Abstract
Purpose:
Recent studies demonstrate the role of the tumor microenvironment in tumor progression. However, strategies used to overcome the malignant phenotypes of cancer cells modulated by the microenvironment have not been thoroughly explored. In this study, we evaluated the therapeutic efficacy of a newly developed monoclonal antibody targeting microfibril associated protein 5 (MFAP5), which is secreted predominately by CAFs, in ovarian and pancreatic cancer models.
Experimental Design:
Monoclonal antibodies were developed using human MFAP5 recombinant protein as an antigen in mice and antibodies from hybridoma clones were evaluated for their specificity to human and murine MFAP5. An Octet RED384 system was used to determine the kinetics of binding affinity and the specificity of the antibody clones, which were followed by epitope mapping and functional characterization by in vitro assays. The therapeutic efficacy of a lead anti-MFAP5 antibody clone 130A in tumor suppression was evaluated by ovarian tumor- and pancreatic tumor-bearing mouse models.
Results:
Three hybridoma clones, which produced antibodies with high affinity and specificity to MFAP5, were selected for functional studies. Antibody clone 130A, which recognizes a common epitope shared between human and murine MFAP5 protein, were further selected for in vivo studies. Results showed that clone 130A down-regulated MFAP5-induced collagen production in CAFs, suppressed intratumoral microvessel leakiness, and enhanced paclitaxel bioavailability in both ovarian and pancreatic cancer mouse models.
Conclusions:
These data suggest that MFAP5 blockade using an immunologic approach inhibits fibrosis, induces tumor vessel normalization and enhances chemosensitivity in ovarian and pancreatic cancer, and can be used as a novel therapeutic agent.
Keywords: MFAP5, Monoclonal antibody, Ovarian cancer, Pancreatic cancer, Cancer associated fibroblasts, Fibrosis, Angiogenesis
Introduction
The tumor microenvironment, composed primarily by fibroblasts, endothelial cells, lymphocytic infiltrates and extracellular matrix proteins, can directly affect cancer cell growth, migration, and differentiation (1), thereby presenting a unique aspect of diagnosing and treating cancer. Cancer associated fibroblasts (CAFs) are primarily responsible for producing the structural components of the stromal microenvironment, which is mostly composed of collagen type I, II and IV as well as fibronectin (1). CAFs also produce secreted factors such as cytokines and growth factors, which maintain normal tissue homeostasis by signaling to other cell components in the stroma, such as immune, fat, vascular, smooth muscle and epithelial cells. CAFs in the tumor stromal microenvironment exhibit altered secretion of extracellular proteins as well as paracrine growth factors, which modify the niche of tumor microenvironment and promote cancer cell proliferation, migration, and invasion.
Microfibril associated protein 5 (MFAP5), a 25-kD glycoprotein has recently been shown to be up-regulated in CAF of multiple tumor types including non-small cell lung cancer (2), pancreatic (3), ovarian (4), prostate (5), and breast cancer (6). In addition, over-expression of MFAP5 in CAFs has been shown to be associate with poor prognosis in ovarian cancer (4), and used as a diagnostic marker for prostate cancer early detection (5). MFAP5 has a RGD binding motif, which can bind αvβ3 integrin to enhance angiogenesis and ovarian cancer metastasis potential through the activation of calcium-dependent FAK/EREK/LPP and FAK/CREB/TNNC1 signaling pathway (4,7,8). These findings suggest that treatment strategies based on targeting CAF-derived MFAP5 activities may be a new modality in suppressing cancer cell growth and metastasis.
Monoclonal antibodies (MAbs) have been shown to be effective therapeutic agents for a number of human malignancies. A number of them have been approved as new therapeutic agents for the treatment of human cancer in the last decade. For example, Trastuzumab, a humanized anti-HER2/neu MAb has been used alone or in combination with chemotherapy for the treatment of metastatic breast cancer in patients with tumors overexpressing HER2/neu (9,10),(11). Bevacizumab, a recombinant humanized MAb against vascular endothelial growth factor (VEGF) improves survival in colorectal (12) and cervical (13) cancer patients. In addition to antibodies targeting antigens on cancer cells, MAbs targeting immune checkpoint molecules on T cells have recently been approved by the FDA. Pembrolizumab, a MAb targeting programmed cell death 1 (PD-1) and Ipilimumab, another MAb targeting cytotoxic T-lymphocyte associated protein 4 (CTLA-4) on T cells have been developed and used for the treatment of advanced melanoma and other cancer types (14).
In spite of these studies, the efficacy of targeting CAF-derived antigens by MAbs in cancer treatment has not been thoroughly explored. Here, we describe our effort in generating and characterizing the MAbs that target CAF-derived MFAP5, determining the efficacy and toxicity of using one of the antibodies generated in the treatment of ovarian and pancreatic cancer murine models, and evaluating the molecular mechanism by which the anti-MFAP5 antibody suppresses fibrosis and enhances chemosensitivity in those models.
Materials and Methods
Generation of Antibody-producing hybridomas.
Immunization and hybridoma generation procedures were conducted at the University of Texas MD Anderson Cancer Center Monoclonal Antibody Core Facility following established protocols (15–17). Briefly, two 6-week-old female BALB/c mice were immunized once every 3 days with the MFAP5 protein (GenScript USA Inc.) by five injections of 20 ul each of the solution emulsified with adjuvant on the footpad. After the fifth injection, serum samples were obtained from both mice to confirm by ELISA, the presence of serum antibodies against the target. Extra boosts were administered as required. Popliteal lymph nodes from the immunized mice were harvested around day 20 and lymph cells were fused with Sp2/0 myeloma to establish hybridomas, plated under selection media (HAT). Screening for selection of positive clones against the protein, was performed by ELISA using an irrelevant protein as negative control. After selection of hybridoma candidates master cells, MAbs were purified using MabSelect SuRe antibody purification resin (GE healthcare) and eluted with low pH Ag/Ab elution buffer. Validation and quality control tests of purified antibody: specificity (binding screening by ELISA), purity (SDS-PAGE), endotoxin (Lonza Endotoxin kit) and isotype (ELISA Sigma) were conducted following recommendations of Rigor and reproducibility by International Working Group for Antibody Validation (18).
Anti-MFAP5 antibody clone 130A-suppressed ovarian tumor growth in vivo.
To determine the inhibitory effects of anti-MFAP5 monoclonal antibodies on ovarian tumor progression in vivo, 3 × 106 luciferase-labeled OVCA432 cells were intraperitoneally injected into nude mice. One week after tumor cell injection, mice were randomized into the treatment and control groups (12 mice/group). They were injected twice weekly with 15 mg/kg anti-MFAP5 antibody clone 130A or 15 mg/kg control normal mouse IgG, respectively, for a total of 6 weeks. Tumor progression was monitored using an IVIS 200 bioluminescence and fluorescence imaging system. To determine the effect of the anti-MFAP5 antibody on intratumoral microvessel leakiness, 100 μl of 10 mg/mL FITC-dextran (relative molecular mass, 200,000 Da; Sigma-Aldrich) was injected via the tail vein into mice before the mice were sacrificed. Tumor weights were recorded, and 6-μm frozen tissue sections were prepared from tumors harvested using a CM1850 cryostat (Leica Microsystems). FITC-dextran signals were quantified by fluorescent microscopy. All animal studies were conducted under an approved protocol by the Institutional Animal Care and Use Committee (IACUC).
