Abstract
Ebola virus (EBOV) is among the deadliest pathogens known to man causing infrequent outbreaks of hemorrhagic disease. In humans, the case fatality rates in the outbreaks can reach 90%. During the West African epidemic almost 30,000 people were infected and of these over 11,000 fatalities were reported. Currently, we are facing an uncontained larger outbreak in the Democratic Republic of the Congo. Even though EBOV was discovered in 1976, extensive efforts to develop countermeasures, particularly therapeutics and vaccines, started late and there is still no FDA-approved product available. Nevertheless, one candidate vaccine, the rVSV-ZEBOV, is being used in clinical trials during the current outbreak with the hope of ending the human transmission chains. However, adverse reactions to administration of some EBOV vaccines have been reported; therefore, we have developed a safe and efficacious formulation of insect-cell derived adjuvanted protein vaccines. Vaccine candidates containing the EBOV glycoprotein with or without matrix proteins VP24 and VP40 formulated with one of three different adjuvants were tested in guinea pigs for immunogenicity and efficacy against lethal EBOV challenge. The results demonstrated that these vaccine candidates engendered high titers of antigen-specific antibodies in immunized animals and two of these vaccine candidates afforded complete or nearly complete protection against lethal challenge. Interestingly, we found a sex bias in partially protected immunized groups with male guinea pigs succumbing to disease and females surviving. In summary, we developed a safe and immunogenic adjuvanted subunit vaccine uniformly protective against EBOV disease in guinea pigs.
Keywords: Zaire ebolavirus, recombinant protein, glycoprotein, VP24, VP40
1. Introduction
Initially discovered in 1976 when two simultaneous outbreaks of acute hemorrhagic fever occurred in the former Zaire (Zaire ebolavirus) (now the Democratic Republic of the Congo) and southern Sudan (Sudan ebolavirus) [1, 2], members of the genus Ebolavirus have been the cause of sporadic outbreaks in humans throughout the years [3]. These initial outbreaks resulted in 318 cases and 280 deaths (Zaire) [1] and 284 cases and 151 deaths (Sudan)[2], respectively. Until today, a total of five species have been characterized within the genus, with at least four of the members causing severe disease in humans with case fatality rates of up to 90% [1, 4]. Ebolaviruses are enveloped negative-sense RNA viruses within the order Mononegavirales and the family Filoviridae with a genome comprised of 7 genes. The glycoprotein (GP) is found in trimeric spikes on the surface of the envelope, while associated with the membrane are the matrix proteins VP24 and VP40. The nucleoprotein (NP) and viral proteins 30 and 35 (VP30, VP35) associate with the viral RNA and form the nucleocapsid and become part of the replication complex along with the viral RNA-dependent RNA polymerase [5].
Ebolaviruses are the causative agents of Ebola virus disease (EVD) which is characterized by an acute febrile illness that includes fever, chills, myalgia, nausea and diarrhea and can lead to coagulopathy, changes in vascular permeability and release of host inflammatory cytokines resulting in organ damage, bruising, clotting failure and intestinal bleeding [3]. Starting in December 2013, the largest documented epidemic of Ebola virus (EBOV) began in Guinea [6] and resulted in 28,646 cases with 11,323 fatalities throughout Guinea, Sierra Leone and Liberia by its end in March 2016 [7]. The outbreak highlighted the need for effective vaccines and therapeutics to prevent and treat EVD and led to accelerated clinical development programs for various medical countermeasures. Although no vaccines have been approved by the FDA, several candidates are in or have concluded phase 2/3 clinical trials [8]. Despite the efficacy and clinical advancement of several EBOV vaccine candidates, several obstacles remain. While the rVSV-ZEBOV vaccine has been reported to be highly efficacious in prevention of EVD [9], adverse events can be associated with this live-attenuated vaccine [9, 10].
