Abstract
Background:
Although microvascular decompression (MVD) is a durable treatment for medically refractory trigeminal neuralgia, hemifacial spasm, or glossopharyngeal neuralgia attributable to neurovascular conflict, few national studies have analyzed predictors of postoperative complications.
Objective:
To determine the incidence and risk factors for adverse events after MVD.
Methods:
Patients who underwent MVD were extracted from the prospectively collected National Surgical Quality Improvement Program registry (2006–2017). Multivariable logistic regression identified predictors of thirty-day adverse events and unplanned readmission; multivariable linear regression analyzed predictors of a longer hospital stay.
Results:
Among the 1,005 patients evaluated the mortality rate was 0.3%, major neurologic complication rate 0.4%, and 2.8% had a nonroutine hospital discharge. Patient age was not a predictor of any adverse events. Statistically significant independent predictors of both any adverse event (9.2%) and of a longer hospitalization were American Society of Anesthesiologists (ASA) classification III-IV designation and longer operative duration (P≤.03) The thirty-day readmission rate was 6.8%, and the most common reasons were surgical site infections (22.4%) and cerebrospinal fluid leakage (14.3%). Higher ASA classification, diabetes mellitus, and operative time were predictors of readmission (P<.04).
Conclusion:
In this NSQIP analysis, postoperative morbidity and mortality after MVD was very low. Patient age was not a predictor of postoperative complications, while higher ASA classification, diabetes mellitus, and longer operative duration were predictive of any adverse event and readmission. ASA classification provided superior risk stratification compared with the total number of patient comorbidities or laboratory values. These data can assist with preoperative patient counseling and risk-stratification.
Keywords: Adverse Events, Microvascular Decompression, National Surgical Quality Improvement Program, Outcomes, Readmission, Trigeminal Neuralgia
Introduction
Microvascular decompression (MVD) is an effective treatment for medically intractable trigeminal neuralgia (TN), hemifacial spasm, or glossopharyngeal neuralgia attributable to neurovascular conflict.1–10 As an elective procedure performed to optimize quality of life, the risk-benefit ratio of MVD is reduced by postoperative complications.11,12 Therefore, preoperative decision analysis, risk-stratification, patient selection and counseling would be augmented by additional data on patients are at an increased risk of complications.4,5,13,14
While there is a body of literature emphasizing the safety and efficacy of MVD,2,4–6,13–29 it is primarily based upon single-center, retrospective reports performed by high-volume centers2,4,5,13,19,20 and there is a dearth of multicenter data analyzing postoperative outcomes after MVD in practice.2,6,15,17,20 The National Surgical Quality Improvement Program (NSQIP) is an externally validated, prospective, national registry that tracks thirty-day outcomes on patients in different healthcare settings. The goal of the present analysis was to use NSQIP to evaluate the rates and predictors of adverse events after elective microvascular decompression for trigeminal neuralgia, hemifacial spasm, or glossopharyngeal neuralgia.
Methods
Data Source:
The NSQIP registry was used from the years 2006–2017. NSQIP is maintained by the American College of Surgeons (ACS), and includes prospectively collected data on patients undergoing operations at more than 700 academic and community hospitals nationwide. Data are collected by trained surgical reviewers using a uniform protocol; to prevent selection bias, enrollment is performed on an eight-day cycle at each center. NSQIP has been externally validated against physician review,30 and previously used to evaluate neurosurgical outcomes.31–39Patient consent and enrollment into NSQIP is performed by the ACS, which releases a fully deidentified registry; our institutional review board has exempted the deidentified NSQIP database from individual study review.
Inclusion Criteria:
To identify patients undergoing elective microvascular decompression, admissions that met the following criteria were included: 1) were aged 18 years or older; 2) had a Current Procedural Terminology (CPT) code indicating an MVD (61458, 61460); 3) had a diagnosis code of trigeminal neuralgia (International Classification of Diseases, 9th Edition (ICD-9) and 10th edition (ICD-10) codes of 350.x and G50.x, respectively), hemifacial spasm (ICD-9: 351.x, ICD-10: G51.x), glossopharyngeal neuralgia or other cranial nerve disorder not specified (ICD-9: 352.x, ICD-10: G52.x), 4) the attending surgeon’s specialty was neurosurgery; 5) the documented case urgency was non-emergent; 6) the disposition was inpatient; & 7) the operation was performed under general anesthesia.
Covariates:
Pertinent covariates were extracted. Age was categorized based on published literature evaluating MVD (18–50, 51–64, & ≥ 65 years).22,26 American Society of Anesthesiologists (ASA) classification was analyzed dichotomously (I-II, III-IV, missing) given that the majority of patients either had a class II or III designation. Operative time was examined as a categorical variable with divisions at the thirty-minute intervals that approximate the median and upper quartile. In sensitivity analyses, patient age was also evaluated continuously and by quartile, while operative time was also assessed as a continuous variable.
Comorbidities collected by NSQIP and present in more than 10 patients were evaluated individually: smoking, hypertension requiring treatment, dyspnea, chronic obstructive pulmonary disease, diabetes mellitus, and steroid administration. Obesity was defined as a body mass index of 30.0–39.99 kg/m2, while morbid obesity as > 40 kg/m2. Pertinent preoperative laboratory testing were extracted and stratified by key values. Moreover, the total number of recorded comorbidities was calculated for each patient.
Outcomes:
Thirty-day outcomes, including mortality and other adverse events, as well as length of hospital stay were extracted. Complications available in the NSQIP algorithm are neurologic (postoperative hemorrhagic or ischemic stroke and coma), cardiovascular (cardiac arrest or myocardial infarction), hematologic (venous thromboembolism and intraoperative or postoperative red blood cell transfusion), airway (unplanned endotracheal intubation or prolonged mechanical ventilation), renal, and infectious (surgical site infection, pneumonia, urinary tract infection, and sepsis). A major complication was defined using previously defined criteria in a neurosurgical population, and included death, neurologic or cardiopulmonary complications, venous thromboembolism, sepsis, surgical site infection, or reoperation.40
In 2011, NSQIP began collecting data on thirty-day unplanned readmission and discharge disposition, and in 2012 NSQIP started including the primary diagnosis associated with the readmission. Data on reoperation has been collected by NSQIP since its inception, but the specific CPT codes of the reoperation have only been recorded since 2012, and therefore the reasons fro reoperation could only be discerned among patients from 2012–2017. A non-routine discharge was defined as any disposition other than to home.
Missing Data:
Patients with missing data on covariates (including in whom laboratory values were not obtained) were stratified into a separate group for these variables. All missing data on covariates are explicitly denoted. The only data missing on outcomes were for unplanned hospital readmission and discharge disposition from the years prior to 2011, and therefore patients from the years 2007–2010 were not included in analyses of these outcomes.