Anti-MFAP5 antibody clone 130A-increased paclitaxel bioavailability in ovarian tumors.
Nude mice were intraperitoneally injected with 3 × 106 luciferase-labeled OVCA432 cells. One week after cancer cell injection, tumor-bearing mice were intraperitoneally injected with 15 mg/kg isotype control mouse IgG or anti-MFAP5 antibody clone 130A twice weekly for a total of 4 weeks. One dose of Oregon green conjugated paclitaxel (1 mg/kg; Life Technologies) was injected via tail vein using sterile PBS as vehicle into both MFAP5-targeting monoclonal antibody treated and control IgG treated animals 1 hour before they were sacrificed. At the experimental endpoint, tumor weights were recorded, and 6-μm frozen tissue sections were prepared from tumors harvested using a CM1850 cryostat. Oregon Green 488 signals were quantified by fluorescent microscopy and the amount of fluorophore conjugated paclitaxel in the tumor tissue samples was then compared between the two group of mice. While evaluation of the tumor suppressive effect of paclitaxel is not one of the aims of this experiment, it is worth noting that it has been reported that linking Oregon green to paclitaxel increases the polarity of the drug and reduces its toxicity (19).
Immunohistochemical analysis.
Immunolocalization of MFAP5 was performed on a FFPE pancreatic tumor tissue array, which contains samples from 91 patients (HPan-Ade170Sur-01; US Biomax Inc.) using a commercially available anti-MFAP5 (1:500, HPA010553; Sigma-Aldrich) antibody. MFAP5 expression was visualized using a Betazoid 3,3’-diaminobenzidine chromogen kit (Biocare Medical). For tumor samples obtained from mice, immunolocalization of CD34 (1:100, GTX28158; GeneTex) was performed. Stromal MFAP5 expression and CD34+ microvessel density was quantified as previously described (4,8).
Statistical analysis.
The SPSS 20 (IBM Corporation) and Prism 7.0 (GraphPad Software) software programs were used to perform statistical tests. All in vitro experiments were repeated independently in triplicate. A two-tailed Student t-test was used to determine differences in sample means for data with normally distributed means. The Mann-Whitney U test was used for analysis of nonparametric data. P values less than 0.05 were considered statistically significant.
Results
Development and characterization of anti-MFAP5 monoclonal antibodies
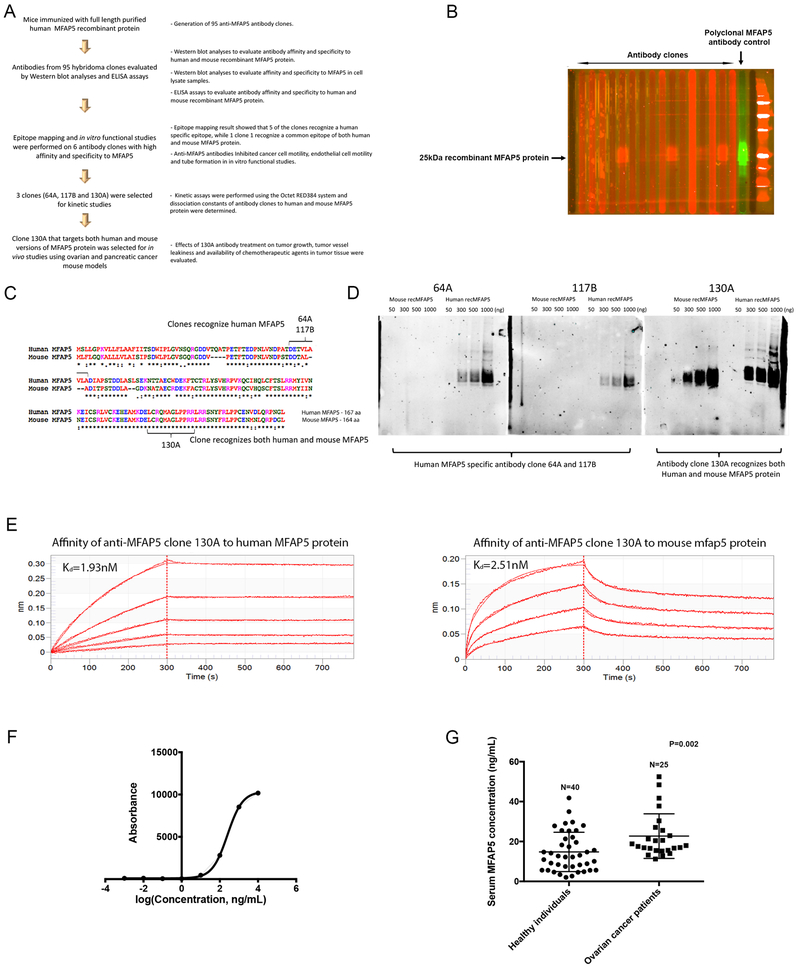
Previous studies showed that down-regulation of endogenous MFAP5 in CAFs suppressed ovarian tumor growth and angiogenesis (4,8), suggesting that targeting CAF-derived MFAP5 may be a new modality in ovarian cancer treatment. Since MFAP5 is a secretory protein, we tested whether blocking MFAP5 using an immunological approach could inhibit tumor growth. First, we developed MFAP5-targeting monoclonal antibodies using purified full-length human MFAP5 recombinant protein (recMFAP5) as an antigen to immunize mice (Fig. 1A). Using an enzyme-linked immunosorbent assay, we identified 95 positive hybridoma clones, which generated MAbs that could bind to human recMFAP5. Western blot analysis using a Bio-Rad multiscreen apparatus was performed to identify antibody clones that had strong binding affinity for human MFAP5 (Fig. 1B). Epitope mapping performed on six of these clones (50B, 52B, 64A, 75B,117B and 130A). Three candidates (antibody clones 64A, 117B and 130A), which have the highest affinity and specificity to MFAP5 protein, were chosen for further studies. Epitope mapping result showed that clone 64A and 117B recognized the same human MFAP5 protein sequence (DETVLAVLA), while clone 130A is specific to a consensus peptide sequence (LCRQMAGLPPRR) common for both human and murine MFAP5 proteins (Fig. 1C and Supplementary Figs. 1A & B). The specificity of each anti-MFAP5 antibody clone was further validated by Western blot analysis (Fig. 1D).
Figure 1. Development and characterization of anti-MFAP5 monoclonal antibodies.
