Abstract
Purpose:
Human papilloma virus (HPV) negative head and neck squamous cell carcinomas (HNSCC) harbor frequent genomic amplification of Fas-Associated Death Domain (FADD), with or without concurrent amplification of Baculovirus inhibitor of apoptosis repeat containing (BIRC2/3) genes encoding cellular Inhibitor of Apoptosis Proteins 1/2 (cIAP1/2). Antagonists targeting cIAP1 have been reported to enhance sensitivity of HPV(−), but not HPV(+) tumors, to Tumor Necrosis Factor family death ligands (TNF, TRAIL) and radiation.
Methods:
We tested a novel dual cIAP/XIAP antagonist ASTX660 in HPV(+) and (−) cell lines in combination with death ligands TNFα and TRAIL, and in preclinical xenograft models with radiation, an inducer of death ligands. The dependence of activity on TNF was examined by antibody depletion.
Results:
ASTX660 sensitized subsets of HPV(−) and (+) HNSCC cell lines to TNFα and TRAIL. These anti-tumor effects of ASTX660 are the result of both apoptosis and/or necroptosis among HPV(−) cells, and primarily by apoptosis (caspase 3 and caspase 8 cleavage) in HPV(+) cells. ASTX660 enhanced restoration of protein expression and inhibitory activity of pro-apoptotic tumor suppressor TP53 in HPV(+) HNSCC. Furthermore, ASTX660 combined with radiotherapy, an inducer of death ligands, significantly delayed growth of both human HPV(−) and HPV(+) human tumor xenografts, an effect attenuated by anti-TNFα pretreatment blockade.
Conclusions:
IAP1/XIAP antagonist ASTX660 sensitizes HPV(+) HNSCC to TNFα via a mechanism involving restoration of TP53.The present findings serve to motivate further studies of dual cIAP/XIAP antagonists and future clinical trials combining these antagonists with radiotherapy to treat both HPV(+) and (−) HNSCC.
Keywords: ASTX660, HPV, inhibitors of apoptosis, radiation therapy, squamous cell carcinoma, TNFα, TRAIL
Introduction
As the sixth most common cancer, head and neck squamous cell carcinoma (HNSCC) accounts for more than 600,000 new cases every year worldwide, with a five-year survival rate nearing 50% (1). HNSCC currently comprises approximately 3% of new cancer diagnoses in the United States (2). More than 90% of all head and neck cancers are squamous cell carcinomas arising from the epithelium of the oral cavity, oropharynx, nasopharynx, larynx, and hypopharynx (3). In recent years, the human papillomavirus (HPV) has been implicated in a continued increase in the incidence of HPV(+) oropharyngeal cancers (4-7). The Cancer Genome Atlas (TCGA) recently analyzed 279 HNSCC, finding that nearly half harbor genomic alterations in cell death pathways (8-10). Approximately 30% of HPV(−) HNSCC carry chromosome 11q13/22 copy gains causing overexpression of Fas-associated death domain (FADD), with or without concurrent amplification of Baculovirus Inhibitor of Apoptosis repeat containing (BIRC2/3) genes that encode cellular Inhibitor of Apoptosis Proteins 1/2 (cIAP1/2). Both FADD and cIAP1/2 are central components of the Tumor Necrosis Factor Receptor superfamily (TNFRSF) signaling pathways that mediate cell death induced by extrinsic ligands (11-13). TNFRSF binding to ligands TNFα, TNF-related apoptosis-inducing ligand (TRAIL), Fas ligand (FasL), or other death agonists typically leads to cell death via FADD and caspase-8 (apoptosis) or Receptor-interacting protein (RIP) kinase (necroptosis) (14-16). However, amplification or overexpression of BIRC2/3 genes and corresponding increased expression of cIAP1/2 can switch the signaling cascade to promote survival by inhibiting caspase-mediated apoptosis and RIP-mediated necroptosis (17). Another IAP family member, X-linked IAP (XIAP), also contributes to inhibition of cell death mediated by the mitochondrial and caspase-3 pathway, which may be activated in response to intrinsic DNA damage TNF-induced reactive oxygen species, or genotoxic chemo- or radiation therapies (18-20).
Second mitochondria-derived activator of caspases (SMAC) mimetics that antagonize IAPs can enhance cell death via these extrinsic or intrinsic pathways. They have been developed as potential therapeutics to reinstate signaling pathways that promote cell death (21-28). We recently reported that Birinapant, a cIAP1 antagonist, sensitizes a panel of HPV(−) human HNSCC lines to death ligands TNFα and TRAIL, but was less active in an HPV(+) line (29). When combined with radiation therapy (XRT) in vivo, Birinapant is a potent inducer of TNFα and can delay or eradicate HPV(−) HNSCC xenografts harboring FADD and cIAP1 gains and overexpression (29). Another SMAC mimetic that preferentially inhibits cIAP1, LCL161, also induced preferential radiosensitization in HPV(−) when compared to HPV(+) HNSCC (30,31). HPV(+) HNSCC usually lack increased FADD and cIAP1 observed in HPV(−) cancers, but could retain some potential for activation of the intrinsic caspase and XIAP-modulated pathway, as a result of incomplete degradation of tumor suppressor protein TP53 via HPV oncoprotein E6 (18-20,32,33). However, the role of combined cIAP and XIAP inhibition in HNSCC remains unknown.
ASTX660 is a novel orally bioavailable, synthetic non-peptidomimetic dual cIAP/XIAP antagonist agent under evaluation in a Phase I/II clinical trial in patients with advanced solid tumors and lymphomas (34-36). In the present study, we sought to investigate the effects of dual cIAP/XIAP antagonist ASTX660 in preclinical models of HNSCC, with particular focus on the potential of such antagonists in treating both HPV(−) and (+) cancer. Using both in vitro and xenograft mouse model techniques, we confirmed our previous findings that dual cIAP/XIAP inhibition combined with TNFRSF ligands or XRT significantly inhibits HPV(−) HNSCC and further extended this finding to HPV(+) HNSCC. Furthermore, we demonstrate that TP53 plays a central role in mediating the anti-tumor effects of cIAP/XIAP inhibition in HPV(+) HNSCC.