Statistical Analysis:
Statistical analyses were conducted using STATA 13 (STATACorp, College Station, TX). First, descriptive statistics were performed of covariates and outcomes. Multivariable regression models were utilized for three outcomes: any complication, unplanned readmission, and length of hospital stay. Multivariable models were constructed after univariable screen: entry criteria was a probability value less than 0.10 (in any strata of categorical variables) in univariable regression with the predictor as the independent variable and that specific outcome as the dependent variable. Logistic regression evaluated each dichotomous outcome, and linear regression analyzed length of hospital stay (which was evaluated as a continuous variable to maximize available information). When a logistic regression model includes more variables than events, there is concern for statistical optimism; therefore, in such cases (which only occurred for a major complication), automated backwards, selection was subsequently employed with an exit criteria of p>0.10. The calibration of logistic regression models were assessed using the Hosmer-Lemeshow test, while concordance statistics evaluated the discrimination of logistic regression models. R2 was used to evaluate the variance explained by linear regression models. For all analyses, a probability less than 0.05 was considered significant.
Results
Demographics of Study Population:
A total of 1,005 patients were included: their preoperative characteristics are reported and stratified by the development of any postoperative complication in Table 1. The recorded ASA physical classification designation was I for 4.8% (n=48), II for 60.6% (n=609), III for 34.2% (n=344), IV for 0.3% (n=3), and missing for 0.1% (n=1). However, patient age and ASA classification were significantly correlated (P<.001 with a global chi-squared test): patients aged at least 65 were significantly more likely to have ASA class IIIIV designation (odds ratio (OR): 2.05, 95% confidence interval (CI): 1.49–2.83, P<.001), compared to those aged 18–50 years, although there was no significant difference for patients aged 51–64 years (OR: 0.88, 95% CI: 0.65–1.21, P=0.45).
Table 1.
The demographics of the study population, with univariable logistic regression evaluating the development of any postoperative complication.
| Variable | Definition | Total (n= 1,005) | Any Complication (n=92) | No Complication (n=913) | Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|---|---|---|---|
| Diagnosis | Trigeminal Neuralgia | 78.2 | 78.3 | 78.2 | Ref. | -- | -- |
| Hemifacial Spasm | 18.4 | 17.4 | 18.5 | 0.94 | 0.53–1.66 | .83 | |
| GN or Other | 3.4 | 4.4 | 3.3 | 1.32 | 0.45–3.86 | .61 | |
| Age (years) | 18–50 | 42.4 | 44.6 | 42.2 | Ref. | -- | -- |
| 51–64 | 32.6 | 26.0 | 33.3 | 0.74 | 0.44–1.25 | .26 | |
| ≥65 | 25.0 | 29.4 | 24.5 | 1.13 | 0.68–1.89 | .64 | |
| Sex | Male | 32.0 | 35.9 | 31.7 | Ref. | -- | -- |
| Female | 68.0 | 64.1 | 68.4 | 0.83 | 0.53–1.30 | .41 | |
| ASA Class | I-II | 65.4 | 45.7 | 67.4 | Ref. | -- | -- |
| III-IV | 34.5 | 53.3 | 32.6 | 2.41 | 1.56–3.72 | <.001 | |
| Missing | 0.1 | 1.1 | 0.0 | -- | -- | -- | |
| Number of Comorbidities | 0 | 31.8 | 26.1 | 32.4 | Ref. | -- | -- |
| 1 | 36.3 | 29.4 | 37.0 | 0.99 | 0.56–1.74 | .96 | |
| 2 | 22.3 | 28.3 | 21.7 | 1.62 | 0.90–2.90 | .11 | |
| ≥3 | 9.6 | 16.3 | 8.9 | 2.28 | 1.15–4.56 | .02 | |
| Smoking | 13.7 | 15.2 | 13.6 | 1.14 | 0.63–2.08 | .66 | |
| Hypertension | 35.4 | 45.7 | 34.4 | 1.60 | 1.04–2.47 | .03 | |
| Dyspnea | 2.4 | 4.4 | 2.2 | 2.03 | 0.68–6.07 | .21 | |
| COPD | 1.1 | 3.3 | 0.9 | 3.82 | 0.99–14.63 | .05 | |
| Diabetes Mellitus | 7.9 | 17.4 | 6.9 | 2.84 | 1.56–5.16 | .001 | |
| Steroid Usage | 2.2 | 4.4 | 2.0 | 2.26 | 0.75–6.83 | .14 | |
| Body Habitus | Nonobese | 60.8 | 56.5 | 61.2 | Ref. | -- | -- |
| Obese | 32.1 | 29.4 | 32.4 | 0.98 | 0.60–1.59 | .94 | |
| Morbidly Obese | 7.1 | 14.1 | 6.4 | 2.41 | 1.24–4.69 | .01 | |
| Preoperative Sodium | ≥135 mEq/L | 67.1 | 73.9 | 66.4 | Ref. | -- | -- |
| <135 mEq/L | 8.7 | 9.8 | 8.5 | 1.03 | 0.49–2.14 | .89 | |
| Not Obtained | 24.3 | 16.3 | 25.1 | 0.58 | 0.33–1.04 | .07 | |
| Preoperative Creatinine | <1.4 mg/dL | 76.1 | 78.3 | 75.9 | Ref. | -- | -- |
| >1.4 mg/dL | 1.7 | 3.3 | 1.5 | 2.06 | 0.58–7.35 | .26 | |
| Not obtained | 22.2 | 18.5 | 22.6 | 0.79 | 0.46–1.38 | .41 | |
| Preoperative White Blood Cell Count (cells/μL) | 4,000–11,000/μL | 74.0 | 76.1 | 73.8 | Ref. | -- | -- |
| <4,000/μL | 4.7 | 6.5 | 4.5 | 1.41 | 0.58–3.44 | .45 | |
| >11,000/μL | 2.1 | 3.3 | 2.0 | 1.60 | 0.46–5.58 | .46 | |
| Not obtained | 19.2 | 14.1 | 19.7 | 0.70 | 0.38–1.29 | .25 | |
| Preoperative Hematocrit | ≥36% | 72.7 | 73.9 | 72.6 | Ref. | -- | -- |
| <36% | 8.5 | 10.9 | 8.2 | 1.30 | 0.64–2.63 | .47 | |
| Not obtained | 18.8 | 15.2 | 19.2 | 0.78 | 0.43–1.42 | .42 | |
| Operative Time | < 180 minutes | 65.6 | 58.7 | 66.3 | Ref. | -- | -- |
| 180–210 minutes | 15.5 | 9.8 | 16.1 | 0.69 | 0.33–1.42 | .31 | |
| > 210 minutes | 18.9 | 31.5 | 17.6 | 2.02 | 1.24–3.27 | .004 |
All data are presented as percentages and statistically significant differences with univariable logistic regression are bolded.
Abbreviations: ASA: American Society of Anesthesiologists, COPD: chronic obstructive pulmonary disease; GN: glossopharyngeal neuralgia.
Adverse Events:
The total thirty-day cumulative incidence and time to event of adverse events are presented in Table 2. When stratified by ASA physical classification designation, among patients with ASA classification I-II, 6.4% experienced any complication, the mean hospital stay was 2.8 (standard deviation (SD) 2.0) days, and 4.7% were readmitted. Among patients with ASA classification III-IV, 14.1% experienced any complication, the mean hospital stay was 3.3 (SD: 2.5) days, and 10.1% were readmitted.
Table 2.