(A) A flow diagram illustrating the overall workflow of therapeutic anti-MFAP5 monoclonal antibody development. (B) Western blot analyses were performed to identify antibody clones with high binding affinity and specificity to MFAP5. (C) Epitope mapping results showing that whereas clones 64A and 117B recognized human MFAP5, clone 130A recognized both human and murine MFAP5 protein sequences. (D) Western blot analyses validating the specificity of two clones of mouse monoclonal antibodies against human MFAP5 (64A and 117B) and one clone of an antibody against both human and mouse MFAP5 (130A). (E) Kinetic assay results showed that the dissociation constant (Kd) of clone 130A for human and mouse MFAP5 protein were at 1.93nM and 2.51nM respectively, suggesting that clone 130A has high binding affinity to both human MFAP5 and mouse Mfap5 protein (F) A titration curve for the 130A antibody generated by ELISA and recombinant MFAP5 protein at various concentrations. (G) ELISA results demonstrating that serum samples obtained from high grade serous ovarian cancer patients had significantly higher levels of MFAP5 than the healthy individuals did.
Focusing on the three antibody clones, binding kinetics experiments were performed using the Octet RED384 system (Pall FortéBio LLC). Kinetic assays showed that the dissociation constant (Kd) of clone 130A for human and mouse MFAP5 protein were at 1.93nM and 2.51nM respectively, suggesting that clone 130A has high binding affinity to both human MFAP5 and mouse MFAP5 protein (Fig. 1E). On the other hand, the dissociation constant (Kd) of clone 64A and 117B for human MFAP5 protein were at 0.48nM and 6.7nM respectively (Supplementary Figs. 1C & D).
To determine whether the antibody recognized native secretory MFAP5, ELISAs using 130A antibody (Fig. 1F) were performed on serum samples obtained from healthy individuals and pre-operative age-matched patients with advanced stage high grade serous ovarian cancer. The results showed that serum samples obtained from cancer patients had significantly higher levels of MFAP5 than the serum from healthy individuals (Fig. 1G). These data suggest that MFAP5 is a tumor associated antigen and circulating MFAP5 can be detected by the 130A anti-MFAP5 antibody.
Anti-MFAP5 antibody suppresses ovarian cancer cell and endothelial cell motility in vitro
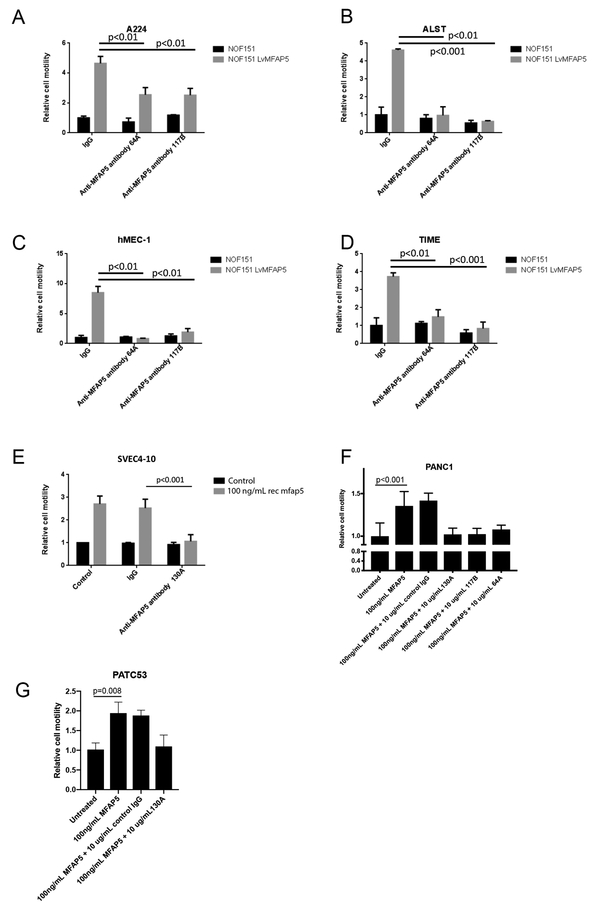
To determine the biological effector functions of monoclonal anti-MFAP5 antibodies, the effects of antibody clones 64A, 117B and 130A on ovarian tumor motility and angiogenesis were determined. In Boyden chambers, A224 and ALST human ovarian cancer cells were co-cultured with ovarian fibroblasts NOF151 transfected with MFAP5 or the control vector. Cancer cell motility was determined in the presence of 64A and 117B antibody or control IgG. The results showed that ovarian cancer cells co-cultured with ovarian fibroblasts transfected with MFAP5 had a significant higher motility potential than the mock transfectant did, and the motility promoting effect of MFAP5 was abrogated in the presence of the monoclonal MFAP5-blocking antibodies but not the isotype control (Figs. 2A & B). MFAP5 expression of MFAP5 transfected NOF151 cells is comparable to that of primary CAF cultures (Supplementary Fig. 2). These data suggest that the MFAP5-blocking antibodies developed effectively suppressed the effect of CAF-derived MFAP5 on ovarian cancer cell motility in vitro.
Figure 2. Anti-MFAP5 antibody suppresses cancer cell and endothelial cell motility in vitro.
(A,B) Motility assays performed on αVβ3-expressing human ovarian cancer cell lines A224 and ALST co-cultured with MFAP5-overexpressing fibroblasts or control fibroblasts in the presence of human IgG or anti-human MFAP5 antibody clones 64A, 117B and 130A demonstrated that the anti-MFAP5 monoclonal antibodies markedly inhibited MFAP5-stimulated ovarian cancer cell motility. (C,D) Human microvascular endothelial cell lines hMEC-1 and TIME co-cultured with MFAP5-overexpressing fibroblasts or control fibroblasts in the presence of human IgG or anti-human MFAP5 antibody clones 64A and 117B demonstrated that the anti-MFAP5 monoclonal antibodies markedly inhibited MFAP5-stimulated microvascular endothelial cell motility. (E) Motility assays on SVEC4–10 mouse endothelial cells cultured in media supplemented with recombinant mouse mfap5 or PBS demonstrated that antibody clone 130A markedly inhibited mfap5-induced mouse endothelial cell motility. (F) Motility assays demonstrated that MFAP5-induced PANC1 human pancreatic cancer cell motility was markedly suppressed by the presence of anti-MFAP5 monoclonal antibody clones 64A, 117B and 130A. (G) For PDX cell line PATC53, the motility promoting effect of MFAP5 was abrogated in the presence of the anti-MFAP5-blocking antibody but not by the control IgG
Besides the anti-motility effect on cancer cells, the anti-angiogenic effect of antibody clone 64A and 117B was determined. Human microvascular endothelial cells hMEC-1 and TIME were co-cultured with CAFs transfected with MFAP5 or the control vector and cell motility was determined in the presence MFAP5-blocking antibodies or control IgG. The results showed that hMEC-1 and TIME cells co-cultured with CAFs transfected with MFAP5 had a significant higher cell motility potential than the mock transfectant, and the effect was abrogated in the presence of 64A and 117B antibodies but not by the control IgG (Figs. 2C & D). In addition, for MAb clone 130A, which also recognizes and binds murine MFAP5, motility assays were performed on SVEC4–10 mouse endothelial cells treated with recombinant mouse MFAP5 protein in the presence of 130A antibody or the control IgG. The results showed that SVEC4–10 cells treated with recombinant mouse MFAP5 has significantly higher motility rates than the control buffer and the effect was abrogated in the presence of 130A antibody but not by the control IgG (Fig. 2E). These data suggest that the MFAP5-blocking antibodies suppressed human and mouse endothelial cell motility in vitro.
