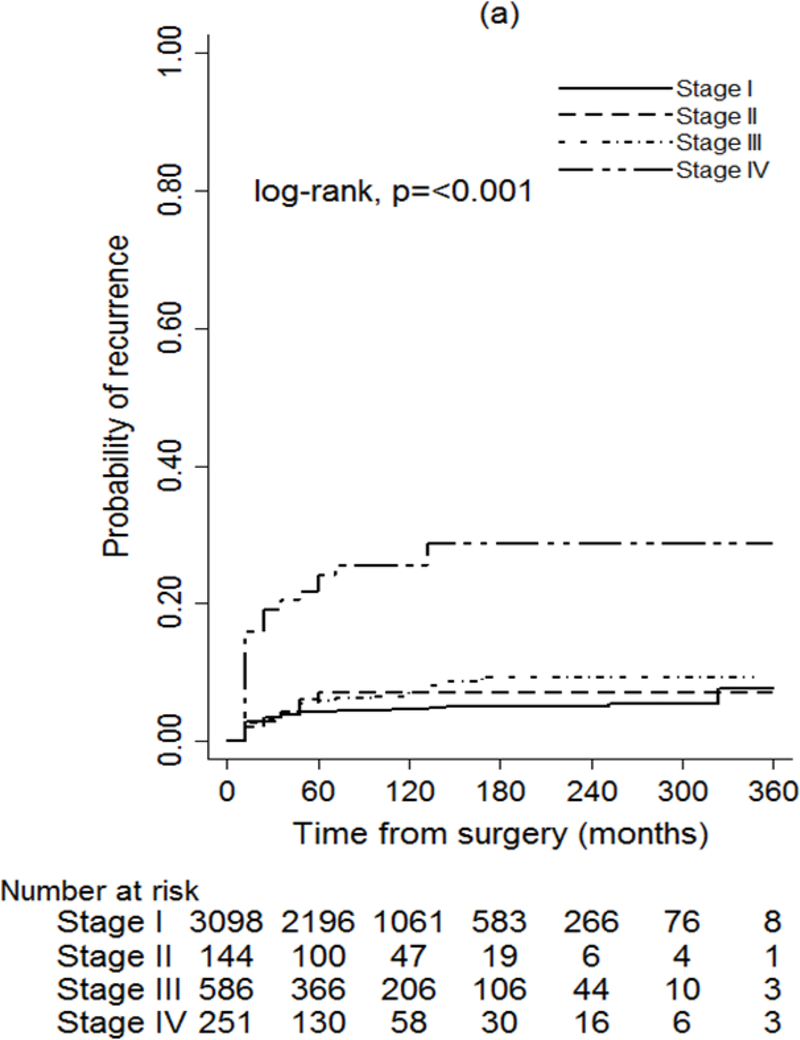
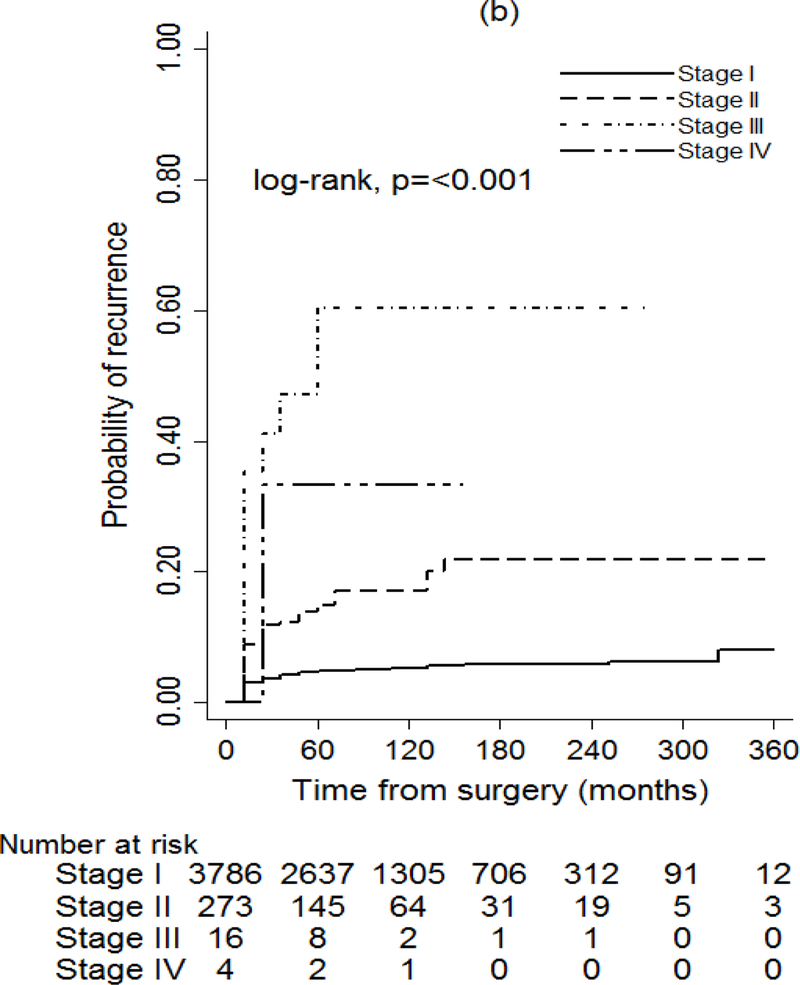
The seventh and eighth editions of the AJCC/UICC classification were both significantly associated with PTC recurrence (P < 0.001) (Fig. 2). Application of the eighth edition achieved a greater separation of recurrence curves based on disease stage than use of the seventh edition; this was especially true for stages I–III. The predicted probabilities of recurrence were higher in the eighth versus the seventh edition across all stages (Table S6, supporting information).
Fig. 2.
Probability of recurrence for patients with papillary thyroid cancer (PTC) using (a) 7th edition and (b) 8th edition of the AJCC/UICC TNM staging system
After adjustment for patient sex, tumour multifocality, extrathyroidal extension, vascular invasion and year of surgery, the seventh and eighth editions of the AJCC/UICC were both found to be associated with the risk of recurrence (P < 0.001) (Table 2). The eighth edition appeared to provide a better fit to the data than the seventh edition in predicting a patient’s probability of recurrence (P < 0.001).
Table 2.
Univariable and multivariable proportional hazards analysis of prognostic factors for recurrence
| Univariable analysis | Multivariable* | |||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| Stage, 7th edition | < 0.001 | < 0.001 | ||
| II versus I | 1.4 (0.7, 2.7) | 2.12 (1.06, 4.24) | ||
| III versus I | 1.5 (1.5, 2.1) | 0.51 (0.35, 0.75) | ||
| IV versus I | 7.2 (5.4, 9.6) | 1.96 (1.42, 2.70) | ||
| Stage, 8th edition | < 0.001 | < 0.001 | ||
| II versus I | 4.3 (3.2, 5.8) | 1.98 (1.44, 2.73) | ||
| III versus I | 14.6(7.5, 28.5) | 3.61 (1.18, 7.19) | ||
| IV versus I | 13.7 (3.4, 55.1) | 8.45 (2.08, 34.61) | ||
Values in parentheses are 95 per cent confidence intervals. Patients with M1 disease were excluded from the analyses.
Included in the multivariable analysis for each stage: sex, tumour multifocality, extrathyroidal extension, vascular invasion and year of surgery.