The thirty-day cumulative incidence and time to event of postoperative complications.
| Complication | Thirty-Day Cumulative Incidence (%) | Time to Event, days, median (IQR) |
|---|---|---|
| Death | 0.3 | |
| Any Complication | 9.2 | 11 (7–18) |
| Major Complication | 5.3 | 11.5 (5–21) |
| Reoperation | 3.6 | 10 (7–19) |
| Unplanned Readmission (n=845) | 6.8 | 11 (7–17) |
| Non-Routine Hospital Discharge (n=841) | 1.9 | 6.5 (4.5–8.5) |
| Neurological Complications | 0.4 | |
| Stroke | 0.4 | - |
| Coma > 24 hours | 0 | - |
| Cardiovascular Complications | 0.2 | - |
| Hematologic Complications | ||
| Venous Thromboembolism | 0.5 | 7 (5–17) |
| Red Blood Cell Transfusion | 0.2 | - |
| Airway Complications | 0.5 | 8 (3–12) |
| Renal Complications | 0 | - |
| Infectious Complications | ||
| Any Infectious Complication | 3.4 | 13.5 (5–21) |
| Surgical Site Infection | 1.7 | 21 (17–26) |
| Pneumonia | 0.3 | 3 (1–5) |
| Urinary Tract Infection | 1.2 | 8 (2–9.5) |
| Sepsis | 0.4 | 20 (10–22) |
Abbreviations: IQR = interquartile range.
A major complication was defined using previously published criteria and included death, a neurologic or cardiopulmonary complication, venous thromboembolism, sepsis, surgical site infection, or reoperation
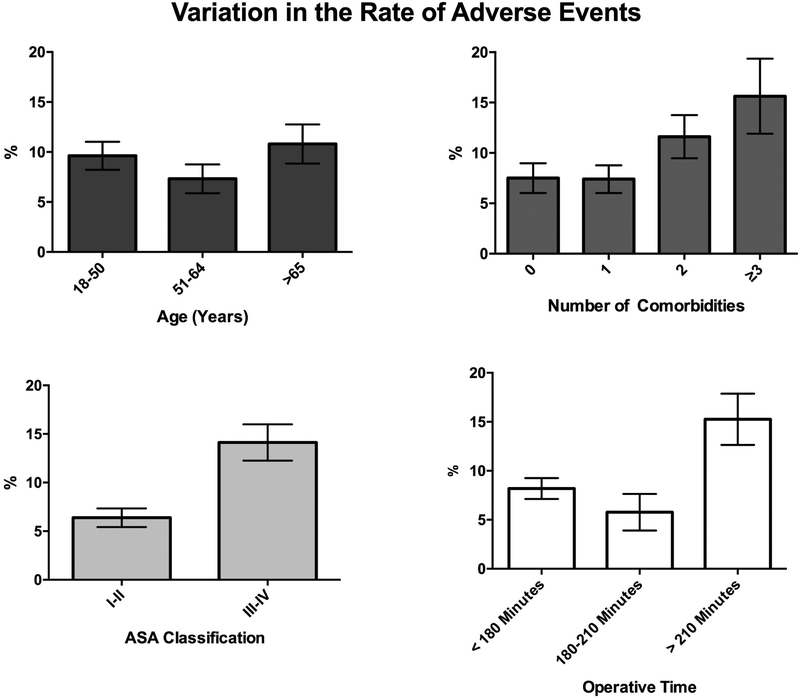
A multivariable logistic regression model evaluated the predictors of developing any adverse event. Predictors were first screened with univariable logistic regression (Table 1): ASA classification III-IV designation, at least three comorbidities, hypertension, diabetes mellitus, morbid obesity, and longer operative time were associated with adverse events (Figure 1). In the multivariable model (Table 3), however, the only independent predictors were ASA class III-IV designation (P=.01), diabetes mellitus (P=.04), and longer operative time (P=.02). Likewise, a multivariable logistic regression model was constructed evaluating predictors of a major complication; statistically significant independent predictors were chronic obstructive pulmonary disease (P=.04), diabetes mellitus (P=.007), preoperative steroid usage (P=.048), and operative time longer than 210 minutes (P=.01).
Figure 1.
Variations in the unadjusted thirty-day adverse event rate (and its associated standard error) by A) patient age, B) the number of comorbidities, C) ASA physical classification designation, and D) operative time.
Table 3.
Multivariable logistic regression models evaluating the predictors of developing any postoperative complication, a major complication, and of an unplanned readmission.
| Predictor | Definition | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|
| Any Adverse Event | ||||
| ASA Class | I-II | Ref. | -- | -- |
| III-IV | 1.85 | 1.14–3.00 | .01 | |
| Number of Comorbidities | 0 | Ref. | ||
| 1 | 0.83 | 0.41–1.66 | .59 | |
| 2 | 0.97 | 0.36–2.61 | .96 | |
| ≥3 | 0.76 | 0.18–3.14 | .71 | |
| Hypertension | 1.18 | 0.62–2.25 | .62 | |
| COPD | 2.09 | 0.46–9.40 | .34 | |
| Diabetes Mellitus | 2.35 | 1.03–5.36 | .04 | |
| Body Habitus | Nonobese | Ref. | -- | -- |
| Obese | 0.81 | 0.42–1.56 | .53 | |
| Morbidly Obese | 1.56 | 0.69–3.49 | .28 | |
| Operative Time | < 180 Minutes | Ref. | -- | -- |
| 180–210 Minutes | 0.71 | 0.34–1.48 | .36 | |
| > 210 Minutes | 1.82 | 1.10–3.01 | .02 | |
| C-Statistic | 0.65 | |||
| Hosmer-Lemeshow | .44 | |||
| Major Complication | ||||
| COPD | 4.60 | 1.05–20.2 | .04 | |
| Diabetes Mellitus | 2.85 | 1.34–6.07 | .007 | |
| Steroid Usage | 3.29 | 1.01–10.7 | .048 | |
| Operative Time | < 180 Minutes | Ref. | -- | -- |
| 180–210 Minutes | 0.60 | 0.21–1.75 | .35 | |
| > 210 Minutes | 2.21 | 1.19–4.10 | .01 | |
| Unplanned Readmission | ||||
| Female Sex | 0.63 | 0.36–1.10 | .10 | |
| ASA Class | I-II | Ref. | -- | -- |
| III-IV | 1.88 | 1.02–3.48 | .04 | |
| Number of Comorbidities | 0 | Ref. | -- | -- |
| 1 | 0.67 | 0.29—1.53 | .34 | |
| 2 | 0.68 | 0.23—1.99 | .49 | |
| ≥3 | 0.44 | 0.09–2.19 | .34 | |
| Smoking | 2.05 | 0.89–4.75 | .09 | |
| Diabetes Mellitus | 3.51 | 1.28–9.58 | .01 | |
| Body Habitus | Nonobese | Ref. | -- | -- |
| Obese | 0.70 | 0.30–1.64 | .41 | |
| Morbidly Obese | 1.83 | 0.68–4.90 | .23 | |
| Operative Time | < 180 Minutes | Ref. | -- | -- |
| 180–210 Minutes | 0.78 | 0.31–1.97 | .60 | |
| > 210 Minutes | 1.98 | 1.06–3.69 | .03 | |
| C-statistic | 0.70 | |||
| Hosmer-Lemeshow | .56 |
Abbreviations: ASA = American Society of Anesthesiologists, CI = confidence interval. Statistically significant differences are bolded.