Anti-MFAP5 antibody suppresses pancreatic cancer cell motility in vitro
We previously showed that MFAP5 expression levels were markedly higher in ovarian CAFs than in normal ovarian fibroblasts, and high stromal MFAP5 protein levels correlated with reduced survival in ovarian cancer patients (4). To evaluate the feasibility of applying MFAP5-targeting therapeutic agent as a treatment regimen for pancreatic ductal adenocarcinoma (PDAC), a type of cancer which is often supported and protected by the dense stromal component (20), immunostaining was performed on tumor tissue samples and the corresponding normal adjacent tissue samples obtained from 64 PDAC patients. Staining results suggested that while the majority of normal adjacent tissue is negative for MFAP5 expression, expression levels of MFAP5 was significantly higher in pancreatic CAFs (P<0.001) (Supplementary Fig. 3A). In addition, survival analysis and log-rank test showed that high stromal MFAP5 expression in patients with PDAC is significantly associated to the reduction of overall survival duration (N=91, P<0.001) (Supplementary Fig. 3B). Cox survival analysis adjusted with age and sex showed that high stromal MFAP5 expression in PDAC has a hazard ratio of 2.79 (N=91, P<0.001). These results indicated that the use of anti-MFAP5 antibody in the treatment of PDAC could be beneficial.
To evaluate the inhibitory roles of monoclonal anti-MFAP5 antibodies on PDAC cell in vitro, the effect of antibody clones 64A, 117B and 130A on PDAC cell motility was determined. In Boyden chambers, PANC1 human pancreatic cancer cells were treated with recombinant MFAP5 protein and antibodies, and cancer motility was determined by the number of cells that migrated through the porous membrane. Motility assay results showed that ovarian cancer cells treated with MFAP5 had a significant higher motility than untreated cells, and the motility promoting effect of MFAP5 was abrogated in the presence of the anti-MFAP5-blocking antibodies but not by the control IgG (Fig. 2F). Similarly, for PDAC PDX cell line PATC53, which was derived from a pancreatic cancer patient harboring a KRAS G12D mutation and a p53 R306* mutation, treatment with recombinant MFAP5 increased cancer cell motility, and the motility promoting effect of MFAP5 was abrogated in the presence of the anti-MFAP5-blocking antibody but not by the control IgG (Fig. 2G)
Anti-MFAP5 antibody suppresses tumor growth in vivo
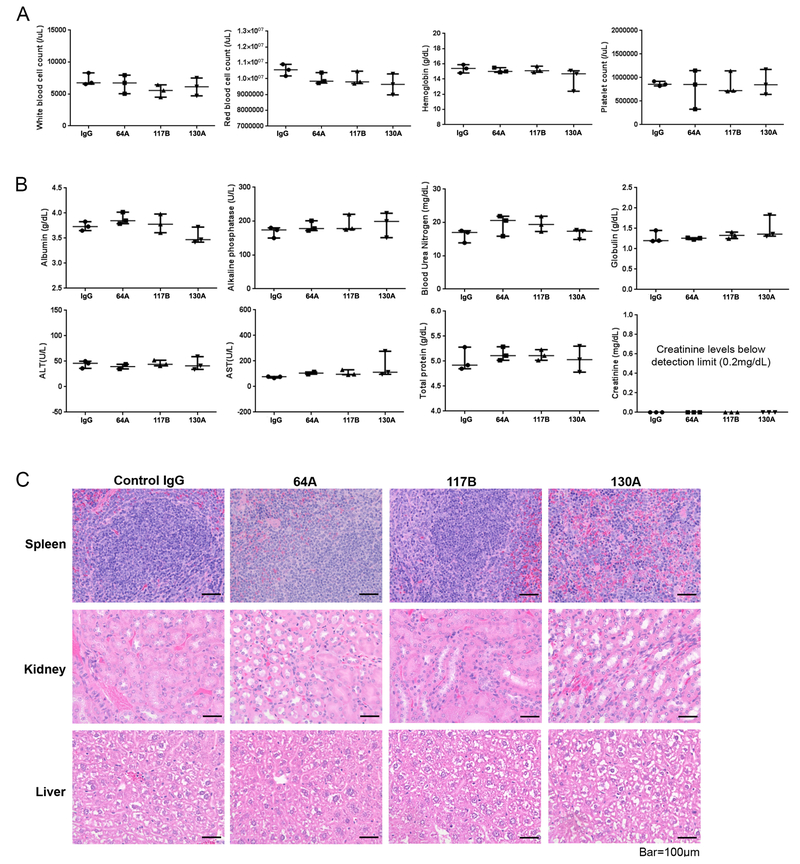
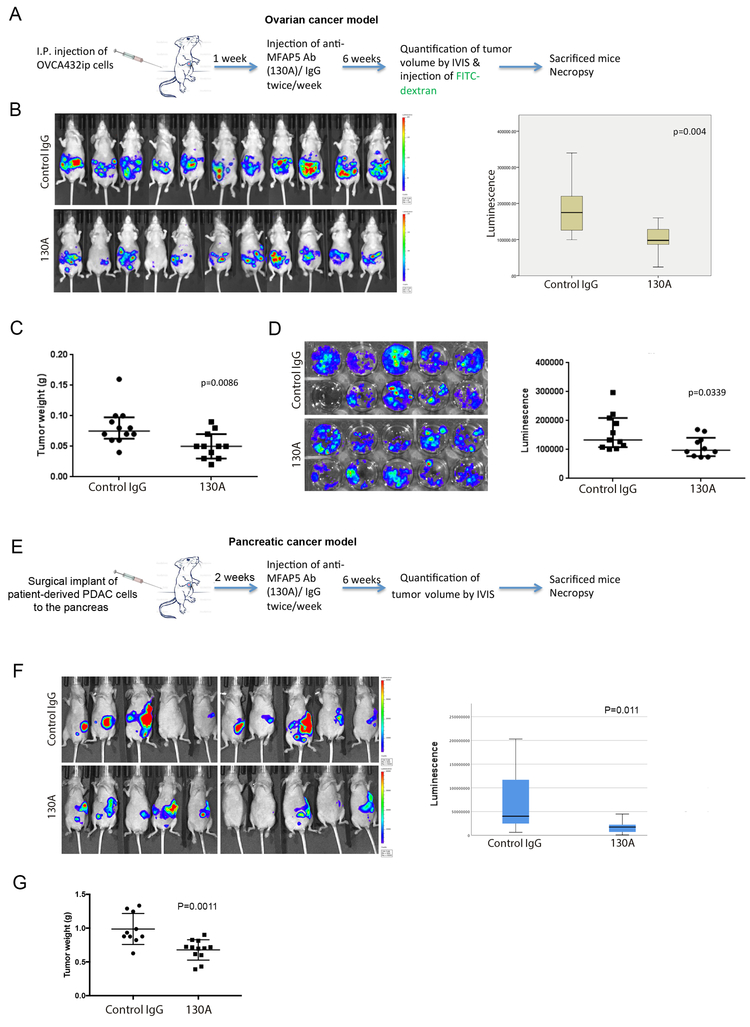
Next, the inhibitory effect of antibody clone 130A, which can recognize and block mouse stromal MFAP5 protein, on tumor growth and angiogenesis were evaluated using in vivo models. We monitored tumor progression in nude mice injected intraperitoneally with luciferase-labeled OVCA432 ovarian cells treated with either 130A (15mg/kg) or control normal mouse IgG (15mg/kg; 12 mice/ group). A dosage of 15mg/kg (twice per week) was used since similar dosages have been used successfully in other FDA-approved antibody treatments targeting different tumor associated antigens. In addition, toxicity studies of monoclonal anti-MFAP5 antibodies showed that mice treated with MAbs (15mg/kg, twice per week for two weeks) had no adverse effects in complete blood counts, serum ALT, AST, alkaline phosphatase and urea nitrogen levels, and major organ histology (Figs. 3A to 3C), suggesting that 15 mg/kg is an optimal dose which can be used for mouse treatment (Fig. 4A). The results showed that mice treated with 130A had significantly lower luciferase activity and tumor weight than those treated with normal mouse IgG (Figs. 4B & C).