+B: Disease-specific survival
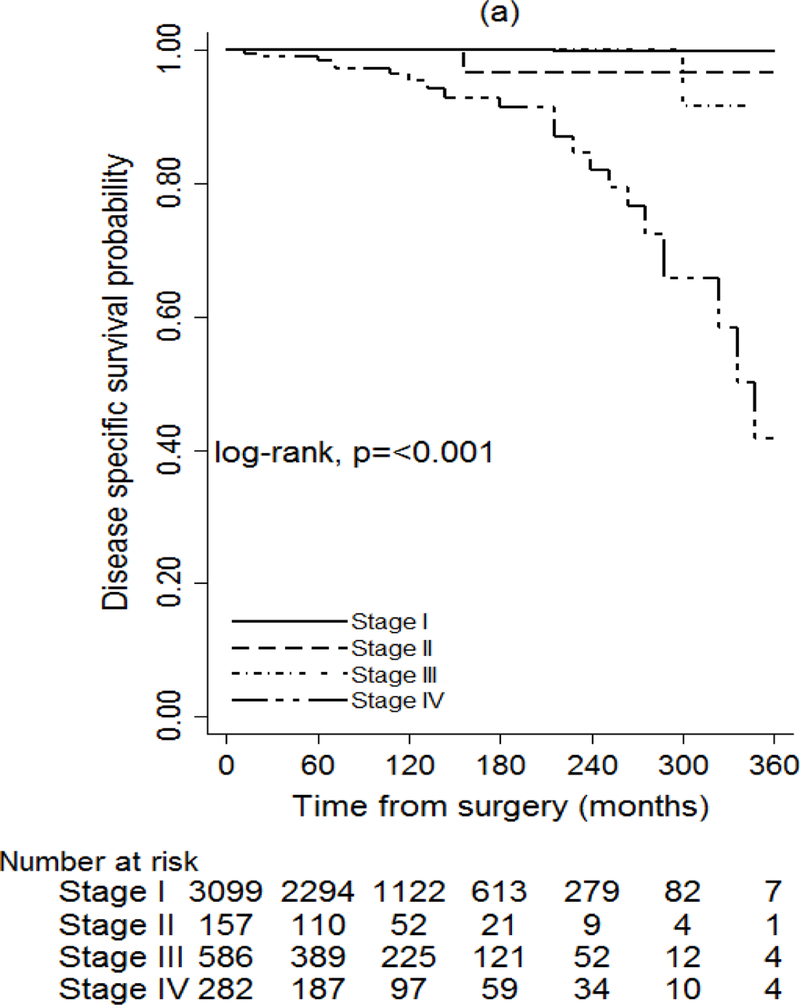
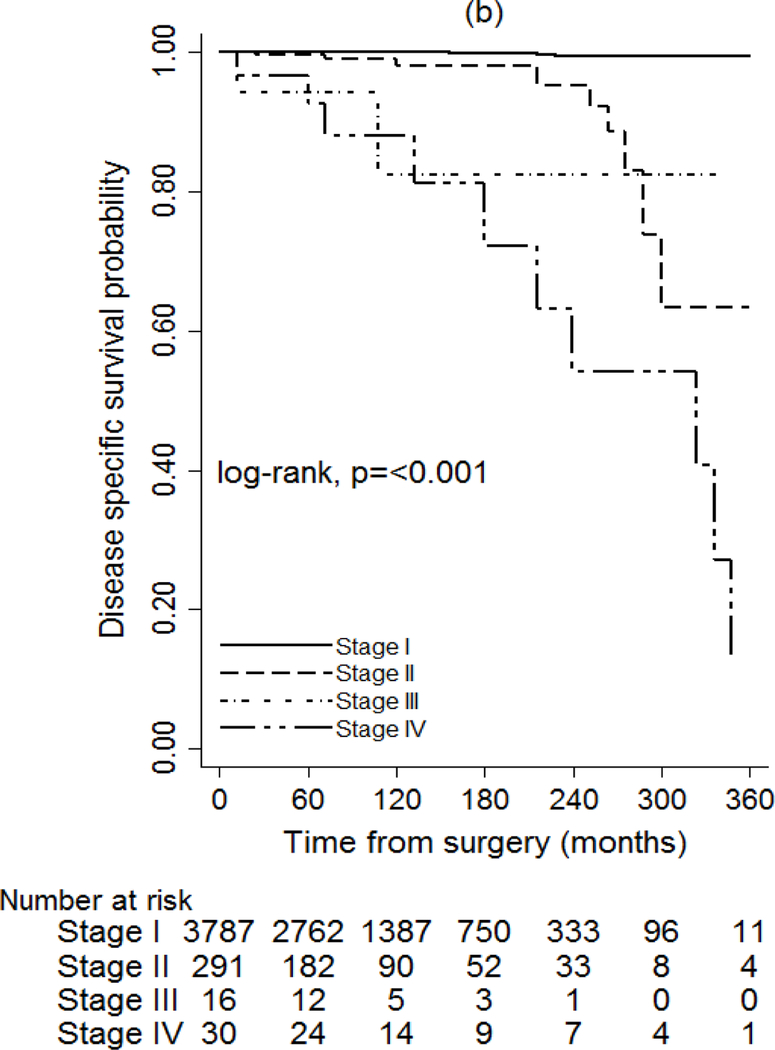
The seventh and eighth AJCC/UICC editions were also associated with the probability of DSS (P < 0.001), but the eighth edition discriminated better between stages (Fig. 3). According to the eighth edition, the DSS rate at 15 years was 100 per cent for stage I, 97.9 per cent for stage II, 82 per cent for stage III and 81 per cent for stage IV (Table S7, supporting information). The impact of recurrence on DSS was assessed in two separate models by adjusting for stage at diagnosis only, using the seventh and eighth editions. The hazard ratio was 18.83 (95 per cent c.i. 3.76 to 94.38) when adjusting for stage according to the seventh edition (P = 0.004), compared with 26.80 (5.37 to 133.81) for the eighth edition (P < 0.001) (Table 3).