A major complication was defined using previously published criteria and included death, a neurologic or cardiopulmonary complication, venous thromboembolism, sepsis, surgical site infection, or reoperation.
Length of Hospital Stay:
The mean length of hospital stay was 3.0 (SD: 2.2) days. Multivariable linear regression analyzed predictors of a longer hospitalization (Table 4) after univariable screen; one model was constructed only based on preoperative and operative characteristics, while the second model also included postoperative complications. In the first model, ASA classification III-IV (P=.004), hypertension (P=.02), and longer operative time (P=.03) were independently associated with a longer length of hospital stay (when assessed continuously). In the second model, these variables remained significant, while postoperative cardiac (P=.001), airway (P=.02), and venous thromboembolic (P=.001) complications were also associated with a longer hospital stay. The R2 values of the first and second model were 2.8 and 6.1%, respectively.
Table 4.
Multivariable linear regression model analyzing the predictors of a longer hospitalization after MVD.
| Predictor | Definition | Coefficient (Days) | 95% CI | P-Value |
|---|---|---|---|---|
| Model 1: Preoperative and Operative Predictors | ||||
| ASA Class | I-II | Ref. | -- | -- |
| III-IV | 0.45 | 0.14 – 0.76 | .004 | |
| Missing | 1.04 | −3.31 – 5.40 | .64 | |
| Number of Comorbidities | 0 | Ref. | ||
| 1 | 0.08 | −0.27 – 0.43 | .66 | |
| 2 | −0.14 | −0.61 – 0.33 | .56 | |
| ≥3 | −0.54 | −1.25 – 0.17 | .13 | |
| Hypertension | 0.44 | 0.06 – 0.81 | .02 | |
| Diabetes Mellitus | 0.49 | −0.14 – 1.11 | .13 | |
| Operative Time | < 180 Minutes | Ref. | -- | -- |
| 180–210 Minutes | −0.04 | −0.43 – 0.34 | .84 | |
| > 210 Minutes | 0.39 | 0.03 – 0.75 | .03 | |
| Model 2: Preoperative, Operative, and Postoperative Predictors | ||||
| ASA Class | I-II | Ref. | -- | -- |
| III-IV | 0.36 | 0.06 – 0.67 | .02 | |
| Missing | 1.19 | −3.10 – 5.47 | .59 | |
| Number of Comorbidities | 0 | Ref. | -- | -- |
| 1 | 0.10 | −0.25 – 0.45 | .57 | |
| 2 | −0.06 | −0.52 – 0.41 | .81 | |
| ≥3 | −0.36 | −1.06 – 0.35 | .32 | |
| Hypertension | 0.43 | 0.07 – 0.80 | .02 | |
| Diabetes Mellitus | 0.17 | −0.46 – 0.80 | .59 | |
| Operative Time | < 180 Minutes | Ref. | -- | -- |
| 180–210 Minutes | −0.02 | −0.40 – 0.36 | .93 | |
| > 210 Minutes | 0.36 | 0.00 – 0.71 | .048 | |
| Postoperative Complications | Airway | 2.38 | 0.36 – 4.40 | .02 |
| Cardiac | 5.24 | 2.05 – 8.43 | .001 | |
| VTE | 3.53 | 1.37 – 5.68 | .001 | |
| Pneumonia | 2.41 | −0.05 – 4.87 | .06 | |
Statistically significant differences are bolded.
Abbreviations: ASA = American Society of Anesthesiologists, CI = confidence interval. Linear regression was utilized to identify pre-operative clinical factors associated with prolonged hospital stay; positive coefficients are factors that are associated with longer stays, while negative values are associated with shorter stays.
Reoperation:
The thirty-day reoperation rate for the entire study period was 3.6% (n=36), and the associated CPT code for the reoperation was available (from years 2012–2017) for 63.9% of cases (n=22). The most common recorded reoperations were repair of cerebrospinal fluid leakage or pseudomeningocele (43.5%, n=10), debridement of a surgical site infection (26.1%, n=6), posterior fossa decompression (8.70%, n=2), percutaneous radiofrequency treatment of the trigeminal nerve (8.70%, n=2), as well as microvascular decompression, ventricular shunt, and cranioplasty (4.3% each, n=1). Given the comparatively small number of reoperation events, no multivariable models of this outcome were constructed.
Unplanned Readmission:
The total thirty-day readmission rate from 2011–2017 was 6.8% (n=57), and the associated diagnosis was recorded in 86.0% of readmitted patients (n=49). The most common reasons for readmission were surgical site infection (22.4%, n=11), central nervous system complications (14.3%, n=7), cerebrospinal fluid leakage (14.3%, n=7), headache (10.2%, n=5), and meningitis (8.2%, n=4). Multivariable logistic regression identified ASA physical classification designation III-IV (P=.01), diabetes mellitus (P=.01), and longer operative time (P=.03) as independent predictors of an unplanned readmission.
Patient Age and Operative Time:
As patient age and operative time were analyzed as categorical variables in the primary analysis, further sensitivity analysis was performed of these variables. When evaluated continuously or categorically by quartile, patient age was not significantly associated with the development of any complication, an extended hospitalization, or unplanned readmission in univariable analysis. Thus, patient age does not meet entry criteria into any multivariable models regardless how it is evaluated.
The multivariable models of any adverse event, a major complication, length of hospital stay, and an unplanned reoperation were also constructed using operative time as a continuous variable. Longer operative duration remained a statistically significant predictor in each multivariable model—of any adverse event (by sixty-minute intervals, OR: 1.23, 95% CI: 1.01–1.50, P=.04), a major complication (OR: 1.31, 95% CI: 1.03–1.66, P=0.03), a longer hospitalization (beta-coefficient: 0.18 days, 95% CI: 0.04–0.31, P=.01), and an unplanned hospital readmission (OR: 1.28, 95% CI: 1.01–1.63, P=.04).