Recombinant Drosophila S2 cell derived viral proteins have been successfully used in the development of vaccines against various flaviviruses [11–14] and have been shown to protect a wide range of animal models from these diseases including nonhuman primates (NHPs) [12–14]. Previous work done in mice has shown the potential of a properly adjuvanted recombinant subunit EBOV vaccines to be immunogenic and protective against lethal challenge with mouse adapted EBOV (ma-EBOV) [15]. This study showed that both GPI-0100 (saponin-based) and CoVaccine HT™ (nanoemulsion) adjuvants are capable of eliciting higher humoral and cell-mediated immune responses than unadjuvanted proteins or formulations using other adjuvants such as ISA51 and Ribi R-700. The study also demonstrated that formulations using multiple viral proteins (GP, VP24 and VP40) induce stronger antibody responses to whole irradiated EBOV when compared to GP, VP24, or VP40 alone, as expected. Challenge with ma-EBOV demonstrated uniform protection in mice receiving GP with CoVaccine HT™ or all three viral proteins with either CoVaccine HT™ or GPI-0100. High levels of protection (70–90%) were seen in mice that received GP alone, with GPI-0100 or all three proteins without adjuvant [15]. VP24 was also shown to induce protection via cell-mediated immunity demonstrating potential for this as an additional vaccine antigen. Here we show the efficacy of recombinant EBOV GP, VP24 and VP40 produced from insect cells with three different adjuvants in the EBOV guinea pig model. In contrast to mice, this animal model recapitulates some of the coagulopathy seen in NHPs and humans, and therefore, helps to further develop and refine protective vaccine formulations [16, 17].
2. Materials and Methods
2.1. Protein expression and purification
Expression and purification of recombinant subunit proteins was conducted as described previously [15]. In addition to single-step purification yielding a >90% pure EBOV GP preparation, the same material was subjected to size-exclusion chromatography using a Superdex 200 column (GE Healthcare, Piscataway, NJ) equilibrated in phosphate-buffered saline (PBS) to separate trimer and high molecular weight (HMW) fractions generating three distinct protein lots: HMW, trimer and a mixed population collected between the two. Monomeric GP was discarded and not used for animal studies as it is presumed to not be in the proper conformation.
2.2. Ethics and Biosafety statement
All work with animals was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition. All procedures were reviewed and approved by the appropriate Institutional Animal Care and Use Committees at Lovelace Biomedical and Environmental Research Institute (LBERI) and the Rocky Mountain Laboratories (RML), DIR, NIAID, NIH. All work with EBOV was conducted in the animal biosafety level 4 (ABSL4) facility at the Rocky Mountain Laboratories, DIR, NIAID, NIH in Hamilton, MT.
2.3. Guinea pig immunogenicity – vaccine formulation and immunization of guinea pigs
For the immunogenicity studies, Hartley guinea pigs were immunized using three different adjuvants with different modes of action; 1, an emulsion-based adjuvant, CoVaccine HT™ (an emulsion of squalane with immunostimulatory sucrose fatty acid sulphate esters and an adjuvant of BTG International Ltd, London, United Kingdom) [18] was used at a dose of 1 mg; 2, a saponin-based, immunomodulatory adjuvant, GPI-0100 (Hawaii Biotech, Inc., Honolulu, HI) [19, 20] was used at a dose of 100 μg; 3, Alhydrogel® 85 (“Alum”; Brenntag, Reading, PA) was used at 1 mg per dose. Groups of 8 or 16 male and female Hartley guinea pigs (>5 weeks old) were obtained from Charles River Laboratories, acclimated for 14 days and vaccinated intramuscularly (i.m.) three times in the hind legs with individual subunit proteins at the indicated dose and formulated with one of the selected adjuvants at 3-week intervals. Vaccine formulations were prepared fresh for each vaccination from frozen antigen stocks, adjuvant stock solutions and sterile PBS at PanThera Biopharma to reach the desired dose within a final volume of 0.2 mL and were sent refrigerated to the facility for immunizations within 3–4 days from preparation. Negative control groups received equivalent doses of GPI-0100 adjuvant in PBS only (also prepared fresh for each administration). Pre-challenge serum samples were collected under anesthesia on study days 0, 20, 38/39 and 56 for study 1 or days 0, 21, 42 and 63 for study 2 to allow serological analysis of the vaccine induced responses.
2.4. Vaccine efficacy against EBOV challenge
Guinea pig-adapted (gpa-) EBOV [21] was propagated and titered on Vero E6 cells and used for the challenge part of this study. Groups of 8–16 previously immunized Hartley guinea pigs were transferred into the ABSL4 facility, acclimated to the new environment and challenged intraperitoneally (i.p.) with 1,000 LD50 (10 focus-forming units) of gpa-EBOV. Animals were observed daily for signs of illness and lethality. Surviving animals were euthanized 28 days after challenge. Serum samples were collected from each animal at their respective final study day.