Fig. 3.
Unadjusted disease-specific survival of patients with papillary thyroid cancer based on staging according to the seventh and eighth editions of the AJCC/UICC TNM staging system Footnote to Fig. 3 a Seventh edition, b eighth edition. a,b P < 0.001 (log rank test).
Table 3.
Assessment of the impact of stage at diagnosis on probability of recurrence and recurrence on survival
| 7th edition | 8th edition | |||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| Impact of stage at diagnosis on probability of recurrence | < 0.001 | < 0.001 | ||
| II versus I | 2.12 (1.06, 4.24) | 1.98 (1.44, 2.73) | ||
| III versus I | 0.51 (0.35, 0.75) | 3.61 (1.18, 7.19) | ||
| IV versus I | 1.96 (1.42, 2.70) | 0.38* | 8.45 (2.08, 34.61) | 0.001* |
| Impact of recurrence on survival | ||||
| Adjusted for stage | 18.83 (3.76, 94.38) | 0.004 | 26.80 (5.37, 133.81) | < 0.001 |
Values in parentheses are 95 per cent confidence intervals. Patients with M1 disease were excluded from the analyses.
P for linear trend.
+A: Discussion
A reliable and easily available prognostic classification system is needed for PTC recurrence to provide clinicians with the information they need to tailor initial therapy and surveillance schedules to the individual patient’s risk of developing recurrent disease. Large registries with adequate recurrence data are lacking and, as a result, it is largely single-centre studies5,12 that have investigated the risk of recurrence of PTC. This present longitudinal study evaluated the effectiveness of the seventh and eighth editions of the AJCC/UICC staging system in predicting recurrence of PTC.
The AJCC/UICC staging system was designed to predict death, not recurrence. To assess the risk of recurrent disease in patients with PTC, the ATA6 created a risk classification system based on selected clinicopathological tumour characteristics (BRAF mutation, vascular invasion, number and size of involved LNs) and the effectiveness of initial therapy, including RAI8–13. Given the level of diagnostic evaluation required, the ATA risk classification system may be less accessible to many clinicians than the AJCC/UICC staging system; the latter system is widely known and used worldwide, and therefore its potential use in predicting recurrence as well as survival is appealing.
The present study highlights that the risk of recurrent disease (6.4 per cent here) is not only a meaningful outcome on its own, but also a factor that influences the survival of patients with PTC. It has demonstrated that stage-adjusted disease recurrence is highly associated with DSS, especially when the eighth edition of the AJCC/UICC system is used. Leboulleux and colleagues13 first emphasized the risk of persistent disease (7 per cent) in a French cohort based on 148 patients with PTC who had LN metastases and/or extrathyroidal tumour extension. The international literature has reported diverse results over time. A locoregional recurrence rate of 24 per cent was reported by Mazzaferri and colleagues15 with 40 years of follow-up. Recent literature15–17 suggests better outcomes, with recurrence rates ranging between 3 and 10 per cent, consistent with that in the present study. However, these studies shared a significant common limitation as they did not describe and underline the importance of the initial surgical management strategy. The present study has the advantage of having data from systematic prophylactic LNDs, allowing better consideration of the role of locoregional metastatic disease as a factor in the estimation of recurrence. It has been reported that the risk of recurrence is related to the number of LNs removed and the LN ratio (number of positive LNs/number of LNs removed)18–20. The impact of this factor is likely to be small in the authors’ institution, because operative management of PTC was homogeneous during the study interval, with a large number of resected LNs. Moreover, a systematic and aggressive approach to lateral LND resulted in a relatively high N1b rate. Regardless of this, it is clear that fastidious preoperative LN evaluation with ultrasonography and thorough initial LND are essential for reducing the risk of future reoperations21,22.