Discussion
The safety and efficacy of MVD for the treatment of medically intractable trigeminal neuralgia, hemifacial spasm, or glossopharyngeal neuralgia attributable to neurovascular conflict has been established by single center reports,1–6,13–26,28,41–65 While a comprehensive review of prior institutional analyses of MVD is beyond the scope of the present study, it is worth noting a few key studies that reported very low complication rates. In 1996, Barker et al. published a series of 1,336 MVD operations performed at a single center:4 the perioperative mortality rate was only 0.1% and major neurologic adverse events were extremely rare. In 2007, Sindou et al. reported a series of 362 patients, and complications were rare, with one patient experiencing gait disturbances due to cerebellar ischemia, three patients experiencing diplopia secondary to trochlear nerve palsy, three experiencing facial nerve palsy, and seven experiencing hearing loss.66 In a more recent institutional report of 250 cases, Broggi et al. found a similar low incidence of complications,6 including no deaths, and a 0.4% rate of perioperative cerebral infarction. Likewise, in a review of 1,1174 patients who underwent MVD for hemifacial spasm, reported complications were rare and included hearing loss in 1.1%, facial weakness in 0.7%, CSF leakage in 0.25%, and cerebellar infarction in 0.17%.67
However, there remains a paucity of prospective, multicenter data evaluating the outcomes, effectiveness, morbidity, and adverse events of MVD in practice, and few studies have evaluated postoperative outcomes after MVD nationally. In 2011, Rughani et al. used the National Inpatient Sample (NIS), an administrative claims dataset, to evaluate complications after MVD when stratified by patient age.26 Likewise, Kalkanis et al.used the NIS to demonstrate a volume-outcomes relationship for MVD, whereby superior outcomes (such as discharge disposition) were seen at high-volume centers. Moreover, Kundu and Rolston68 as well as Wang et al..69 have used national billing datasets to evaluate trends in the treatment of trigeminal neuralgia, reporting that the use of microvascular decompression is increasing in the United States compared to other procedural treatments; this underscores the importance of evaluating postoperative outcomes after MVD. Nevertheless, administrative billing datasets such as the NIS have limited discernment between comorbidities and postoperative complications from coding identifiers; on the other hand, prospectively collected surgical registries such as NSQIP explicitly evaluate postoperative complications. Arnone et al. used NSQIP to evaluate reoperation after MVD, reporting diabetes and morbid obesity to be significant risk factors.70 However, the authors only evaluated patients from 2007–2014 and had a comparatively small sample size of 506 patients; thus, no significant predictors of readmission were discerned, potentially due to an underpowered analysis. Additionally, they did not examine other adverse events or length of hospital stay.
In the present analysis, patients who underwent elective MVD were extracted from NSQIP to identify the rates and predictors of 30-day adverse events, a major complication, an extended hospitalization, and unplanned readmission in a nationally accrued population. Very low morbidity was seen after MVD, as the thirty-day mortality and major neurologic complication rates were 0.3% and 0.4%, respectively. Additionally, each individual complication had quite low thirty-day rates, with the most common being surgical site infections (1.7%). Multivariable logistic regression models were constructed to identify statistically significant independent predictors of any postoperative adverse event and an unplanned readmission, and predictors of both were ASA classification III-IV designation, diabetes mellitus, and longer operative time. Additionally, significant predictors of a major complication were diabetes mellitus, COPD, preoperative steroid usage, and longer operative duration, while higher ASA classification, hypertension, and longer operative time were predictors of an extended hospitalization, underscoring their relationship with the efficiency of postoperative care.
The relationship between patient age and outcomes after MVD—both postoperative complications and pain relief—is debated. Bick et al.reported in a retrospective, single-institution study that patients older than 60 years in fact had greater pain relief after MVD compared to those younger than 60 years.71 However, one of the greatest concerns about surgical decompression in older patients are postoperative complications, and some authors have reported that older age is a predictor of complications after MVD: Rughani et al.. found that age greater than 65 years was associated with a higher incidence of postoperative cardiac, thromboembolic, and cerebrovascular complications.26 On the other hand, some single-center studies have not found that postoperative complications vary by age when careful patient selection is employed.22,24,72,73 In the present analysis, older age was not an independent predictor any adverse events, readmission, or an extended hospitalization. Moreover, age was not predictive of outcomes regardless how the data were analyzed: categorically (using clinically pertinent divisions), by quartile, or continuously. This suggests that age alone may not be an optimal mode for preoperative risk stratification. Nevertheless, older patient age was found to be significantly associated with higher ASA physical classification designation, and ASA classification was found in this analysis to be a significant predictor of an adverse event, an unplanned readmission, and a longer hospitalization (after accounting for postoperative complications). Therefore, ASA classification may be a better indicator of a patient’s preoperative health status than age, the total number of comorbidities, or any specific comorbidity.
The ASA physical status classification was established in 1963 for the preoperative assessment of suitability of a patient to undergo a major operation. The system ranks patients on the severity of comorbid and the presenting illness, ranging from I, a healthy individual, to V, a moribund person who is not expected to survive without the operation. Among neurosurgical patients, classifications II-IV are the most common, which describe mild systemic disease (II), severe systemic disease (III), and severe systemic disease that is a constant threat to life (IV)—the prevalence of which were 60.6%, 34.2%, and 0.3% in the present patient population, respectively. Examples of ASA class II include smoking, social alcohol drinker, class I obesity, and controlled hypertension, diabetes, or lung disease. Class III designation includes poorly controlled hypertension, diabetes, or lung disease, morbid obesity, alcohol dependence, moderate reduction of ejection fraction, dialysis dependence, and either cardiovascular or cerebrovascular events sustained more than 90 days prior. Patients with greater severity of comorbid diseases (such as recent myocardial infarction), but who are undergoing elective surgeries, receive classification IV designation. Although the ASA scale is used internationally for patients undergoing any major operation,74 few studies have shown its utility an intracranial setting, and this analysis highlights its use in risk-stratifying patients prior to MVD.75–77
Additionally, in this analysis, longer operative time was an independent predictor of adverse events, prolonged hospitalization, and unplanned readmission. Although operative time was analyzed as a categorical variable in the primary analysis, these relationships persisted when operative time was evaluated continuously. Several neurosurgical studies have reported that longer operative time is a risk factor for perioperative complications, including venous thromboembolism and surgical site infections.78–80 This relationship has been hypothesized to be multifactorial, partially attributable to duration of anesthesia, as well as a maker for more complex surgical cases.
Another advantage of NSQIP is the inclusion of the specific reasons for reoperation and readmission. Surgical site infections were the most common individual complication, and the most common indications for both readmission and reoperation were surgical site infections and CSF leakage. Therefore surgeons, should balance operative speed with a meticulous closure, including of the Dura mater, and be cognizant that some of the most significant complications after this elective operation are related to closure.
There are several noteworthy limitations of this study. The NSQIP algorithm collects the same variables and postoperative complications for all patients regardless of surgical specialty. Thereby procedure-specific predictors and adverse events are not routinely available, unless specific complications merited 30-day reoperation or readmission, in which case NSQIP captures these occurrences through ICD and CPT coding. Thus, several pertinent specific variables and outcomes could not be evaluated including prior treatment for TN, the duration of symptoms, postoperative cranial nerve palsies including hearing loss, dysphagia, and postoperative improvement in preoperative symptoms.3,5,6,20,26,29,46,48 Additionally, as NSQIP only collects data on patients undergoing open surgery, this study could not compare MVD with other medical or interventional (including percutaneous) management strategies for TN.
Nevertheless, NSQIP has many distinct advantages in evaluating postoperative outcomes.32,33,35,37,39 These include greater generalizability than typical single-center reports, as NSQIP accrues patients nationally from varied healthcare settings, and therefore may be more representative of patients undergoing MVD in the United States. Additionally, NSQIP provides data on indications for reoperation and readmission, which can inform postoperative management and follow-up. Large, multicenter analysis of neurosurgical outcomes can be used to identify of risk factors for adverse outcomes and guiding pre-operative counseling, decision analysis, and risk-stratification.