2.5. Analysis of antibody responses by ELISA
Individual guinea pig serum samples were titrated for IgG specific to the recombinant EBOV GP and VP40 by standard ELISA technique using plates coated with purified recombinant antigen. The titers presented are defined as the dilution of antiserum yielding 50% maximum absorbance values (EC50) and were determined using a sigmoidal dose response curve fitting algorithm (Prism, Graphpad Software, San Diego, CA).
2.6. Statistical analysis
Determination of significant differences in EBOV GP-specific IgG titers and weight changes post infection between animal groups vaccinated with different vaccine formulations was done using an unpaired t-test (Prism, Graphpad Software, San Diego, CA). Significant differences in survival between immunized (or non-immunized control) groups subsequently challenged were determined by the Fisher exact probability test (GraphPad Prism). Kaplan-Meier survival curves were compared using the log-rank (Mantel-Cox) test for significant differences (Graph Pad Prism). For all tests p < 0.05 was considered significant.
3. Results
3.1. Vaccine immunogenicity in guinea pigs
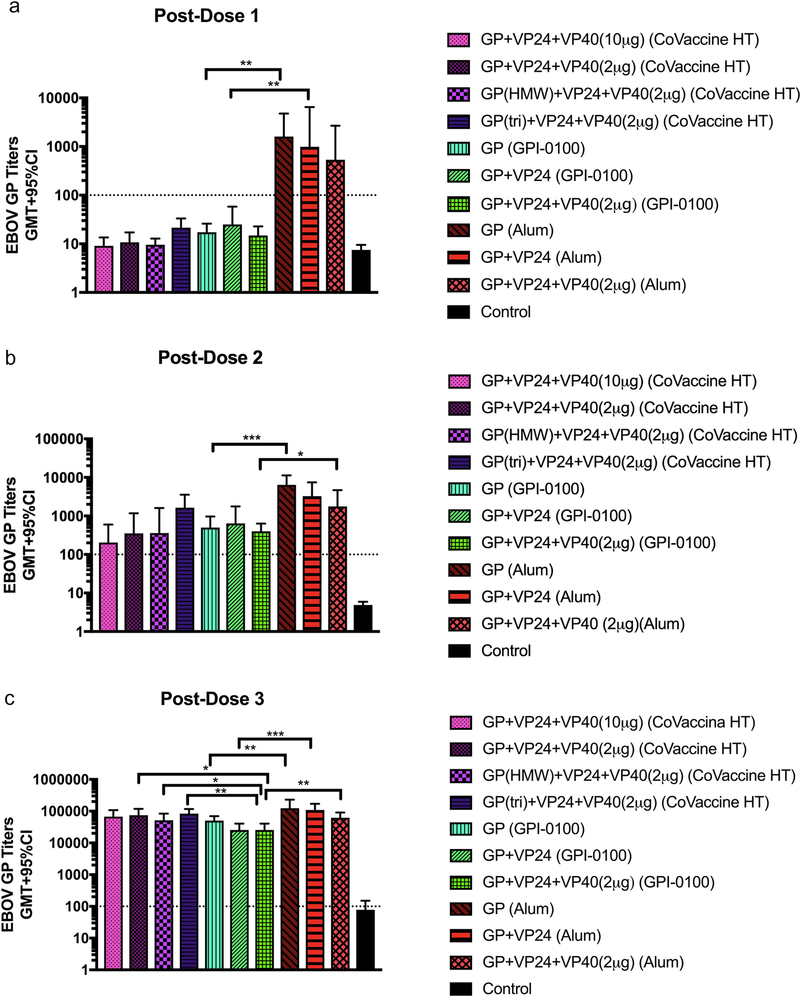
Immunogenicity and efficacy of various candidate vaccine formulations were assessed in groups of 7 or 8 Hartley guinea pigs receiving three immunizations in 3-week intervals. Formulations containing CoVaccine HT™ were designed to allow for comparison of multi-antigen formulations as earlier work in mice showed greater humoral responses to EBOV VP40 in comparison to GP and VP24. Therefore, equal amounts of all three antigens (10 μg) or an “antigen balanced” vaccine with 10 μg each of GP and VP24, but only 2 μg of VP40 were used in this study. In addition, antigen balanced vaccines were formulated that contained either the trimeric GP or HMW GP fractions consisting of dimers of trimers and larger aggregates prepared by an additional size-exclusion chromatography step used to separate properly assembled oligomers from monomeric GP. In the GPI-0100 and Alum containing groups, animals were given vaccines with either 10 μg of GP only, 10 μg each of GP + VP24, or 10 μg each of GP and VP24 with 2 μg of VP40. An assessment of GP-specific IgG titers using ELISA (Fig. 1A) showed that after the first dose titers in all groups receiving the Alum-adjuvanted vaccines were higher than in other adjuvant groups. In addition, in groups that received GP only or GP + VP24 the titers were significantly higher than the corresponding GPI-0100 groups (Fig. 1A). This difference remained consistent after dose 2, with titers in the Alum groups being higher than for other adjuvant groups (Fig. 1B). After the third dose, titers were similar for all adjuvant groups, although some differences between groups were still seen (Fig. 1C). Animals in the GPI-0100 group that received all three antigens had significantly lower titers than those in the corresponding Alum and CoVaccine HT™ groups. Formulations containing GPI-0100 that used GP only or GP + VP24 had significantly lower titers than corresponding formulations containing Alum. Control animals that received GPI-0100 alone did not develop titers above the assay cutoff at any point (Fig. 1).