Figure 3. Toxicity analysis of therapeutic anti-MFAP5 monoclonal antibodies using animal models of ovarian cancer.
(A) Complete blood counts for anti-MFAP5 antibody clone 64A-, 117b-, or 130A-treated mice and PBS-treated control mice revealing no observable adverse effects from anti-MFAP5 antibody administration. (B) Chemistry test results generated from serum samples collected from anti-MFAP5 antibody clone 64A-, 117b-, and 130A-treated mice compared with PBS-treated control mice revealing no significant toxic effects of the antibody-based treatment to the animal’s kidneys and livers. For scatter plots, upper and lower bars indicate the interquartile range of the records, and the middle line indicates the median measurements. (C) Hematoxylin- and eosin-stained images of spleen, kidney, and liver tissue samples obtained from mice given treatment with antibody clone 64A, 117B, or 130A compared with those obtained from PBS-treated control animals. No significant toxic effects of antibody-based treatment were observed. Representative images were selected from three individual replicates. Bar, 100 μm.
Figure 4. MFAP5-targeting monoclonal antibody suppress ovarian and pancreatic cancer progression in mice.
(A) Schematic of the procedures used to evaluate the effects of treatment with an anti-mfap5 antibody on ovarian cancer progression in vivo. (B) Bioluminescence measurement of ovarian tumor progression in the control IgG and anti-MFAP5 monoclonal antibody treatment groups of mice showed significantly lower luciferase activity in the anti-MFAP5 antibody-treated groups than in the control group (P=0.004). In the box plot, the boxes represent the interquartile range of the records, and the lines across the boxes indicate the median luminescence signals. The whiskers indicate the highest and lowest values among the records that are no more than 1.5 times greater than the interquartile range. (C) After necropsy, the tumor weight was recorded for each animal, significantly reduced tumor dry weights were detected in animal treatment with an anti-MFAP5 antibody (P=0.0086). (D) Schematic of the procedures used to evaluate the effects of treatment with an anti-MFAP5 antibody on pancreatic cancer progression in vivo. (E) Bioluminescence measurement of pancreatic tumor progression in the control IgG and anti-MFAP5 monoclonal antibody treatment groups of mice showed significantly lower luciferase activity in the anti-mfap5 antibody-treated groups than in the control group (P=0.011). In the box plot, the boxes represent the interquartile range of the records, and the lines across the boxes indicate the median luminescence signals. The whiskers indicate the highest and lowest values among the records that are no more than 1.5 times greater than the interquartile range. (F) After necropsy, the tumor weight was recorded for each animal, significantly reduced tumor weights were detected in animal treatment with an anti-MFAP5 antibody (P=0.0011).
Besides using the ovarian cancer xenograft mouse model, experiments were performed on a PDAC patient-derived tumor xenograft (PDX) cell line PATC53 to determine the efficacy of 130A in suppressing PDAC progression. PDX cell line were injected into the pancreas of nude mice. They were treated with 15 mg/kg 130A or the control IgG twice a week for 6 weeks (Fig.4D). The results showed that mice treated with 130A had a significant lower luminescence signals and tumor weight than those treated with IgG, suggesting that MFAP5 blockade by the 130A antibody suppresses PDAC growth in vivo (Figs. 4E & F).
In additional to clone 130A, to evaluate the therapeutic efficacy of antibody clones 64A and 117B, which target only MFAP5 of human origin, OVCA3, a MFAP5 expressing human ovarian cancer cell line, was used to study the therapeutic effect of targeting human cancer cell-derived MFAP5 in the tumor microenvironment. Luciferase labeled OVCA3 cancer cells were intraperitoneally injected into nude mice and animals were subsequently treated with either 64A, 117B or control normal mouse IgG at the dosage of 15mg/kg. Experimental results showed that treatment of anti-MFAP5 monoclonal antibody clones 64A and 117B significantly suppressed OVCA3 ovarian tumor growth in mice (Supplementary Fig.4).
Anti-MFAP5 antibody increases chemosensitivity in animal models
Since our previous studies showed that stromal MFAP5 confers chemoresistance in ovarian tumor (4,8), we determine whether MFAP5 blockade by the anti-MFAP5 antibody could enhance chemosensitivity in an ovarian tumor mouse model. OVCA432 tumor-bearing nude mice were treated with paclitaxel together with 130A or the control IgG twice a week. Tumor progression was monitored using the IVIS bioluminescence imaging system. Eight weeks after initial drug treatment, all the animals were sacrificed and their tumor weights and ex vivo tumor luminescence signals were recorded. The results showed that bioluminescence signals of mice and the tumor weight were significantly lower in mice treated with 130A than the IgG did (Fig. 5A and 5B), suggesting that MFAP5 blockade by the antibody enhances the sensitivity of paclitaxel treatment.