Conclusions
In this analysis of a nationally accrued, prospectively collected registry, MVD performed for trigeminal neuralgia, hemifacial spasm, or glossopharyngeal neuralgia was associated with very low postoperative thirty-day morbidity and mortality. Patient age was not a predictor of any adverse events. Higher American Society of Anesthesiologists classification designation and longer operative time were predictors of any adverse event, an extended hospitalization, and an unplanned hospital readmission. The most common reasons for reoperation and readmission were surgical site infections and CSF leakage. ASA classification was found to be a superior mode of risk stratification than the total number of comorbidities alone; these data may augment preoperative patient counseling and risk-stratification.
Funding:
D.J.C.: NIH training grant T32 CA001009
Abbreviations:
- ACS
American College of Surgeons
- ASA
American Society of Anesthesiologists
- CSF
cerebrospinal fluid
- CPT
Current Procedural Terminology
- ICD
International Classification of Diseases
- MVD
microvascular decompression
- NIS
Nationwide Inpatient Sample
- NSQIP
National Surgical Quality Improvement Program
- TN
trigeminal neuralgia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work was presented in abstract form at the American Association of Neurological Surgeons Annual Meeting, May 2017 in Los Angeles, California, Podium Presentation, AANS/CNS Section on Pain.
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
References
- 1.Barba D, Alksne JF. Success of microvascular decompression with and without prior surgical therapy for trigeminal neuralgia. Journal of neurosurgery.1984;60(1):104–107. [DOI] [PubMed] [Google Scholar]
- 2.Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery.1995;36(1):64–68; discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza N, Illingworth RD. Trigeminal neuralgia treated by microvascular decompression: a long-term follow-up study. British journal of neurosurgery.1995;9(1):13–19. [PubMed] [Google Scholar]
- 4.Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. The New England journal of medicine.1996;334(17):1077–1083. [DOI] [PubMed] [Google Scholar]
- 5.Jannetta PJ. Outcome after microvascular decompression for typical trigeminal neuralgia, hemifacial spasm, tinnitus, disabling positional vertigo, and glossopharyngeal neuralgia (honored guest lecture). Clinical neurosurgery.1997;44:331–383. [PubMed] [Google Scholar]
- 6.Broggi G, Ferroli P, Franzini A, Servello D, Dones I. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry.2000;68(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumot C, Brinzeu A, Berthiller J, Sindou M. Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta neurochirurgica.2017;159(2):237–249. [DOI] [PubMed] [Google Scholar]
- 8.Acevedo JC, Sindou M, Fischer C, Vial C. Microvascular decompression for the treatment of hemifacial spasm. Retrospective study of a consecutive series of 75 operated patients--electrophysiologic and anatomical surgical analysis. Stereotactic and functional neurosurgery.1997;68(1–4 Pt 1):260–265. [DOI] [PubMed] [Google Scholar]
- 9.Teton ZE, Holste KG, Hardaway FA, Burchiel KJ, Raslan AM. Pain-free survival after vagoglossopharyngeal complex sectioning with or without microvascular decompression in glossopharyngeal neuralgia. Journal of neurosurgery.2019:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Kandan SR, Khan S, Jeyaretna DS, Lhatoo S, Patel NK, Coakham HB. Neuralgia of the glossopharyngeal and vagal nerves: long-term outcome following surgical treatment and literature review. British journal of neurosurgery.2010;24(4):441–446. [DOI] [PubMed] [Google Scholar]
- 11.Bartek J Jr., Gulati S, Unsgard G, et al. Standardized reporting of adverse events after microvascular decompression of cranial nerves; a population-based single-institution consecutive series. Acta neurochirurgica.2016;158(9):1775–1781. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Wang H, Fan Z, Fan Z. Complications in retrosigmoid cranial nerve surgery. Acta oto-laryngologica. 2010;130(2):247–252. [DOI] [PubMed] [Google Scholar]
- 13.Resnick DK, Jannetta PJ, Lunsford LD, Bissonette DJ. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis. Acta oto-laryngologica.1996;46(4):358–361; discussion 361–352. [DOI] [PubMed] [Google Scholar]
- 14.Pollack IF, Jannetta PJ, Bissonette DJ. Bilateral trigeminal neuralgia: a 14-year experience with microvascular decompression. Journal of neurosurgery.1988;68(4):559–565. [DOI] [PubMed] [Google Scholar]
- 15.Cutbush K, Atkinson RL. Treatment of trigeminal neuralgia by posterior fossa microvascular decompression. The Australian and New Zealand journal of surgery.1994;64(3):173–176. [DOI] [PubMed] [Google Scholar]
- 16.Linskey ME, Jho HD, Jannetta PJ. Microvascular decompression for trigeminal neuralgia caused by vertebrobasilar compression. Journal of neurosurgery.1994;81(1):1–9. [DOI] [PubMed] [Google Scholar]
- 17.Fields HL. Treatment of trigeminal neuralgia. The New England journal of medicine. 1996;334(17):1125–1126. [DOI] [PubMed] [Google Scholar]
- 18.Lovely TJ, Jannetta PJ. Microvascular decompression for trigeminal neuralgia. Surgical technique and long-term results. Neurosurgery clinics of North America.1997;8(1):11–29. [PubMed] [Google Scholar]
- 19.Resnick DK, Levy EI, Jannetta PJ. Microvascular decompression for pediatric onset trigeminal neuralgia. Neurosurgery.1998;43(4):804–807; discussion 807–808. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. Journal of neurosurgery.1999;90(1):1–8. [DOI] [PubMed] [Google Scholar]
- 21.Kabil MS, Eby JB, Shahinian HK. Endoscopic vascular decompression versus microvascular decompression of the trigeminal nerve. Minimally invasive neurosurgery : MIN.2005;48(4):207–212. [DOI] [PubMed] [Google Scholar]
- 22.Sekula RF, Marchan EM, Fletcher LH, Casey KF, Jannetta PJ. Microvascular decompression for trigeminal neuralgia in elderly patients. Journal of neurosurgery.2008;108(4):689–691. [DOI] [PubMed] [Google Scholar]
- 23.Sindou M, Leston JM, Decullier E, Chapuis F. Microvascular decompression for trigeminal neuralgia: the importance of a noncompressive technique--Kaplan-Meier analysis in a consecutive series of 330 patients. Neurosurgery.2008;63(4 Suppl 2):341–350; discussion 350–341. [DOI] [PubMed] [Google Scholar]
- 24.Sekula RF Jr., Frederickson AM, Jannetta PJ, Quigley MR, Aziz KM, Arnone GD. Microvascular decompression for elderly patients with trigeminal neuralgia: a prospective study and systematic review with meta-analysis. Journal of neurosurgery.2011;114(1):172–179. [DOI] [PubMed] [Google Scholar]