Materials and Methods
Cell Lines
A panel of five HPV(−) [UM-SCC-11B, UM-SCC-22B, UM-SCC-38, UM-SCC-46, and UM-SCC-74A] and six HPV(+) [UM-SCC-47, UM-SCC-104, UPCI-SCC-90, UPCI-SCC-152, UD-SCC-2, and 93VU147T] HNSCC cell lines were used for the experiments in the present study. The UM-SCC cells were obtained from Drs. Thomas E. Carey, Mark E. Prince, Carol R. Bradford at the University of Michigan (Ann Arbor, MI). The UPCI-SCC cells were obtained from Drs. Robert Ferris and Susanne Gollin at the University of Pittsburgh (Pittsburgh, PA). The UD-SCC-2 cells were obtained from Drs. Thomas K. Hoffman and Henning Bier from the University of Dusseldorf (Dusseldorf, Germany) and maintained as previously described (37). The 93VU147T cells originated from the Free University of Amsterdam (Amsterdam, The Netherlands) and were acquired as previously described (38-40). The unique genotypes of these cells have been previously validated, confirmed to be mycoplasma free by PCR, and were maintained as previously described (40). All cell lines were stored in liquid nitrogen and cultured for no longer than 3 months or 15 passages before experimental use. For in vitro experiments, cells were harvested with 0.25% Trypsin-EDTA (Life Technologies) and viability was determined using propidium iodide exclusion. Prior to experiments, all cells were confirmed to be Mycoplasma negative (MycoAlert Kit, Thermo Fisher).
siRNA Knockdown of target mRNAs
HNSCC cells were transfected with 40nM of non-targeting control (sc-37007) or TP53 siRNA (sc-29435) using siRNA Transfection Reagent (sc-29528) in reduced-serum siRNA Transfection Medium (sc-36868, Santa Cruz Biotechnology) according to the manufacturer’s instructions. To evaluate cell proliferation of transfected cells, HNSCC cells were plated at 5.0×103 cells per well in 96-well plates and allowed to adhere overnight prior to transfection. Cells were transfected for 24 hours, then allowed to grow for another 48 hours, before being treated as indicated. TP53 knockdown was verified by quantitative RT-PCR as previously described (41) and flow cytometry (Supplemental Figure S5).
Reagents and Antibodies
ASTX660 was obtained from Astex Pharmaceuticals through a cooperative research and development agreement with the National Institute on Deafness and Other Communication Disorders (NIDCD). Recombinant human TNFα and TRAIL were obtained from R&D Systems. XTT kits to assess cell density were obtained from Sigma-Aldrich. Pan-caspase inhibitor ZVAD (FMK001, R&D Systems), caspase 8 inhibitor ZIETD (550380, BD Biosciences), and RIP1 inhibitor Necrostatin-1 (N9037, Sigma-Aldrich) were used to differentiate apoptotic and necroptotic cell death. Pifithrin-α (63208-82-2, Sigma-Aldrich) a selective TP53 inhibitor, was used at a concentration shown to functionally inhibit TP53 reporter activity in UM-SCC cell lines (41).
The following primary antibodies were used for Western blots: anti-cIAP1 (AF8181), anti-cIAP2 (AF8171), anti-XIAP (AF8221), anti-MDM2 (AF1244, R&D Systems); anti-caspase 8 (9746S), anti-caspase 3 (9662S), anti-p21 (2947S), anti-Bcl-2 (15071S), anti-Bcl-xL (2764S), anti-Bax (2774S, Cell Signaling Technology); anti-MLKL (EPR17514, abcam), and anti-TP53 (OP43, EMD Millipore). All but one of the same primary antibodies were used for immunohistochemistry (IHC), as a different anti-TP53 antibody (2527S, Cell Signaling Technology) was required for IHC. Additional anti-Ki-67 (9027T, Cell Signaling Technology) and In Situ Cell Death Detection kits (11684817910, Sigma-Aldrich) were also used for IHC. Antibodies used for flow cytometry included p53-PE (Biolegend, #645806), p-p53-Ser46 F11 (Santa Cruz, #sc101764), and cleaved caspase 8-PE (Cell Signaling Technology, #12602S). The secondary antibody used for p-p53-Ser46 was a PECy7 Rat anti-mouse IgG1 (Biolegend, #406613).
Cell proliferation and viability assays
HNSCC cells were plated at 5.0×103 cells per well in 96-well plates and allowed to adhere overnight prior to drug treatments. Cells were then treated with 0.01% DMSO control or ASTX660 at indicated concentrations ± recombinant human TNFα (20 ng/mL) or TRAIL (50 ng/mL). When using ZVAD (20 μg/mL), ZIETD (20 μg/mL), and Necrostatin (20 μg/mL) to differentiate apoptotic and necroptotic cell death, or Pfithrin-α (50 uM) to inhibit cellular TP53, those agents were added to the cells at the same time point as any ASTX660. When evaluating proliferation of cells transfected with either control or TP53 siRNA, indicated ASTX660 treatments were added after cells could grow for 48 hours following the completion of transfection. Cell density was measured by XTT kits to determine half-maximal inhibitory concentration (IC50) following 72 hours of treatment using the nonlinear four-parameter regression function in GraphPad Prism 7.
Western blot
HNSCC cells were plated in 10-cm dishes overnight before treatment with 0.01% DMSO control or ASTX660 at indicated concentrations ± recombinant human TNFα (20 ng/mL). At 12, 24, and 48 hours after treatment, whole-cell lysates were collected and stored in NP-40 lysis buffer (Invitrogen) supplemented with 1% Halt protease inhibitor and 1% Halt phosphatase inhibitor cocktails from ThermoFisher. Collected lysates were vortexed and frozen at −80°C prior to use. Protein concentrations were determined using the Pierce BCA Protein Assay Kit (ThermoFisher). Equal amounts of lysates (15μg) for each line were loaded into and separated by 4-12% gradient Bis-Tris gel (Invitrogen) electrophoresis and transferred for 6 minutes to PVDF membranes using the Invitrogen iBlot 2 system according to manufacturer’s instructions. Membranes were blocked for one hour in Odyssey blocking buffer (LI-COR) and incubated overnight at 4°C with primary antibody diluted in Odyssey blocking buffer as specified by manufacturer’s instructions with 0.2% Tween 20 (Sigma-Aldrich). Membranes were then washed with 0.05% Tween 20 in PBS before incubation with species-appropriate Odyssey secondary antibodies (LI-COR) for 1 hour at room temperature. Quantification of protein expression by Western blotting was performed using protein densitometry normalized to β-actin with ImageJ 1.51a software.
Flow cytometry
Cells were fixed after 48 hours of treatment with 2% paraformaldehyde, then permeabilized overnight in ice-cold 100% methanol. Cells were then stained in primary antibody (p53 F11), and followed with secondary/conjugated antibodies. Analysis was performed on a BD Fortessa Cytometer with BD FACS Diva software, then analyzed with FlowJo. Isotype controls were also used for each antibody to verify low levels of nonspecific background staining with each treatment group.
In vivo Mouse Experiments
All animal experiments were conducted under protocol 1322-16 approved by the NIDCD Animal Care and Use Committee, in compliance with the Guide for the Care and Use of Laboratory Animal Resource (1996) National Research Council. Female athymic nu/nu mice aged 4-6 weeks were obtained from Charles River and housed in a pathogen-free animal facility. Each mouse was injected in the right hind leg with 2.5×106 UM-SCC-46 cells or 1.0×107 UPCI-SCC-90 cells in Matrigel. UM-SCC-46 and UPCI-SCC-90 tumors grew for 7 and 18 days, respectively, before being randomized into treatment groups. Treatments included ASTX660 (16 mg/kg/day for two nonconsecutive weeks, by oral gavage), XRT (daily weekday treatments of 2 Gy each), or a combination of the two. Antibodies against TNFα (200 μg twice weekly, IP) were also administered to assess the role of the death ligand in the mechanism through which ASTX660 and XRT inhibit tumor growth. In a subset of randomly selected mice, identifiable tumors were harvested following the first week of treatment (day 8) for IHC.