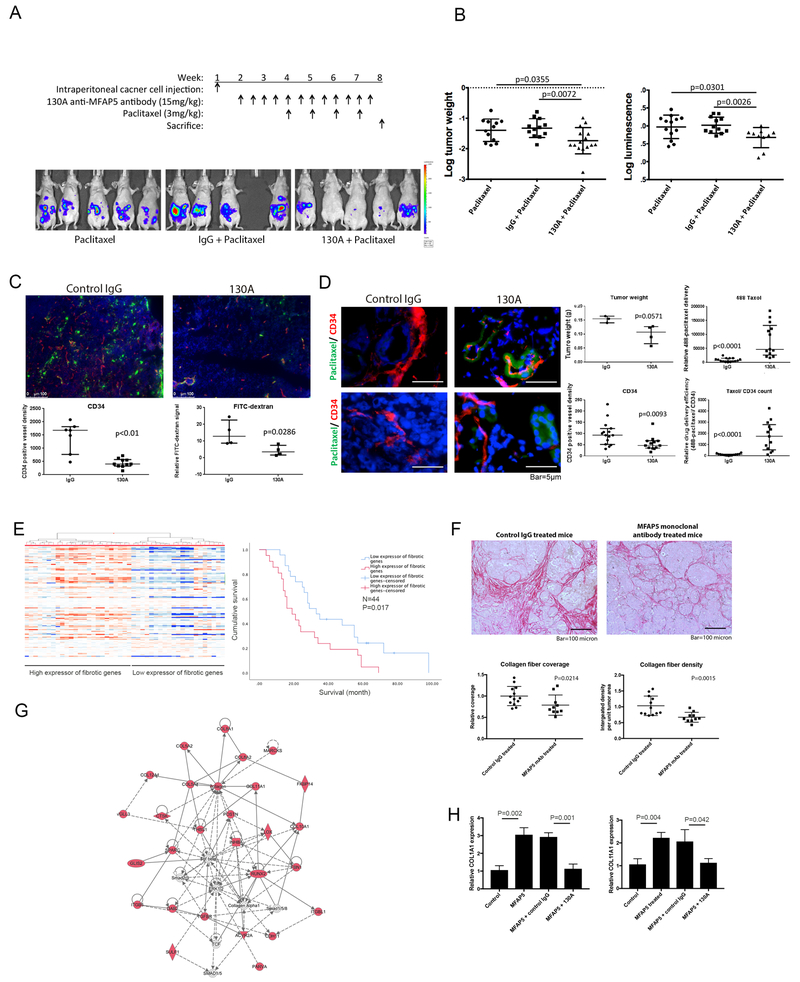
Figure 5. MFAP5-targeting monoclonal antibody increase the bioavailability of the chemotherapeutic agent paclitaxel and reduces cancer fibrosis in mice.
(A) Dosing schedule for combination therapy with an anti-MFAP5 antibody and paclitaxel in an ovarian cancer animal model. (B) Tumor progression was quantified in terms of both tumor weight and ex vivo bioluminescence. Experimental results indicated that the combination treatment with the anti-mfap5 antibody and paclitaxel resulted in markedly smaller tumors than did single-agent treatment with paclitaxel. (C) Confocal microscopic images demonstrating lower CD34+ microvessel density (red) and FITC-dextran (green) signals around tumor microvessels in anti-MFAP5 antibody-treated mice than in IgG-treated mice, suggesting that treatment with the anti-mfap5 antibody reduced tumor angiogenesis and intratumoral microvessel leakiness in vivo. Red, CD34; green, dextran; blue, nuclei; scale bar, 100 μm. (D) Fluorescent microscopic images showing significantly greater delivery of Oregon Green 488-conjugated paclitaxel (green) to tumors despite lower intratumoral MVD in mice given an anti-mfap5 antibody than in control mice given IgG. These results suggested that the anti-mfap5 antibody facilitated systematic delivery of paclitaxel to ovarian tumors. Red, CD34; green, paclitaxel; blue, nuclei; bar, 5 μm. (E) Stromal expression heatmap of fibrosis related genes showing two group of ovarian cancer patients expressing high and low levels of fibrotic genes. Ovarian cancer patients express high level of fibrotic genes had a median survival duration of 19 months (95%CI = 12.3 – 25.7 months), whereas patients express low level of fibrotic genes had a median survival duration of 33 months (95%CI = 22.0 – 44.0 months) (P=0.017). (F) Picrosirius red staining of collagen on tumor tissues obtained from mice treated with 130A antibody or the control IgG showed that tumors in mice treated with 130A had significantly lower collagen coverage and intensity in cancer associated stromal tissue than in those treated with IgG. (G) Pathway analysis on 176 genes that demonstrated significant positive correlation with MFAP5 expression in CAFs (Pearson correlation coefficient >0.7, Pearson correlation P values and Benjamini-Hochberg adjusted P values <0.05) suggested a collagen enriched key signaling network. (H) qRT-PCR analysis demonstrated that recombinant MFAP5 protein induces COL1A1 and COL11A1 mRNA expression in ovarian fibroblasts, which was abrogated in the presence of 130A anti-MFAP5 antibody.
To determine the mechanism by which 130A suppressed tumor progression and chemoresistance in vivo, histologic evaluation of ovarian tumors obtained from mice treated with 130A and the control IgG was performed. Since increased angiogenesis has been shown to be associated with increased chemoresistance in tumor tissues (21,22), we determined whether 130A treatment suppressed angiogenesis and fibrosis in the tumor tissue. Immunolocalization of CD34 positive microvessels showed that tumor tissue in mice treated with 130A had a significant lower microvessel density than those treated with IgG (Fig. 5C), suggesting that MFAP5 blockade by the 130A antibody inhibits angiogenesis, most likely through preventing the binding of MFAP5 to αVβ3 integrin on endothelial cells and activating calcium dependent FAK/ERK/LPP signaling pathway as we previously reported (7,8).
To determine whether vessel normalization played a role in mediating the effect of MFAP5 blockade in enhancing paclitaxel sensitivity, intratumoral microvessel leakiness was determined by injecting FITC-dextran into tail veins of mice before they were sacrificed. The results showed that perivascular FITC-dextran signals in ovarian tumors were significantly lower in the 130A-treated mice than in the control mice, suggesting that MFAP5 blockade reduced intratumoral microvessel leakiness (Fig. 5C).
To evaluate whether the reduced intratumoral microvessel leakiness resulting from 130A antibody treatment affected the bioavailability of systemically administered drugs in ovarian tumors, OVCA432 tumor-bearing mice were treated with 130A or the control IgG for 4 weeks before intravenous injection of Oregon Green 488-conjugated paclitaxel into each animal. Fluorescent microscopy was then performed on tumor tissue sections. The results showed that tumor tissues obtained from mice treated with 130A had significantly stronger greater Oregon Green 488 signals than those treated with the control IgG (Fig. 5D), suggesting that MFAP5 blockade increases paclitaxel bioavailability in the tumor tissue and thus enhances paclitaxel sensitivity.