- 25.Oesman C, Mooij JJ. Long-term follow-up of microvascular decompression for trigeminal neuralgia. Skull base : official journal of North American Skull Base Society [et al. ].2011;21(5):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rughani AI, Dumont TM, Lin CT, Tranmer BI, Horgan MA. Safety of microvascular decompression for trigeminal neuralgia in the elderly. Clinical article. Journal of neurosurgery.2011;115(2):202–209. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Choi HJ, Ko HC, Choi SK, Lim YJ. Long term outcomes of gamma knife radiosurgery for typical trigeminal neuralgia-minimum 5-year follow-up. Journal of Korean Neurosurgical Society.2012;51(5):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakker NA, Van Dijk JM, Immenga S, Wagemakers M, Metzemaekers JD. Repeat microvascular decompression for recurrent idiopathic trigeminal neuralgia. Journal of neurosurgery.2014;121(4):936–939. [DOI] [PubMed] [Google Scholar]
- 29.Sivakanthan S, Van Gompel JJ, Alikhani P, van Loveren H, Chen R, Agazzi S. Surgical management of trigeminal neuralgia: use and cost-effectiveness from an analysis of the Medicare Claims Database. Neurosurgery.2014;75(3):220–226; discussion 225–226. [DOI] [PubMed] [Google Scholar]
- 30.Sellers MM, Merkow RP, Halverson A, et al. Validation of new readmission data in the American College of Surgeons National Surgical Quality Improvement Program. Journal of the American College of Surgeons.2013;216(3):420–427. [DOI] [PubMed] [Google Scholar]
- 31.Lieber BA, Appelboom G, Taylor BE, Malone H, Agarwal N, Connolly ES Jr. Assessment of the “July Effect”: outcomes after early resident transition in adult neurosurgery. Journal of neurosurgery.2015:1–9. [DOI] [PubMed] [Google Scholar]
- 32.McGirt MJ, Godil SS, Asher AL, Parker SL, Devin CJ. Quality analysis of anterior cervical discectomy and fusion in the outpatient versus inpatient setting: analysis of 7288 patients from the NSQIP database. Neurosurgical focus.2015;39(6):E9. [DOI] [PubMed] [Google Scholar]
- 33.Lieber BA, Appelboom G, Taylor BE, et al. Preoperative chemotherapy and corticosteroids: independent predictors of cranial surgical-site infections. Journal of neurosurgery.2015:1–9. [DOI] [PubMed] [Google Scholar]
- 34.Lim S, Parsa AT, Kim BD, Rosenow JM, Kim JY. Impact of resident involvement in neurosurgery: an analysis of 8748 patients from the 2011 American College of Surgeons National Surgical Quality Improvement Program database. Journal of neurosurgery.2015;122(4):962–970. [DOI] [PubMed] [Google Scholar]
- 35.McCutcheon BA, Ciacci JD, Marcus LP, et al. Thirty-Day Perioperative Outcomes in Spinal Fusion by Specialty Within the NSQIP Database. Spine.2015;40(14):1122–1131. [DOI] [PubMed] [Google Scholar]
- 36.Dasenbrock HH, Devine CA, Liu KX, et al. Thrombocytopenia and craniotomy for tumor: A National Surgical Quality Improvement Program analysis. Cancer.2016. [DOI] [PubMed] [Google Scholar]
- 37.Lieber BA, Han J, Appelboom G, et al. Association of Steroid Use with Deep Venous Thrombosis and Pulmonary Embolism in Neurosurgical Patients: A National Database Analysis. World neurosurgery.2016. [DOI] [PubMed] [Google Scholar]
- 38.Kim BD, Smith TR, Lim S, Cybulski GR, Kim JY. Predictors of unplanned readmission in patients undergoing lumbar decompression: multi-institutional analysis of 7016 patients. Journal of neurosurgery Spine.2014;20(6):606–616. [DOI] [PubMed] [Google Scholar]
- 39.Dasenbrock HH, Liu KX, Devine CA, et al. Length of hospital stay after craniotomy for tumor: a National Surgical Quality Improvement Program analysis. Neurosurgical focus.2015;39(6):E12. [DOI] [PubMed] [Google Scholar]
- 40.Lukasiewicz AM, Grant RA, Basques BA, Webb ML, Samuel AM, Grauer JN. Patient factors associated with 30-day morbidity, mortality, and length of stay after surgery for subdural hematoma: a study of the American College of Surgeons National Surgical Quality Improvement Program. Journal of neurosurgery.2016;124(3):760–766. [DOI] [PubMed] [Google Scholar]
- 41.Taarnhoj P Decompression of the posterior trigeminal root in trigeminal neuralgia. A 30-year follow-up review. Journal of neurosurgery.1982;57(1):14–17. [DOI] [PubMed] [Google Scholar]
- 42.Zorman G, Wilson CB. Outcome following microsurgical vascular decompression or partial sensory rhizotomy in 125 cases of trigeminal neuralgia. Neurology.1984;34(10):1362–1365. [DOI] [PubMed] [Google Scholar]
- 43.Szapiro J Jr., Sindou M, Szapiro J. Prognostic factors in microvascular decompression for trigeminal neuralgia. Neurosurgery.1985;17(6):920–929. [DOI] [PubMed] [Google Scholar]
- 44.Treatment of trigeminal neuralgia. The New England journal of medicine. 1987;316(11):692–693. [DOI] [PubMed] [Google Scholar]
- 45.Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. Journal of neurosurgery.1989;71(3):359–367. [DOI] [PubMed] [Google Scholar]
- 46.Dahle L, von Essen C, Kourtopoulos H, Ridderheim PA, Vavruch L. Microvascular decompression for trigeminal neuralgia. Acta neurochirurgica.1989;99(3–4):109–112. [DOI] [PubMed] [Google Scholar]
- 47.Sindou M, Amrani F, Mertens P. [Microsurgical vascular decompression in trigeminal neuralgia. Comparison of 2 technical modalities and physiopathologic deductions. A study of 120 cases]. Neuro-Chirurgie.1990;36(1):16–25; discussion 25–16. [PubMed] [Google Scholar]
- 48.Klun B Microvascular decompression and partial sensory rhizotomy in the treatment of trigeminal neuralgia: personal experience with 220 patients. Neurosurgery.1992;30(1):49–52. [DOI] [PubMed] [Google Scholar]
- 49.Sun T, Saito S, Nakai O, Ando T. Long-term results of microvascular decompression for trigeminal neuralgia with reference to probability of recurrence. Acta neurochirurgica.1994;126(2–4):144–148. [DOI] [PubMed] [Google Scholar]
- 50.Meneses MS, Clemente R, Russ HH, et al. [Microsurgical treatment of trigeminal neuralgia. A study of 50 cases]. Neuro-Chirurgie.1995;41(5):349–352. [PubMed] [Google Scholar]
- 51.Pamir MN, Zirh TA, Ozer AF, Keles GE, Baykan N. Microvascular decompression in the surgical management of trigeminal neuralgia. Neurosurgical review.1995;18(3):163–167. [DOI] [PubMed] [Google Scholar]
- 52.Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: Outcome on spasm and complications. A review. Neuro-Chirurgie.2018;64(2):106–116. [DOI] [PubMed] [Google Scholar]
- 53.Kondo A Follow-up results of microvascular decompression in trigeminal neuralgia and hemifacial spasm. Neurosurgery.1997;40(1):46–51; discussion 51–42. [DOI] [PubMed] [Google Scholar]