Figure 1: Immunogenicity in guinea pigs.
Geometric mean titers (GMT+95%CI) of EBOV GP-specific IgG determined using ELISA in serum from guinea pigs following the first (A), second (B) and third (C) vaccine dose for each adjuvant and antigen formulation. Serum samples were collected 3 weeks following each vaccination. Statistical significance was determine using an unpaired t-test (*p<0.05, **p<0.01, ***p<0.001). The control group received GPI-0100 adjuvant only. The negative assay cutoff is indicated by the dotted line.
3.2. Protective efficacy in guinea pigs
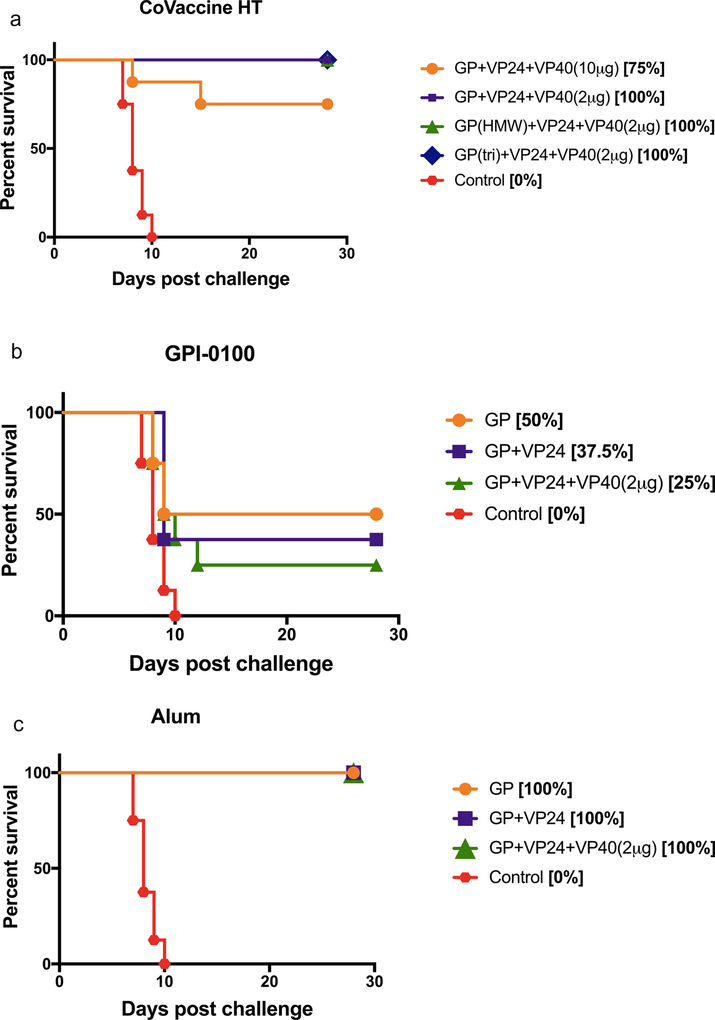
Four weeks after the 3rd vaccine dose, animals in all groups were challenged with 1,000 LD50 of gpa-EBOV. Amongst the groups receiving formulations adjuvanted with CoVaccine HT™, all antigen balanced formulations were uniformly protective against lethal challenge (Fig. 2A). Only the group that received equal amounts of all three antigens plus CoVaccine HT™ resulted in 75% survival (6/8). Animals in groups that received formulations with GPI-0100 showed varying levels of protection, although no group was uniformly protected using this adjuvant (Fig. 2B). The highest level of protection was seen in animals that received GPI-0100 with GP alone where 50% (4/8) of the animals survived the lethal challenge. The lowest protection was seen in the group receiving all three antigens, with only 25% (2/8) survival, while GP + VP24 showed 37.5% (3/8) protection against challenge. All non-surviving animals succumbed to infection between days 8–12 post-challenge showing no significant increase in time to death compared to control animals that succumbed between days 7–10. Alum was shown to be the most potent adjuvant for these antigens in guinea pigs, as all of the animals in groups receiving formulations with Alum survived challenge (Fig. 2C). This is consistent with the rapid and robust antibody responses observed (Fig. 1). Survival in all groups vaccinated with either CoVaccine HT™ or Alum adjuvanted formulations was statistically significant (p<0.05) compared to the adjuvant control group (0/8 survivors). Among the GPI-0100 adjuvanted vaccine groups, only the survival in the GP only group was significantly different from the control group. Comparison of the Kaplan-Meier survival curves (Fig. 2) confirmed the results of the contingency analysis, yielding statistically significant differences between vaccinated and control animals.