MFAP5 blockage by anti-MFAP5 antibody reduces cancer fibrosis
In addition to tumor angiogenesis, mediators secreted by CAFs, which constitute the fibrotic microenvironment, have also been shown to be associated with tumor progression and increased chemoresistance (22–25). To evaluate the prognostic significance of a fibrotic microenvironment in ovarian cancer tissue, correlation studies between the fibrotic gene signature in microdissected CAFs in ovarian tumor tissue samples and patient survival rates were performed. The results showed that patients whose cancer stroma had the fibrotic gene signature (Supplementary Table 1) had significantly lower survival rates (Fig. 5E). Ovarian cancer patients expressing high level of fibrotic genes had a median survival duration of 19 months (95%CI = 12.3 – 25.7 months), whereas patients expressing low level of fibrotic genes had a median survival duration of 33 months (95%CI = 22.0 – 44.0 months) (P=0.017). To further determine whether MFAP5 blockade by 130A antibody could reduce fibrosis in tumors developed from both ovarian and pancreatic mouse models, Picrosirius red staining, which is used for the visualization of collagen fibers, was first performed on tumor tissues obtained from mice treated with 130A or the control IgG. The results showed that tumors in treated mice had significantly lower Picrosirius red staining coverage and intensity in cancer associated stromal tissue than in control group (Fig. 5F, Supplementary Fig. 5). In order to confirm the presence of fibrosis of at the time of antibody treatment initiation, Picrosirius red staining was performed on mouse tumor tissue samples harvested at week 2 after initial tumor cell injection. Our staining results confirmed the presence of collagen I positive stroma within the tumor tissue (Supplementary Fig. 6), suggested that fibrosis is present in that time point and antibody treatment was likely started after the onset of fibrosis. Overall, our data suggest that MFAP5 blockade inhibits fibrosis in both ovarian and pancreatic tumor tissues expression, and MFAP5 may regulate genes associated with fibrosis in CAFs in an autocrine fashion. To test this hypothesis, Pearson Correlation studies were performed on expression levels of MFAP5 and other genes using transcriptome generated from microdissected CAFs. We identified expression of 176 genes that demonstrated significant positive correlation with MFAP5 expression in CAFs (Pearson correlation coefficient >0.7, Pearson correlation P values and Benjamini-Hochberg adjusted P values <0.05) (Supplementary Table 2). Further analysis on the 176 genes using the Ingenuity Pathway Analysis software program identified a collagen enriched key signaling network, which is involved in extracellular matrix and connective tissue disorder (Fig. 5G), suggesting that high MFAP5 expressed by CAFs may contribute to a collagen rich, fibrotic tumor microenvironment. To further determine whether MFAP5 regulates the expression of key fibrosis-related genes in CAFs, qRT-PCR analyses on two of the key fibrosis-related genes, COL1A1 and COL11A1, whose expression showed significant correlation with MFAP5 expression in CAF, were performed on human fibroblasts treated with exogenous MFAP5. The results showed markedly higher expression of COL1A1 and COL11A1 in fibroblasts treated with MFAP5 than those treated with the control solvent. (Fig. 5H), In addition, CAFs treated with MFAP5 in the presence of 130A anti-MFAP5 antibody demonstrated significantly lower levels of COL1A1 and COL11A1 expression than those treated with MFAP5 in the presence of control IgG (Fig. 5H). These data suggest that MFAP5 up-regulates COL1A1 and COL11A1 in CAFs in an autocrine manner.
Discussion
In this study, we demonstrated the development of an anti-MFAP5 monoclonal antibody which could down-regulate MFAP5-induced collagen production in CAFs, suppress intratumoral microvessel leakiness, and enhance paclitaxel bioavailability in both ovarian and pancreatic cancer models. MFAP5 is a pro-tumorigenic and pro-angiogenic protein, which is up-regulated in CAFs in both ovarian and pancreatic cancer patients. Our previous studies on MFAP5 demonstrated its crucial roles in promoting ovarian tumor metastasis, stimulating tumor angiogenesis and enhancing cancer cells’ resistance to chemotherapeutic agent through the reduction in drug delivery via the tumor vascular system (4,8). In the present study, treating tumor-bearing mice with a newly developed MFAP5-targeting MAb suppressed ovarian and pancreatic tumors progression with no observable toxic effects. Based on The Human Protein Atlas constructed by Uhlen and colleagues, MFAP5 expression was detected only in 1 out of 81 analyzed normal tissue cell types at an expression level of medium level or higher. mRNA analyses showed that MFAP5 is expressed by about 50% of fibrosarcoma and normal fibroblasts during wound healing. On the other hand, VEGF expression was detected in 75 out of 80 analyzed normal tissue cell types at medium or high levels (26,27). The low endogenous expression level of MFAP5 by normal tissue may contribute to the low treatment related toxicity observed in our animal studies. Furthermore, our data demonstrated increased paclitaxel delivery after treating ovarian tumors with an anti-MFAP5 monoclonal antibody and that combining paclitaxel with that antibody improved the efficacy of paclitaxel in ovarian cancer treatment, indicating that targeting stromal MFAP5 with MAbs can potentiate the therapeutic efficacy of cancer chemotherapy.
The idea that therapeutic antibodies could serve as “magic bullets” in cancer therapy has a long history and achieved noticeable success in recent years. The current anti-MFAP5 antibody clones could be further modified as immunoconjugate therapy by conjugation with drugs, toxins or radioisotopes to carry enhanced killing capacity directly to the tumors. While treatment efficacy could be context specific, CAF-targeting for cancer treatment is believed to have two benefits: 1) the continuous support from CAFs is critical to tumor progression and 2) stromal cells, including CAFs, are genetically more stable than cancer cells, which can accumulate adaptive mutations during drug treatment to acquire resistance (28–30). On the other hand, chimeric or humanized anti-MFAP5 MAbs could be developed. These humanized or chimeric MAbs have a longer half-life in the patients’ blood stream, and enables better interactions with human effector cells in the patients (31). Although the anti-MFAP5 MAbs generated have KD values in the low nanomolar range (10−9), indicating high level of affinity, these values can be further improved by performing affinity maturation.
This is the first report demonstrating that MFAP5 blockade reduced fibrosis in both ovarian and pancreatic cancers through down-regulation of fibrosis-related genes including COL1A1 and COL11A1. Fibrosis in tumor tissue has been shown to increase tissue matrix stiffness, which promotes tumor progression and confers chemoresistance (32–34). Fibrosis-related genes such as COL11A1 has been shown to confer cisplatin resistance in ovarian cancer cells (35). These data suggest that that MFAP5 blockade suppresses fibrosis through down-regulating of fibrosis-related genes such as COL11A1 as we observed in our study, which subsequently enhances chemosensitivity of cancer cells. Our data also demonstrated for the first time, that MFAP5 transcriptionally up-regulated fibrosis-related genes including COL1A1 and COL11A. While the molecular mechanism by which MFAP5 regulates these collagen genes in CAFs remain to be elucidated, it is likely that MFAP5 may bind to alphaVbeta3 integrin, which subsequently activates collagen genes through integrin/ERK mediated signaling pathways as we described previously (4,7,8). In fact, activation of alphaVbeta3/ERK signaling pathway by TGF-beta has been shown to activate collagen genes in fibroblasts (36). In additional to cancer, many diseases involve fibrotic conditions. Further evaluation on whether MFAP5 also plays a role in the excess formation of fibrous tissue in other diseases and whether MFAP5-targeting therapy would offer clinical benefits is warranted.