Immunohistochemistry
Harvested samples of UM-SCC-46 or UPCI-SCC-90 xenograft tumors were embedded in OCT, and frozen tissue sections were generated by Histoserv, Inc. (Germantown, MD). For IHC, tissue sections were fixed using 4% formalin, permeabilized with methanol, and quenched with 3% H2O2. Sections to be stained with goat antibodies were blocked using rabbit serum from the VECTASTAIN Elite ABC Kit (PK-6105), while sections to be stained with rabbit antibodies were blocked using goat serum from another VECTASTAIN Elite ABC Kit (PK-6101). Primary antibodies were diluted in 3% BSA in TBS and placed on tissue sections overnight at 4°C. Sections were then washed with 0.1% Triton X-100 in TBS before incubation with species-appropriate secondary antibodies (VECTASTAIN Elite ABC Kit) diluted in 3% BSA in TBS for 30 minutes at room temperature. Sections were washed once more then stained using the VECTASTAIN Elite ABC Kit along with the DAB Enhancing Solution. Counterstain was achieved using hematoxylin (Gill’s solution, Sigma-Aldrich). Ethanol and Histo-Clear (National Diagnostics) were used to dehydrate the sections. Permount (Fisher Scientific) was used to mount the slides. Slides were imaged using the Aperio ScanScope (Leica Biosystems). Histoscores for cytoplasmic staining and % positive nuclei for nuclear staining were calculated using the ImageScope software and reported as the average of 10 randomly selected, non-overlapping regions from the stained slides (Leica Biosystems). Standard hematoxylin and eosin staining of representative tumor specimens from each treatment group was also performed by Histoserv (Germantown, MD).
Statistical Analysis
Laboratory-generated data were analyzed using GraphPad Prism 7. Descriptive statistics are presented as means with standard deviations or counts with percentages. Continuous variables were compared using one-way ANOVA and post hoc Tukey’s multiple comparisons tests. Kaplan-Meier analysis was used to estimate survival following tumor inoculation and Gehan-Breslow-Wilcoxon tests were used to compare survival distributions. All values of p<0.05 were considered statistically significant. Whole exome and transcriptomics sequencing were performed using SOLiD4 platform (Applied Biosystems, Foster City, CA) as previously described (42). Copy number alterations were analyzed using CONTRA (43) and visualized with IGV (44) software. Read counts of the genes were normalized using DESeq (45) R package (version 3.2.0) and the upper quartile normalization method.
Results
ASTX660 Sensitizes HPV(−) and HPV(+) Tumor Cells to Death by TNFα and TRAIL
We selected a panel of five HPV(−) and six HPV(+) human HNSCC cell lines that differed in copy alterations for chromosome 11q13/22 genes FADD and IAP1 and chromosome X gene XIAP (Supplemental Figure S1; ref 42). We examined sensitivity to ASTX660 by XTT assay across a range of concentrations (1 nM-10 μM) with and without TNFα and TRAIL at concentrations previously shown to be active in combination with IAP antagonists (29). Among those tested, the HPV(−) UM-SCC-46 cells were the most sensitive to ASTX660 alone (IC50 4.3 nM), while the remaining HPV(−) or HPV(+) cells were resistant to ASTX660 alone up to 1 μM (Supplemental Tables S1, 2). When combined with TNFα and TRAIL, ASTX660 significantly enhanced sensitivity of HPV(−) lines UM-SCC-11B, UM-SCC-22B, UM-SCC-46, and UM-SCC-74A, that exhibit copy gains and express FADD +/− IAP1 or XIAP1, or wild-type TP53, but not UM-SCC-38, a control lacking copy altered FADD, IAP1 or XIAP1 (Figure 1, Supplemental Figure 1; refs 29, 42). Less expectedly, ASTX660 in combination with death ligands also inhibited several HPV(+) lines, including UM-SCC-47, UPCI-SCC-90, UPCI-SCC-152, which lack consistent copy gains in FADD, IAP1 or XIAP (Supplemental Figure 1). We quantified whether the effects of the combination are consistent with additivity, synergy or antagonism among drug combinations, using the Bliss independence method (Supplemental Table S3). The synergistic effects for ASTX660 in combination with death ligands were observed for most cell lines except for UM-SCC-38, which suggested additivity with TRAIL, or 93VU147T and UDSCC-2, where antagonism was observed.
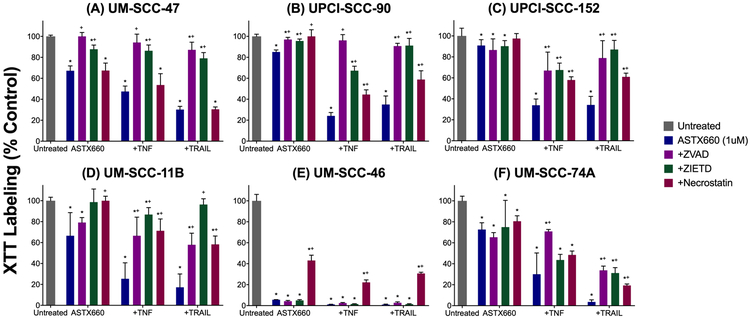
Figure 1.
ASTX660 enhances human HNSCC death with TNFα and TRAIL. HPV(−) (A-B) and HPV(+) (C-D) cell lines were treated with ASTX660 (1μM), TNFα (20 ng/mL), TRAIL (50 ng/mL), or combinations of these agents, then assessed following 72 hours by XTT assay. XTT labeling (% control is displayed. *p<0.05, significance in comparison with untreated cells. +p<0.05 comparison vs cells treated with ASTX660 only. =p<0.05 comparison against cells treated with TNFα only.
ASTX660 in the Presence of TNFα or TRAIL Induces Cell Death through Necroptosis and/or Apoptosis
To clarify the mechanisms through which ASTX660 sensitizes tumor cells to TNFα or TRAIL, we assessed the effects of pan-caspase (ZVAD), caspase-8 (ZIETD), and RIPK1 (necrostatin) inhibitors on cell density in the three most sensitive HPV(+) and HPV(−) cell lines (Figure 2). All three HPV(+) lines (UM-SCC-47, UPCI-SCC-90, and UPCI-SCC-152) demonstrated rescue from inhibitory effects of ASTX660 and death ligands with caspase inhibitors ZVAD and ZIETD, while necrostatin had a much less significant effect (Figure 2A-C). These findings suggest that HPV(+) tumor cell sensitivity to ASTX660 with TNFα or TRAIL is predominantly mediated by caspase-dependent apoptotic cell death. Among HPV(−) cells, UM-SCC-11B appeared to be susceptible to a mixed caspase- and RIPK1-dependent mechanisms of cell death, with all three inhibitors restoring cell density among cells treated with ASTX660 together with either TNFα or TRAIL (Figure 2D). UM-SCC-46 cell death was most strongly reversed with necrostatin, when compared with ZVAD or ZIETD, indicating a predominantly RIPK1-mediated, necroptotic cell death (Figure 2E). In contrast, UM-SCC-74A cell death was reversed most with ZVAD and ZIETD, supporting a predominantly caspase-mediated apoptotic cell death (Figure 2F). The effects of ZVAD and ZIETD in attenuating ASTX660+TNFα mediated degradation of caspase-8 and/or −3 were confirmed in the UM-SCC-47 and UM-SCC-46 cell lines in control experiments (Supplemental Figure 2).
Figure 2.