- 54.Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery.2002;50(4):705–710; discussion 710–701. [DOI] [PubMed] [Google Scholar]
- 55.Sarsam Z, Garcia-Finana M, Nurmikko TJ, Varma TR, Eldridge P. The long-term outcome of microvascular decompression for trigeminal neuralgia. British journal of neurosurgery.2010;24(1):18–25. [DOI] [PubMed] [Google Scholar]
- 56.Sampson JH, Grossi PM, Asaoka K, Fukushima T. Microvascular decompression for glossopharyngeal neuralgia: long-term effectiveness and complication avoidance. Neurosurgery.2004;54(4):884–889; discussion 889–890. [DOI] [PubMed] [Google Scholar]
- 57.Sindou M, Leston J, Howeidy T, Decullier E, Chapuis F. Micro-vascular decompression for primary Trigeminal Neuralgia (typical or atypical). Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta neurochirurgica.2006;148(12):1235–1245; discussion 1245. [DOI] [PubMed] [Google Scholar]
- 58.Danenbaum M, Lega BC, Suki D, Harper RL, Yoshor D. Microvascular decompression for hemifacial spasm: long-term results from 114 operations performed without neurophysiological monitoring. Journal of neurosurgery. 2008;109(3):410–415. [DOI] [PubMed] [Google Scholar]
- 59.Kndo A Follow-up results of using microvascular decompression for treatment of glossopharyngeal neuralgia. Journal of neurosurgery. 1998;88(2):221–225. [DOI] [PubMed] [Google Scholar]
- 60.Barker FG 2nd, Jannetta, Bissonette, Shields, Larkins, Jho. Microvascular decompression for hemifacial spasm. Journal of neurosurgery.1995;82(2):201–210. [DOI] [PubMed] [Google Scholar]
- 61.Taha JM, Tew JM Jr., Long-term results of surgical treatment of idiopathic neuralgias of the glossopharyngeal and vagal nerves. Neurosurgery.1995;36(5):926–930; discussion 930–921. [DOI] [PubMed] [Google Scholar]
- 62.Huang CI, Chen IH, Lee LS. Microvascular decompression for hemifacial spasm: analyses of operative findings and results in 310 patients. Neurosurgery.1992;30(1):53–56; discussion 56–57. [DOI] [PubMed] [Google Scholar]
- 63.Miller JP, Acar F, Burchiel KJ. Classification of trigeminal neuralgia: clinical, therapeutic, and prognostic implications in a series of 144 patients undergoing microvascular decompression. Journal of neurosurgery.2009;111(6):1231–1234. [DOI] [PubMed] [Google Scholar]
- 64.Lu VM, Goyal A, Graffeo CS, Perry A, Jonker BP, Link MJ. Glossopharyngeal Neuralgia Treatment Outcomes After Nerve Section, Microvascular Decompression, or Stereotactic Radiosurgery: A Systematic Review and Meta-Analysis. World neurosurgery.2018;120:572–582.e577. [DOI] [PubMed] [Google Scholar]
- 65.Jani RH, Hughes MA, Ligus ZE, Nikas A, Sekula RF. MRI Findings and Outcomes in Patients Undergoing Microvascular Decompression for Glossopharyngeal Neuralgia. Journal of neuroimaging : official journal of the American Society of Neuroimaging.2018;28(5):477–482. [DOI] [PubMed] [Google Scholar]
- 66.Sindou M, Leston J, Decullier E, Chapuis F. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. Journal of neurosurgery.2007;107(6):1144–1153. [DOI] [PubMed] [Google Scholar]
- 67.Hyun SJ, Kong DS, Park K. Microvascular decompression for treating hemifacial spasm: lessons learned from a prospective study of 1,174 operations. Neurosurgical review.2010;33(3):325–334; discussion 334. [DOI] [PubMed] [Google Scholar]
- 68.Kundu B, Rolston JD. Nationwide Shift From Percutaneous Rhizotomy to Microvascular Decompression for Treatment of Trigeminal and Other Cranial Nerve Neuralgias. Headache.2018;58(10):1675–1679. [DOI] [PubMed] [Google Scholar]
- 69.Wang DD, Ouyang D, Englot DJ, et al. Trends in surgical treatment for trigeminal neuralgia in the United States of America from 1988 to 2008. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia.2013;20(11):1538–1545. [DOI] [PubMed] [Google Scholar]
- 70.Arnone GD, Esfahani DR, Papastefan S, et al. Diabetes and morbid obesity are associated with higher reoperation rates following microvascular decompression surgery: An ACSNSQIP analysis. Surgical neurology international.2017;8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bick SK, Huie D, Sneh G, Eskandar EN. Older Patients Have Better Pain Outcomes Following Microvascular Decompression for Trigeminal Neuralgia. Neurosurgery.2019;84(1):116–122. [DOI] [PubMed] [Google Scholar]
- 72.Gunther T, Gerganov VM, Stieglitz L, Ludemann W, Samii A, Samii M. Microvascular decompression for trigeminal neuralgia in the elderly: long-term treatment outcome and comparison with younger patients. Neurosurgery.2009;65(3):477–482; discussion 482. [DOI] [PubMed] [Google Scholar]
- 73.Ashkan K, Marsh H. Microvascular decompression for trigeminal neuralgia in the elderly: a review of the safety and efficacy. Neurosurgery.2004;55(4):840–848; discussion 848–850. [DOI] [PubMed] [Google Scholar]
- 74.Hackett NJ, De Oliveira GS, Jain UK, Kim JY. ASA class is a reliable independent predictor of medical complications and mortality following surgery. International journal of surgery (London, England).2015;18:184–190. [DOI] [PubMed] [Google Scholar]
- 75.Puffer RC, Mallory GW, Burrows AM, Curry TB, Clarke MJ. Patient and Procedural Factors That Influence Anesthetized, Nonoperative Time in Spine Surgery. Global spine journal.2016;6(5):447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verlaan JJ, Choi D, Versteeg A, et al. Characteristics of Patients Who Survived < 3 Months or > 2 Years After Surgery for Spinal Metastases: Can We Avoid Inappropriate Patient Selection? Journal of clinical oncology : official journal of the American Society of Clinical Oncology.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sherrod BA, Johnston JM, Rocque BG. Risk factors for unplanned readmission within 30 days after pediatric neurosurgery: a nationwide analysis of 9799 procedures from the American College of Surgeons National Surgical Quality Improvement Program. Journal of neurosurgery Pediatrics.2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimmell KT, Walter KA. Risk factors for venous thromboembolism in patients undergoing craniotomy for neoplastic disease. Journal of neuro-oncology.2014;120(3):567–573. [DOI] [PubMed] [Google Scholar]
- 79.Kimmell KT, Jahromi BS. Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. Journal of neurosurgery.2015;122(5):1004–1011. [DOI] [PubMed] [Google Scholar]
- 80.De la Garza-Ramos R, Abt NB, Kerezoudis P, et al. Deep-wound and organ-space infection after surgery for degenerative spine disease: an analysis from 2006 to 2012. Neurological research.2016;38(2):117–123. [DOI] [PubMed] [Google Scholar]