External validity was confirmed by benchmarking the AJCC/UICC stage distribution of this French cohort against the results of a recent study4 in the USA based on Surveillance, Epidemiology, and End Results and National Cancer Database information. The distribution and downstaging of disease in the eighth edition were very similar those in the present study, and appeared to have strong face validity. Interestingly, the eighth edition seems to influence the downstaging of patients with T3 tumours in particular. This is most likely explained by the fact that patients who harboured PTC with minimal extrathyroidal extension and who were explicitly categorized in previous editions as having T3 tumours are no longer identified as such in the eighth edition. However, overall agreement on the identification and pathological reporting of minimal extrathyroidal extension is poor, and there are conflicting results from other studies23–26 on the prognostic relevance of minimal extrathyroidal extension. Analysis of data concerning the potential relevance of minimal extrathyroidal extension to patient outcomes should continue. Another major change in the eighth edition that leads to downstaging of a significant number of patients is the increase in the age cut-off point from 45 years (7th edition) to 55 years. The present findings confirmed that this change contributes to downstaging in 23.8 per cent of patients. It appears that young patients have excellent outcomes unless they have distant metastases at presentation.
One of the limitations of this study is its retrospective design; it is possible that the relatively large proportion of the patients who received postoperative RAI (62.6 per cent) could have had an impact on the measured risk of recurrence5. It was noted that the rate of RAI decreased overall between the two time intervals, as did the rate of disease recurrence. However, the authors believe that an association between the decline in rate of RAI and recurrence cannot be assumed from this analysis. The reasons for the decline over time in rate of recurrence are clearly multifactorial and were not analysed specifically. The surgical approach of undertaking LND routinely in this cohort was not in accordance with the 2015 ATA guidelines6; it is not applied widely in surgical practice, and is still debated. For example, in the present cohort, systematic prophylactic lateral LN dissection showed a high rate of occult N1b disease. However, the aim of the present study was to assess this risk based on the pathological results after initial surgery, and then to apply these to the new AJCC/AICC staging system. The long duration of follow-up in this study resulted in increased identification of recurrent disease and disease-specific death from PTC. Because of the small numbers of patients assessed as having stage III and IV according to the eighth edition, the risk of recurrent disease might have been underestimated for these groups; larger multi-institutional cohort studies are needed to validate the present findings. Information on molecular profiles of tumours was not available, nor on the number and size of metastatic LNs. The strengths of this study include the large size of the cohort, standardized treatment over time, and long-term follow-up with few missing data.
It is difficult to ascertain from retrospective studies how many patients who undergo remedial treatment with surgery and/or RAI truly have recurrent disease after being assigned disease-free status, and how many have persistent PTC. The ATA guidelines offer no specific timeline for making a clear distinction between recurrent and persistent disease. In the present study, the majority of patients experienced recurrence within the first 2 years after surgery, suggesting that some patients may have had persistent disease. Given these complexities, persistence and true recurrence were included in the global term recurrent disease; from the patient perspective, these two clinical scenarios are likely to have the same impact on treatment.
Although the eighth edition of the AJCC/UICC staging system was developed to predict mortality, this study has demonstrated that it also predicts PTC recurrence, and that stage for stage, the eighth edition is a better fit for disease recurrence and DSS than the seventh edition. Ultimately, other predictive factors, such as molecular markers and pathological characteristics of the LN, must be integrated into the assessment of recurrence. Nevertheless, the ubiquitous AJCC/UICC eighth edition is an excellent tool for this application, and can potentially offer a robust means of helping physicians better predict patient outcomes and the need for intensive long-term surveillance.
Supplementary Material
+A: Acknowledgements
J.A.S. is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by Novo Nordisk, GlaxoSmithKline, Astra Zeneca and Eli Lilly.
Footnotes
Disclosure: The authors declare no other conflict of interest.