ASTX660 with TNFα or TRAIL causes cell death by necroptosis and/or apoptosis. HPV(−) (A-C) and HPV(+) (D-F) cells were treated with ASTX660 (1μM), TNFα (20 ng/mL), TRAIL (50 ng/mL), or combinations of these agents, with or without ZVAD (20 μg/mL), ZIETD (20 μg/mL), or Necrostatin (20 μg/mL) then assessed following 72 hours by XTT assay. *p<0.05 comparing cells treated with ZVAD/ZIETD/Necrostatin to cells not treated with caspase/RIP1 inhibitors.
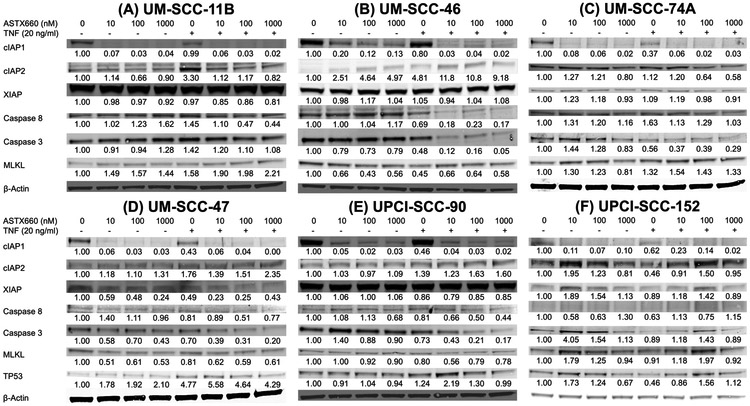
Expression levels of key cell death pathway proteins following treatment with ASTX660, with or without TNFα, were assessed by Western blot of whole-cell lysates from the same six cell lines following 48 hours of treatment (Figure 3). Consistent with the established mechanism of action for ASTX660, cIAP1 levels decreased by at least 80% across all six cell lines when treated with as little as 10 nM ASTX660 alone (36). Inhibition of cIAP1 tended to be accompanied by an increase cIAP2 levels, a compensatory effect observed previously with other cIAP1 inhibitors (29). While ASTX660 inhibits the actions of XIAP (34-36), it does not degrade the protein, consistent with our observations of minimal changes in XIAP levels with ASTX660 treatments.
Figure 3.
Changes in protein expression with ASTX660 treatment with or without TNFα. Cells were treated with ASTX660 at indicated concentrations with or without TNFα (20 ng/mL) then lysed following 48 hours. Equal amounts of lysates (15 μg) were loaded into each well.
Changes in protein levels for UM-SCC-11B with treatments with ASTX660 were consistent with our previous findings of mixed apoptotic/necroptotic cell death, since levels of full-length caspase 8 decreased, indicating activation by cleavage, and the concomitant increase of necroptosis marker MLKL with ASTX660 and TNFα (Figure 3A). While RIPK1 inhibitor necrostatin restored UM-SCC-46 cell density with ASTX660 and TNFα treatment, Western blotting nevertheless revealed significant caspase-8 and caspase-3 protein degradation with ASTX660 and TNFα treatment (Figure 3B), which was less prominent in prior studies with a predominantly cIAP1 inhibitor (29). In highly sensitive UM-SCC-46 cells, decreases in MLKL levels were observed with increasing doses of ASTX660 alone at 48hrs when widespread cell death was observed, whereas increasing doses of ASTX660 in the presence of TNFα increased MLKL expression relative to TNFα alone. Moreover, we observed mixed apoptotic/necroptotic cell death for UM-SCC-74, as ASTX660 and TNFα treatment induced significant caspase-3 cleavage concomitant with mild increases in MLKL (Figure 3C). As a negative control, a TNFα and IAP inhibitor resistant HPV(−) UM-SCC-38 cell line lacking FADD or cIAP amplification (29), demonstrated no discernable changes in levels of caspase-3, caspase-8, or MLKL proteins with ASTX660 and TNFα (Supplemental Figure S3). For the HPV(+) cell lines, we observed a predominantly apoptotic mechanism demonstrated by degradation of caspase-8 and/or caspase-3 protein, and limited changes in MLKL expression (Figures 3D-F). Unexpectedly, TNFα and/or ASTX660 enhanced pro-apoptotic tumor suppressor protein TP53 expression in HPV(+) UM-SCC-47, and weakly in UPCI-SCC90 and −152 (Figures 3D-F). We independently confirmed that combined treatment increased intracellular TP53 immunostaining by flow cytometry in UM-SCC-47, whereas UPCI-SCC-90 and the HPV(−) lines UM-SCC-74A with wtTP53, or UM-SCC-46 with mtTP53, showed minimal change or reduced TP53 or phospho-p53-serine46 (Supplemental Figure 4A-C). Using the chemotherapy drug and DNA damage agent cisplatin as a control inducer of TP53, we confirmed that it can induce higher total TP53 protein MFI in HPV(+) line UM-SCC-47 than in UPCI-SCC-90 with weaker TP53 expression (Supplemental Figure S4B). In HPV(−) lines, no induction of TP53 protein by cisplatin was observed in UM-SCC46 with mtTP53, while wtTP53 line UM-SCC-74 showed the greatest response to cisplatin. TNF-α only moderately induced TP53 protein expression in UM-SCC 47 cells. We found phospho-Ser46 TP53, a marker of signal modification, was inducible by cisplatin in all the cell lines, and weakly by ASTX660/TNF in UM-SCC47 and UM-SCC46 (Supplemental Figure S4C). Although we did not observe discrete caspase-3 or 8 cleavage bands in the full Western blots for Figure 3, the degradation observed was accompanied by a trend toward increase in cleaved caspase-8 by specific antibody by flow cytofluorometry (Supplemental Figure S4D). Together, these data suggest ASTX660 enhanced TP53 protein expression and caspase-mediated cell death in a subset of HPV(+) HNSCC.