In conclusion, we demonstrated that targeting MFAP5 using the newly developed antibody can successfully block the downstream signaling network mediated by MFAP5 and subsequently inhibits ovarian and pancreatic cancer progression, promotes chemosensitivity and reduces fibrosis. Furthermore, the toxicity and therapeutic efficacy of the humanized antibody will be evaluated in non-human primates. Patients can be stratified to increase the efficacy of this new antibody treatment, based on the expression levels of stromal MFAP5 in tumor tissue biopsies or the levels of circulating MFAP5. These findings implicate that further development of 130A into a MFAP5-targeting therapeutic monoclonal antibody is warranted. This process will include the generation of a humanized antibody clone which will include its affinity maturation to further improve the binding of the antibody to its antigen.
Supplementary Material
Translational Relevance.
The stromal microenvironment plays important roles in tumor progression and disease prognosis. However, strategies used to overcome the malignant phenotypes of cancer cells modulated by the microenvironment have not been thoroughly explored. This study seeks to evaluate the therapeutic efficacy of a newly developed monoclonal antibody targeting microfibril associated protein 5 (MFAP5), a cancer associated fibroblast-derived secretory protein using ovarian and pancreatic cancer models. The results of this study demonstrated that anti-MFAP5 monoclonal antibody treatment suppressed intratumoral microvessel leakiness, enhanced paclitaxel bioavailability and inhibited tumor progression in both ovarian and pancreatic cancer mouse models, suggested that MFAP5 blockade with the monoclonal antibody can be used as a novel therapeutic approach in treating these diseases and improve patient survival. Furthermore, when used in combination with chemotherapeutic agents, the efficacy of chemotherapy could be enhanced.
Acknowledgments
This study was supported in part by grants RO1CA133057, RO1CA142832, and RC4CA156551; The University of Texas MD Anderson Cancer Center Ovarian Cancer Specialized Program of Research Excellence grant P50CA083639 from the National Institutes of Health; the U.S. Department of Health and Human Services; the Gilder Foundation; by grants W81XWH-17–1–0126, W81XWH-17–1–0146 and W81XWH-16–1–0038 from the Ovarian Cancer Research Program, US Department of Defense; and funding support from the Mary K Chapman Foundation and the Ovarian Cancer Research Fund. The Monoclonal Antibodies Core Facility is supported by the Cancer Center Support Grant NCI P30CA016672. We acknowledge the assistance of Dr. Giulio Draetta from the Department of Genomic Medicine and Dr. Anirban Maitra from the Department of Pathology at MD Anderson Cancer Center on pancreatic cancer related studies.
Footnotes
Conflict of interest:
The authors have declared that no conflict of interest exists
References
- 1.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol 2006;1:119–50 [DOI] [PubMed] [Google Scholar]
- 2.Navab R, Strumpf D, Bandarchi B, Zhu CQ, Pintilie M, Ramnarine VR, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci U S A 2011;108:7160–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Casas PP, Lopez-Fernandez LA. Gene-expression profiling in pancreatic cancer. Expert Rev Mol Diagn 2010;10:591–601 [DOI] [PubMed] [Google Scholar]
- 4.Leung CS, Yeung T-L, Yip K-P, Pradeep S, Balasubramanian L, Liu J, et al. Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential. Nature Communications 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Z, Wang Y, Sawyers A, Yao H, Rahmatpanah F, Xia XQ, et al. Diagnosis of prostate cancer using differentially expressed genes in stroma. Cancer Res 2011;71:2476–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwers B, Fumagalli D, Brohee S, Hatse S, Govaere O, Floris G, et al. The footprint of the ageing stroma in older patients with breast cancer. Breast Cancer Res 2017;19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok SC, Bonome T, Vathipadiekal V, Bell A, Johnson ME, Wong KK, et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell 2009;16:521–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung CS, Yeung T-L, Yip K-P, Wong K-K, Ho SY, Mangala LS, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J Clin Invest 2018;128:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639–48 [DOI] [PubMed] [Google Scholar]
- 11.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673–84 [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin Colorectal Cancer 2004;4 Suppl 2:S62–8 [DOI] [PubMed] [Google Scholar]
- 13.Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017;390:1654–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Vien LT, Xia X, Bover L, Li S. Generation of a monoclonal antibody against the glycosylphosphatidylinositol-linked protein Rae-1 using genetically engineered tumor cells. Biol Proced Online 2014;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin H, Wei G, Sakamaki I, Dong Z, Cheng WA, Smith DL, et al. Novel BAFF-Receptor Antibody to Natively Folded Recombinant Protein Eliminates Drug-Resistant Human B-cell Malignancies In Vivo. Clin Cancer Res 2018;24:1114–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voo KS, Bover L, Harline ML, Vien LT, Facchinetti V, Arima K, et al. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J Immunol 2013;191:3641–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, et al. A proposal for validation of antibodies. Nat Methods 2016;13:823–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MM, Gao Z, Peterson BR. Synthesis of a Fluorescent Analogue of Paclitaxel That Selectively Binds Microtubules and Sensitively Detects Efflux by P-Glycoprotein. Angew Chem Int Ed Engl 2017;56:6927–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med 2012;2:a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, Kavallaris M, et al. Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol 2014;5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields MA, Dangi-Garimella S, Redig AJ, Munshi HG. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem J 2012;441:541–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdani S, Bansal R, Prakash J. Drug targeting to myofibroblasts: Implications for fibrosis and cancer. Adv Drug Deliv Rev 2017;121:101–16 [DOI] [PubMed] [Google Scholar]
- 25.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat 2012;15:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Molecular & cellular proteomics : MCP 2005;4:1920–32 [DOI] [PubMed] [Google Scholar]
- 27.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nature biotechnology 2010;28:1248–50 [DOI] [PubMed] [Google Scholar]
- 28.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer 2004;45 Suppl 2:S163–75 [DOI] [PubMed] [Google Scholar]
- 29.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005;7:513–20 [DOI] [PubMed] [Google Scholar]
- 30.Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol 2015;309:C444–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol 2008;26:1774–7 [DOI] [PubMed] [Google Scholar]
- 32.Joyce MH, Lu C, James ER, Hegab R, Allen SC, Suggs LJ, et al. Phenotypic Basis for Matrix Stiffness-Dependent Chemoresistance of Breast Cancer Cells to Doxorubicin. Front Oncol 2018;8:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011;4:165–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J Biol Chem 2013;288:12722–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rada M, Nallanthighal S, Cha J, Ryan K, Sage J, Eldred C, et al. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 2018 [DOI] [PubMed] [Google Scholar]
- 36.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol 2005;175:7708–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.