+A: References
- 1.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017; 317: 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 2013; 154: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 3.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK et al. AJCC Cancer Staging Manual (8th edn). Springer: New York, 2017. [Google Scholar]
- 4.Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA et al. Projecting survival in papillary thyroid cancer: a comparison of the seventh and eighth editions of the American Joint Commission on Cancer/Union for International Cancer Control staging systems in two contemporary national patient cohorts. Thyroid 2017; 27: 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010; 20: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge SB, & Compton CC (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology, 17(6), 1471–1474.AJCC 7th [DOI] [PubMed] [Google Scholar]
- 8.Ducoudray R, Trésallet C, Godiris-Petit G, Tissier F, Leenhardt L, Menegaux F. Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg 2013; 37: 1584–1591. [DOI] [PubMed] [Google Scholar]
- 9.Hartl DM, Leboulleux S, Al Ghuzlan A, Baudin E, Chami L, Schlumberger M et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg 2012; 255: 777–783. [DOI] [PubMed] [Google Scholar]
- 10.Pacini F, Castagna MG, Brilli L, Pentheroudakis G; ESMO Guidelines Working Group. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(Suppl 7): vii110–vii119. [DOI] [PubMed] [Google Scholar]
- 11.Zerdoud S, Giraudet AL, Leboulleux S, Leenhardt L, Bardet S, Clerc J et al. Radioactive iodine therapy, molecular imaging and serum biomarkers for differentiated thyroid cancer: 2017 guidelines of the French Societies of Nuclear Medicine, Endocrinology, Pathology, Biology, Endocrine Surgery and Head and Neck Surgery. Ann Endocrinol (Paris) 2017; 78: 162–175. [DOI] [PubMed] [Google Scholar]
- 12.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S et al. ; PTC Study Group. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab 2013; 98: 636–642. [DOI] [PubMed] [Google Scholar]
- 13.Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab 2005; 90: 5723–5729. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001; 86: 1447–1463. [DOI] [PubMed] [Google Scholar]
- 15.Yim JH, Kim WB, Kim EY, Kim WG, Kim TY, Ryu JS et al. The outcomes of first reoperation for locoregionally recurrent/persistent papillary thyroid carcinoma in patients who initially underwent total thyroidectomy and remnant ablation. J Clin Endocrinol Metab 2011; 96: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 16.Wang LY, Migliacci JC, Tuttle RM, Shaha AR, Shah JP, Patel SG et al. Management and outcome of clinically evident neck recurrence in patients with papillary thyroid cancer. Clin Endocrinol (Oxf) 2017; 87: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh S, Kim YH, Goh TS, Lee J, Jeong DC, Oh SO et al. Outcome prediction with the revised American joint committee on cancer staging system and American thyroid association guidelines for thyroid cancer. Endocrine 2017; 58: 495–502. [DOI] [PubMed] [Google Scholar]
- 18.Sterpetti AV. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg 2015; 261: e30. [DOI] [PubMed] [Google Scholar]
- 19.Vas Nunes JH, Clark JR, Gao K, Chua E, Campbell P, Niles N et al. Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid 2013; 23: 811–816. [DOI] [PubMed] [Google Scholar]
- 20.Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol 2016; 34: 3434–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young S, Harari A, Smooke-Praw S, Ituarte PH, Yeh MW. Effect of reoperation on outcomes in papillary thyroid cancer. Surgery 2013; 154: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 22.Kouvaraki MA, Lee JE, Shapiro SE, Sherman SI, Evans DB. Preventable reoperations for persistent and recurrent papillary thyroid carcinoma. Surgery 2004; 136: 1183–1191. [DOI] [PubMed] [Google Scholar]
- 23.Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor? Ann Surg Oncol 2011; 18: 1916–1923. [DOI] [PubMed] [Google Scholar]
- 24.Hay ID, Johnson TR, Thompson GB, Sebo TJ, Reinalda MS. Minimal extrathyroid extension in papillary thyroid carcinoma does not result in increased rates of either cause-specific mortality or postoperative tumor recurrence. Surgery 2016; 159: 11–19. [DOI] [PubMed] [Google Scholar]
- 25.Su HK, Wenig BM, Haser GC, Rowe ME, Asa SL, Baloch Z et al. Inter-observer variation in the pathologic identification of minimal extrathyroidal extension in papillary thyroid carcinoma. Thyroid 2016; 26: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin DT, Yu K, Lu RQ, Li X, Xu J, Lei M. Prognostic impact of minimal extrathyroidal extension in papillary thyroid carcinoma. Medicine (Baltimore) 2016; 95: e5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.