ASTX660 Sensitization of HPV(+) Tumor Cells to TNFα through TP53
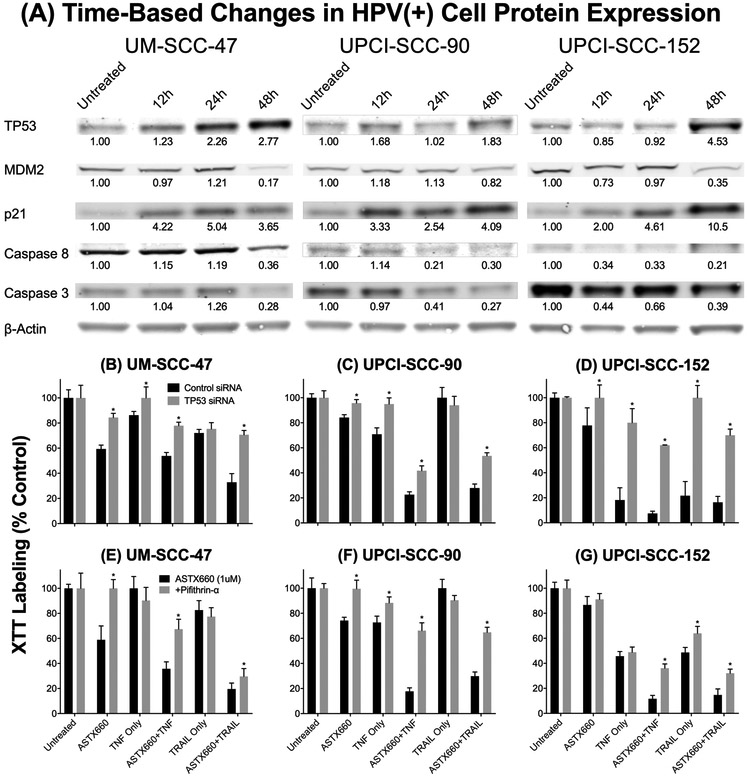
We next investigated the role and time course of effects of ASTX660 on TP53 protein, and the potential relationship to MDM2, which can inversely regulate TP53, in the mechanism of response of HPV(+) tumor cells to ASTX660 and TNFα (Figure 4A). We performed Western blotting of whole cell lysates collected following 12, 24, and 48 hours of treatment with ASTX660 and TNFα. Consistent with earlier findings, TP53 protein increased within 48 hours in all three HPV(+) cell lines, and this increase was mirrored by a corresponding decrease in the TP53 negative regulator MDM2 and an increase in the TP53-inducible downstream cell cycle arrest marker p21. Time-dependent caspase-8 and caspase-3 degradation was observed across all three cell lines, supporting a predominantly apoptotic mechanism of cell death.
Figure 4.
ASTX660 sensitizes HPV(+) tumor cells to TNFα through TP53. (A) Cells were treated with ASTX660 (1 μM) with TNFα (20 ng/mL) then lysed following 12, 24, or 48 hours. Equal amounts of lysates (15 μg) were loaded into each well. (B-D) Cells transfected with control or TP53 siRNA were treated with ASTX660 (1μM), TNFα (20 ng/mL), TRAIL (50 ng/mL), or combinations of these agents. (E-G) Cells were treated with ASTX660 (1μM), TNFα (20 ng/mL), TRAIL (50 ng/mL), or combinations of these agents with or without Pifithrin-α (20 μg/mL). *p<0.05 comparing cells treated with TP53 siRNA or Pifithrin-α to cells not treated with TP53 inhibitors.
The role of TP53 in HPV(+) tumor cell response to ASTX660 with and without TNFα or TRAIL was also assessed by siRNA knockdown of TP53 gene expression (Figures 4B-D) and direct Pifithrin-α-mediated inhibition of TP53 protein (Figures 4E-G). The TP53 siRNA knockdown in two cell lines was confirmed by mRNA using RT-PCR and by protein using flow cytometry (Supplemental Figure S5). Across all three HPV(+) cell lines, cells transfected with TP53 siRNA were significantly more resistant to either ASTX660 with TNFα or ASTX660 with TRAIL compared to cells transfected with control siRNA. Similarly, when TP53 protein was inhibited directly by Pifithrin-α, UM-SCC-47, UPCI-SCC-90, and UPCI-SCC-152 became significantly more resistant to ASTX660 alone, or in combination with TNFα or TRAIL.
ASTX660 Synergizes with XRT to Delay HPV(−) and HPV(+) Xenograft Tumor Growth
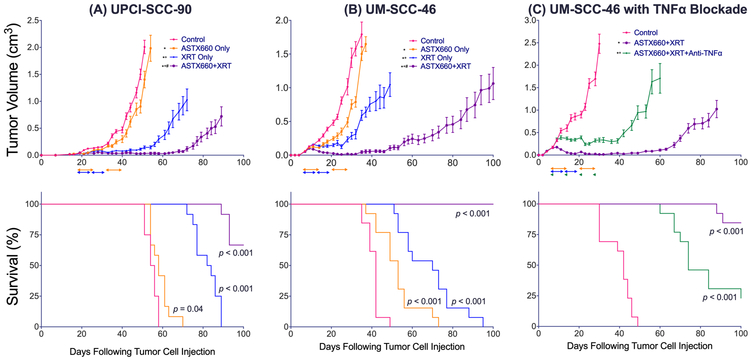
Following our in vitro observations that ASTX660 sensitizes HPV(−) as well as HPV(+) tumor cells to TNFα and TRAIL, we sought to use HPV(−) UM-SCC-46 and HPV(+) UPCI-SCC-90 xenograft models to assess the efficacy of ASTX660 as a monotherapy and in combination with XRT, which stimulates significant production of death ligands such as TNFα in the tumor microenvironment (46,47). Mice were treated according to the schema provided (Supplementary Figure S6). Among UM-SCC-46 tumor-bearing mice, tumors in untreated mice grew rapidly and their median survival was 42 days (Figure 5A). ASTX660 alone modestly delayed tumor growth and provided a significant survival benefit (median survival 53 days, p<0.001), while XRT alone moderately delayed tumor growth and significantly extended survival compared to controls or ASTX660 alone (70 days, p<0.001 for both). When ASTX660 was combined with XRT, tumor growth was markedly delayed and all treated mice survived the full 100 days following inoculation, with the combination therapy providing a significant survival benefit compared to all three other groups (p<0.001). Animal weights remained stable or were not significantly reduced (Supplemental Figure S7A, B), and no other toxicities were observed throughout the experiment.
Figure 5.
ASTX660 synergizes with XRT to delay HPV(−) and HPV(+) xenograft tumor growth and extend survival. (A) 2.5×106 UM-SCC-46 cells were implanted into the right flank of wildtype female athymic nu/nu mice. Mice were randomized into 4 groups (vehicle control, daily ASTX660, XRT, or ASTX660+XRT) of 13 mice each starting 8 days after tumor injection. ASTX660 treatment began on day 8 with daily treatments via oral gavage for two full weeks with one week off in between (orange arrows). XRT was administered in daily weekday doses across two consecutive weeks (blue arrows). (B) Using the same methods as (A), mice were randomized into 3 groups (vehicle control, ASTX660+XRT, or ASTX660+XRT with TNFα depletion). Anti- TNFα antibody was given twice weekly (green arrows). (C) 1.0×107 UPCI-SCC-90 cells were implanted into the right flank of wildtype female athymic nu/nu mice. Mice were randomized into the same groups as (A) starting 19 days after tumor injection. Error bars represent standard error of the mean. *p<0.05 survival difference versus control (exact p-values printed on Kaplan-Meier plots), #p<0.05 survival difference versus ASTX660 only, +p<0.05 survival difference versus XRT only (A, C) or versus ASTX660+XRT (B).
Subsequently, we sought to elucidate the role of TNFα in the mechanism by which ASTX660 synergizes with XRT to delay UM-SCC-46 tumor growth (Figure 5B). We studied this by treating mice with ASTX660+XRT in the setting of TNFα blockade. For this experiment, control mice again rapidly grew tumors with a median survival of 42 days. The ASTX660+XRT combination therapy again significantly delayed tumor growth and extended survival with all but two mice surviving the full 100 days following inoculation (p<0.001). When the ASTX660+XRT treatment was administered to mice depleted of TNFα with anti-TNF antibody, tumor growth was still delayed compared to control mice, but with a reduced median survival of 74 days (p<0.001). Thus, both the delay of tumor growth and the extension of survival achieved by ASTX660+XRT with TNFα depletion were significantly mitigated compared to the benefits achieved by ASTX660+XRT without TNFα blockade, confirming the critical role of TNFα for the anti-tumor effects of ASTX660+XRT.
We also assessed the efficacy of ASTX660 as a monotherapy and in combination with XRT in our HPV(+) UPCI-SCC-90 xenograft model (Figures 5C). Injected tumor cells required a longer period following inoculation before growing into tumors large enough for randomization (18 days). However, control tumors in untreated mice grew rapidly once that critical mass was achieved with a median survival of 55 days. ASTX660 alone moderately delayed tumor growth with a small, though statistically significant, increase in median survival to 58 days (p=0.04). In contrast, XRT alone significantly delayed tumor growth and extended median survival to 83 days (p<0.001). Again, the ASTX660+XRT combination therapy significantly delayed tumor growth and enhanced survival compared with all three other groups, with the majority of mice surviving the full 100 days following inoculation (p<0.001). Taken together, these results suggest that ASTX660+XRT represents a potential therapeutic regimen to treat both HPV(−) and HPV(+) HNSCC.
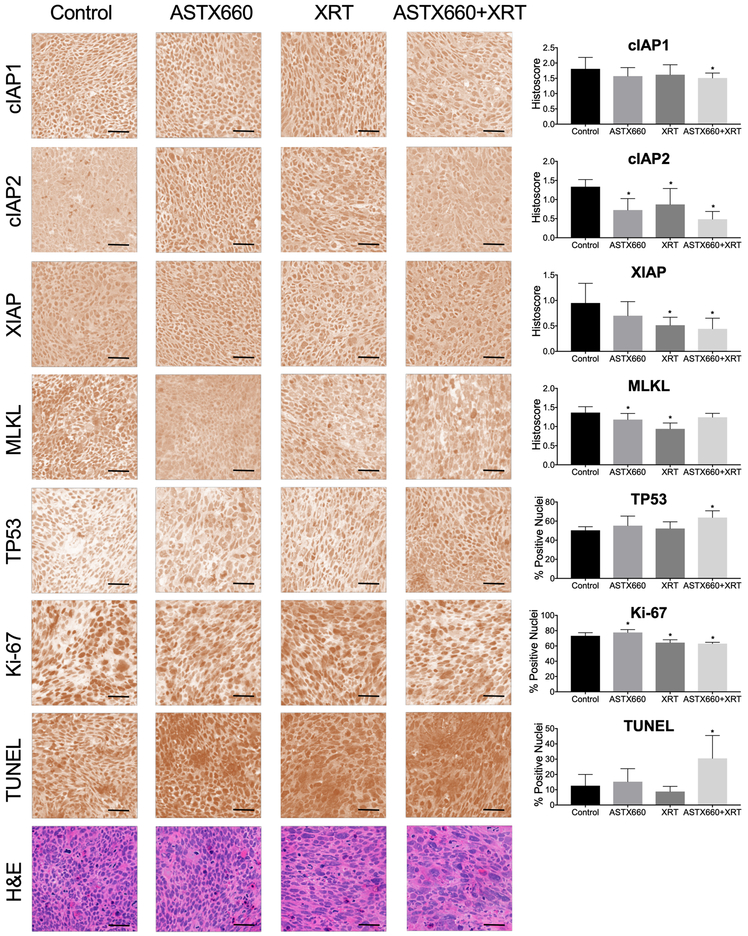
We next examined the effects of treatment on molecular correlative markers in tumor tissues, including IAPs, necroptosis mediator MLKL, TP53, proliferation marker Ki-67, and DNA fragmentation by TUNEL immunostaining, which have been found to be modulated above in vitro or in prior animal studies with IAP inhibitors (Figures 3, 4; ref. 28). We harvested and collected tumor samples on day 8 following the first week of ASTX660, radiation or combined treatment, and performed IHC for selected markers on frozen sections for HPV(+) UPCI-SCC-90 (Figure 6) and HPV(−) UM-SCC-46 (Supplemental Figure S8). For HPV(+) UPCI-SCC-90, we observed significantly reduced cIAP1, as well as IAP2 and XIAP following ASTX660+XRT treatment. Modest but significant decreases in MLKL were observed with ASTX660 or XRT alone, but not for the combination treatment. Increases in TP53 and TUNEL apoptosis marker staining as well as decreased Ki-67 staining were observed with ASTX660+XRT treatment, consistent with induction of proteins with pro-apoptotic and anti-proliferative effects related to TP53 tumor suppressor in vitro. Several areas where diffuse apoptotic body staining is observed by TUNEL assay showed corresponded to similar areas of increased nuclear fragmentation and eosinophilic necrosis by H&E staining in adjacent sections (Figure 6, lower 2 rows).
Figure 6.
In vivo effects of ASTX660 and/or XRT treatment on cIAP1, cIAP2, XIAP, TP53, Ki-67, and TUNEL markers in UPCI-SCC-90 xenograft model. Photomicrographs show immunostaining done on frozen sections of tumors harvested on day 25 deconvoluted to remove background staining. Standard hematoxylin and eosin staining performed by Histoserv, Inc. (Germantown, MD) shown on the bottom row. Bar, 50 μm. Staining quantified by histoscores (cytosolic) or % positive nuclei (nuclear). *p<0.05 versus control.
For HPV(−) UM-SCC-46, we also observed significant reduction of cIAP1 among mice treated with ASTX660 alone, that was enhanced when combined with XRT. ASTX660+XRT was also associated with a modest decrease in both cIAP2 and XIAP expression. In contrast with decrease in MLKL seen in UM-SCC-47, ASTX660 and ASTX660+XRT treatments both significantly increased necroptosis marker MLKL expression in vivo, consistent with preferential rescue observed with necrostatin in UM-SCC-46 in vitro (Figure 2E) and MLKL staining in xenografts in prior studies of birinapant and XRT (28). Proliferation marker Ki-67 was significantly decreased with ASTX660+XRT treatment, while DNA fragmentation marker TUNEL was diffusely but not focally significantly increased, in association with eosinophilic necrosis (Supplemental Figure S8, 2 lower rows).
Discussion
An increasing variety of IAP antagonists have been developed in recent years and are currently in Phase I/II clinical trials for cancer (48-50). It is well-established that these agents are directly cytotoxic towards tumor cells through increased sensitivity to death ligands like TNFα, particularly among HPV(−) HNSCC with BIRC2/3 and FADD overexpression predisposing those cancers to increased sensitivity to IAP inhibition (24-29). However, the role of combined cIAP/XIAP inhibition, as well as the role of such inhibition in HPV(+) HNSCC has not yet been described. Thus, we sought to expand and evaluate the potential role and mechanism of dual cIAP/XIAP inhibition in the treatment of HPV(+) as well as HPV(−) cancers.
In the present study, we utilized a panel of human cell lines as preclinical models of HNSCC to demonstrate that dual cIAP/XIAP inhibition by ASTX660 provides direct anti-tumor effects against both HPV(−) and HPV(+) subtypes. Consistent with previous investigations of cIAP1 antagonists, in vitro treatment of HPV(−) human HNSCC cells with ASTX660 in the presence of TNFα or TRAIL significantly inhibited cell density. This was particularly evident among several cell lines known to overexpress FADD with or without cIAP amplification, while the UM-SCC-38 cell line without such amplification (29) was less sensitive to this treatment. Furthermore, we found that inhibitors of apoptosis and/or necroptosis rescued HPV(−) HNSCCs from inhibition by ASTX660 with TNFα or TRAIL, consistent with our lab’s previous findings (29). In the HPV(−) UM-SCC-46 xenograft model, we observed significant tumor growth delay with ASTX660+XRT treatment (Figure 5A) associated with increased necroptosis marker MLKL as well as TUNEL apoptosis marker expression (Supplemental Figure S4), the latter of which was less prominent in prior studies with an inhibitor that preferentially blocks cIAP1 (29). While it has been presumed that local release of TNFα is the central mediator through which IAP antagonists sensitize tumors to XRT, our experiment utilizing an anti-TNFα antibody (Figure 5B) provides direct evidence for the contribution of TNFα in the anti-tumor effects of IAP inhibition in combination with XRT.
Of note, the shorter schedule of alternating weeks of ASTX660 currently employed in phase I trials modeled in our xenograft studies (Figure S1), significantly delayed but was not curative when combined with 2 weeks of radiation in the UM-SCC-46 xenograft experiment (Figure 5A). By contrast, we observed a higher rate of durable responses with a different cIAP1 antagonist given concurrently for 2 weeks plus an additional week after XRT (29). As this difference may be attributable to differences in extent or duration of IAP inhibition in combination with radiation due to the treatment schedule, further preclinical and clinical studies to optimize the treatment schedule of ASTX660 when combined with XRT could potentially result in a superior anti-tumor response.
In addition to confirming the effects of cIAP/XIAP inhibition in HPV(−) HNSCC, we observed similar efficacy when treating HPV(+) HNSCC across a range of cell lines, with several exhibiting significant sensitization to TNFα and TRAIL. Focused experiments using UM-SCC-47, UPCI-SCC-90, and UPCI-SCC-152 consistently revealed caspase-3 and caspase-8 cleavage to be associated with the anti-tumor effects of ASTX660 in the presence of TNFα or TRAIL in HPV(+) cancers (Figure 2D-F). In contrast with the mixed apoptotic and necroptotic cell death observed among HPV(−) cell lines, RIP1 kinase inhibitor necrostatin only minimally rescued the anti-tumor effects of ASTX660 with TNFα or TRAIL on HPV(+) cell lines, suggesting that the anti-tumor effects of ASTX660 on HPV(+) cells are primarily dependent upon caspase-mediated apoptosis. The results of our recent whole exome and transcriptome analysis (42) provide data that suggest the more resistant HPV(+) lines have different genomic alterations whose role(s) could be explored in future studies. 93-VU-147T has mutations predicted to be deleterious for tumor suppressor TP53 and KEAP1; UD-SCC-2 has mutations in KMT2D and EP300 that can modulate methylation and acetylation, widely affecting transcription; and UM-SCC-104 has mutations in Hippo growth pathway tumor suppressor FAT1 and a frameshift deletion of RB important in G1 checkpoint and DNA synthesis, which are not seen in the sensitive HPV(+) lines (42). Mutations affecting these genes along with copy number alterations shared with more aggressive HNSCC could potentially affect cell death, growth, or DNA repair, meriting investigation in future studies.
The broader clinical relevance of our present findings stems from the unexpected connection established between apoptotic HPV(+) cell death and increased TP53 expression and function enhanced by ASTX660 combined with TNFα. Western blotting revealed consistent increases in TP53 expression in HPV(+) UM-SCC-47, UPCI-SCC-90, and UPCI-SCC-152 when treated with ASTX660 with TNFα. This increased TP53 expression was accompanied by a reduction in the expression of the TP53 regulator MDM2, increased cleavage of caspase-3 and caspase-8, and increased expression of the TP53-inducible cell cycle inhibitor p21 (Figure 3D-F). Furthermore, the central role of TP53 in mediating the anti-tumor effects of cIAP/XIAP inhibition among HPV(+) cancers was confirmed when the efficacy of ASTX660 was significantly diminished by reducing TP53 expression using TP53 siRNA or directly inhibiting TP53 protein activity with Pifithrin-α (Figure 4). Our in vivo findings using the UPCI-SCC-90 xenograft model again showed significant tumor growth delay from ASTX660+XRT treatment (Figure 5C) with accompanying decreases in cIAP1/cIAP2/XIAP expression and increased expression of TP53 protein and the TUNEL cell death marker (Figure 6). Taken together, these data suggest a potential role of cIAP/XIAP inhibition in enhancing TP53 expression in HPV(+) tumors, thus countering a central mechanism through which HPV inactivates TP53.
The present findings are timely and pertinent as several IAP antagonists continue to be developed and studied (48-50). Our understanding of these agents as therapies for not only HNSCC, but also other cancers, is still in its early stages. In recent years, the downstream pro-apoptotic signaling pathways allowing IAP antagonists to sensitize tumor cells to TNFRSF ligands like TNFα have become more evident (24-29); however, alternative mechanisms through which agents like ASTX660 antagonize HPV(+) driven cancers had not yet been described. Identifying mediators such as TP53 and further clarifying this mechanism is critical to understanding the role of IAP antagonists in the treatment landscape of HPV(+) cancer as the incidence of oropharyngeal cancer rises (4-7). Taken together, antagonists of cIAP/XIAP appear to be a potential new medication class offering targeted therapy for HPV(+) as well as HPV(−) HNSCC, particularly when combined with radiotherapy. The present findings should serve to motivate further studies of cIAP/XIAP antagonists and future clinical trials combining dual antagonists like ASTX660 with radiotherapy to treat HNSCC.
Supplementary Material
Translational Relevance.
IAP antagonists are emerging as sensitizers of cancer cells to death ligands such as TNF and TRAIL, which are important in mediating anti-tumor effects of radiation and cytotoxic T lymphocytes. The discovery of combinatorial activity of IAP antagonists with death ligands in HPV(+) HNSCC lacking genomic alterations in death pathways found in HPV(−) tumors, uncovered a role for dual IAP antagonists in restoring expression of tumor suppressor TP53. TP53 is known to enhance cell death via intrinsic mitochondrial and caspase-dependent pathways, that mediate response to DNA damaging cytotoxic therapies. ASTX660 displayed combinatorial activity with radiation in HPV(+) as well as HPV(−) HNSCC xenografts, supporting clinical investigation of dual IAP1/XIAP inhibitors with radiation in both subtypes.
Acknowledgments
This work was supported by NIDCD intramural projects ZIA-DC-000016, 73,74 (CVW), 87 (CTA) and 90 (NS). This work (RX, WY, AD) was supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. Astex Pharmaceuticals provided ASTX660 and research funding under a cooperative research and development agreement with NIDCD (CVW). The authors would like to acknowledge Megan Morisada and Ellen Moore for technical assistance, Dr. James Mitchell and his lab for their assistance with irradiation treatments, and Drs. Cheng-Ming Chiang, and Vassiliki Saloura, for careful external review of the manuscript. This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, Md. (http://biowulf.nih.gov).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 3.Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. [DOI] [PubMed] [Google Scholar]
- 4.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9:665–73. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova A V, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. [DOI] [PubMed] [Google Scholar]
- 12.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–74. [DOI] [PubMed] [Google Scholar]
- 14.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moquin DM, McQuade T, Chan FK-M. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8:e76841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–10. [DOI] [PubMed] [Google Scholar]
- 17.Salvesen GS, Duckett CS. Apoptosis: IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–10. [DOI] [PubMed] [Google Scholar]
- 18.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–21. [DOI] [PubMed] [Google Scholar]
- 20.Schimmer AD, Welsh K, Pinilla C, Wang Z, Krajewska M, Bonneau M-J, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. [DOI] [PubMed] [Google Scholar]
- 21.Long JS, Ryan KM. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene. 2012;31:5045–60. [DOI] [PubMed] [Google Scholar]
- 22.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–24. [DOI] [PubMed] [Google Scholar]
- 23.Derakhshan A, Chen Z, Van Waes C. Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin Cancer Res. 2017;23:1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzinger O, Viertl D, Tsoutsou P, Kadi L, Rigotti S, Zanna C, et al. The radiosensitizing activity of the SMAC-mimetic, Debio 1143, is TNFα-mediated in head and neck squamous cell carcinoma. Radiother Oncol. 2015;116:495–503. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, McEachern D, Li W, Davis MA, Li H, Morgan MA, et al. Radiosensitization of Head and Neck Squamous Cell Carcinoma by a SMAC-Mimetic Compound, SM-164, Requires Activation of Caspases. Mol Cancer Ther. 2011;10:658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raulf N, El-Attar R, Kulms D, Lecis D, Delia D, Walczak H, et al. Differential response of head and neck cancer cell lines to TRAIL or Smac mimetics is associated with the cellular levels and activity of caspase-8 and caspase-10. Br J Cancer. 2014;111:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brands RC, Herbst F, Hartmann S, Seher A, Linz C, Kübler AC, et al. Cytotoxic effects of SMAC-mimetic compound LCL161 in head and neck cancer cell lines. Clin Oral Investig. 2016;20:2325–32. [DOI] [PubMed] [Google Scholar]
- 28.Eytan DF, Snow GE, Carlson SG, Schiltz S, Chen Z, Van Waes C. Combination effects of SMAC mimetic birinapant with TNFα, TRAIL, and docetaxel in preclinical models of HNSCC. Laryngoscope. 2015;125:E118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eytan DF, Snow GE, Carlson S, Derakhshan A, Saleh A, Schiltz S, et al. SMAC Mimetic Birinapant plus Radiation Eradicates Human Head and Neck Cancers with Genomic Amplifications of Cell Death Genes FADD and BIRC2. Cancer Res. 2016;76:5442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Kumar B, Romito M, Teknosa TN, Chakravarti A, Williams TM. Abstract C144: LCL161, a SMAC mimetic, induces preferential radiosensitization in human papillomavirus negative head and neck squamous cell carcinoma through induction of apoptosis. Mol Cancer Ther. 2015;14:C144–C144. [Google Scholar]
- 31.Brands RC, Herbst F, Hartmann S, Seher A, Linz C, Kübler AC, et al. Cytotoxic effects of SMAC-mimetic compound LCL161 in head and neck cancer cell lines. Clin Oral Investig. 2016;20:2325–32. [DOI] [PubMed] [Google Scholar]
- 32.Carter BZ, Mak DH, Schober WD, Koller E, Pinilla C, Vassilev LT, et al. Simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML. Blood. 2010;115:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Brazhnik P, Tyson JJ. Computational analysis of dynamical responses to the intrinsic pathway of programmed cell death. Biophys J. 2009;97:415–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward G, Chessari G, Johnson CN, Lewis J, Rich S, Thompson N. 380 Induction of apoptosis with a novel dual cIAP1/XIAP antagonist in models of melanoma. Eur J Cancer. 2014;50:122. [Google Scholar]
- 35.Mita M, LoRusso P, Gordon M, Oganesian A, Zhang X, Ferraldeschi R, et al. Phase 1 study of the IAP inhibitor ASTX660 in adults with advanced cancers and lymphomas AACR-NCI-EORTC Int Conf Mol Targets Cancer Ther. Philadelphia, PA; 2017. page A091. [Google Scholar]
- 36.Ward GA, Lewis EJ, Ahn JS, Johnson CN, Lyons JF, Martins V, et al. ASTX660, a Novel Non-peptidomimetic Antagonist of cIAP1/2 and XIAP, Potently Induces TNFα-Dependent Apoptosis in Cancer Cell Lines and Inhibits Tumor Growth. Mol Cancer Ther. 2018;17:1381–91. [DOI] [PubMed] [Google Scholar]
- 37.Busch J, Kriegs M, Laban S, Tribius S, Knecht R, Petersen C, et al. HPV-positive HNSCC cell lines but not primary human fibroblasts are radiosensitized by the inhibition of Chk1. Radiother Oncol. 2013;108:495–9. [DOI] [PubMed] [Google Scholar]
- 38.Tang AL, Owen JH, Hauff SJ, Park JJ, Papagerakis S, Bradford CR, et al. Head and neck cancer stem cells: the effect of HPV--an in vitro and mouse study. Otolaryngol Head Neck Surg. 2013;149:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang AL, Hauff SJ, Owen JH, Graham MP, Czerwinski MJ, Park JJ, et al. UM-SCC-104: a new human papillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck. 2012;34:1480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman J, Nottingham L, Duggal P, Pernas FG, Yan B, Xin PY, et al. Deficient TP53 expression, function, and cisplatin sensitivity are restored by quinacrine in head and neck cancer. Clin Cancer Res. 2007;13:6568–78. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H, Yang X, Si H, Saleh AD, Xiao W, Coupar J, et al. Genomic and Transcriptomic Characterization Links Cell Lines with Aggressive Head and Neck Cancers. Cell Rep. 2018;25:1332–1345.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Lupat R, Amarasinghe KC, Thompson ER, Doyle MA, Ryland GL, et al. CONTRA: copy number analysis for targeted resequencing. Bioinformatics. 2012;28:1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallahan DE, Virudachalam S, Sherman ML, Huberman E, Kufe DW, Weichselbaum RR. Tumor necrosis factor gene expression is mediated by protein kinase C following activation by ionizing radiation. Cancer Res. 1991;51:4565–9. [PubMed] [Google Scholar]
- 48.Infante JR, Dees EC, Olszanski AJ, Dhuria S V, Sen S, Cameron S, et al. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2014;32:3103–10. [DOI] [PubMed] [Google Scholar]
- 49.Amaravadi RK, Schilder RJ, Martin LP, Levin M, Graham MA, Weng DE, et al. A Phase I Study of the SMAC-Mimetic Birinapant in Adults with Refractory Solid Tumors or Lymphoma. Mol Cancer Ther. 2015;14:2569–75. [DOI] [PubMed] [Google Scholar]
- 50.Hurwitz HI, Smith DC, Pitot HC, Brill JM, Chugh R, Rouits E, et al. Safety, pharmacokinetics, and pharmacodynamic properties of oral DEBIO1143 (AT-406) in patients with advanced cancer: results of a first-in-man study. Cancer Chemother Pharmacol. 2015;75:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.