Figure 2: Survival in guinea pigs.
Kaplan-Meier survival curves of animals receiving vaccine formulations with CoVaccine HT™ (A), GPI-0100 (B), and Alum (C) following lethal challenge with gpa-EBOV. Control animals received GPI-0100 without antigen. The same control group is shown in panels A-C. Comparison of survival curves by the log-rank (Mantel-Cox) test showed that all vaccinated groups compared to the control group were significantly different (p<0.0001, panel A; p<0.05, panel B; p<0.0001, panel C). Comparisons between vaccinated groups in each panel were not significantly different.
The body weight of all animals was monitored daily following gpa-EBOV challenge as changes in body weight correlate with disease development in this model. Only non-protected animals show significant weight loss after challenge indicating development of EVD-like disease. To allow direct comparison between groups, weight analysis was done by comparing the mean animal weight per group at day 0 to the mean animal weight per group at various days following challenge (Fig. S1). Due to the significant difference in weight between males and females, weight analysis was done separately for both sexes in each group. Animals receiving formulations containing CoVaccine HT™ maintained consistent weights throughout the post-challenge period, with the exception of a small loss in weight in the group that received equal amounts of all three antigens with CoVaccine HT™ suggesting that the antigen-balancing has been successful. This conclusion is supported by statistical analysis of the differences in weight changes post infection between the groups vaccinated with 10 μg of each antigen compared to 10 μg each of GP and VP24 plus 2 μg of VP40 with CoVaccine HT™. Comparing data from individual animals at day 16, differences in percent of day 0 weights were statistically significant (p = 0.0071, unpaired t test, with mean values of 98.77% and 101.3%, respectively, for the 10/10/10 and 10/10/2 groups. Likewise, comparing data from individual animals for all time points combined, statistical analysis yielded p < 0.0001, unpaired t test, with mean values of 98.82% and 100.5%, respectively, for the 10/10/10 and 10/10/2 groups.
Weight loss in animals receiving formulations containing GPI-0100 can be seen in all groups starting at day 8 post-challenge and it should be noted that males showed a bigger weight loss as all males succumbed to lethal challenge (Fig. S2). The group that received GP with GPI-0100 showed consistent weight throughout the experiment and also showed the highest level of protection. The most consistent animal weights post-challenge were seen in all groups receiving vaccine formulations with Alum as an adjuvant, as expected with full protection observed in all of these animals. The lack of weight loss in any of the Alum adjuvanted groups is an indicator of the quality of protection elicited by these vaccine candidates, as none of the animals showed any signs of morbidity or mortality following challenge. As expected, animals that were protected from lethal challenge maintained a consistent weight throughout the study, while those that were not protected lost weight following challenge.
3.3. Effect of sex on immune responses to vaccination and protective efficacy
During the analysis of the first efficacy study described above, it became evident that in groups showing partial protection, a disproportionate number of males succumbed to disease; therefore, the data was further analyzed by sex (Figs. S3, S4). When survival was analyzed by sex in all partially protected groups of the experiment as well as in all experimental groups, differences in survival between both sexes were significant. For partially protected groups p < 0.0001, and for all groups p = 0.0021 (Fisher exact test, 2-tailed).
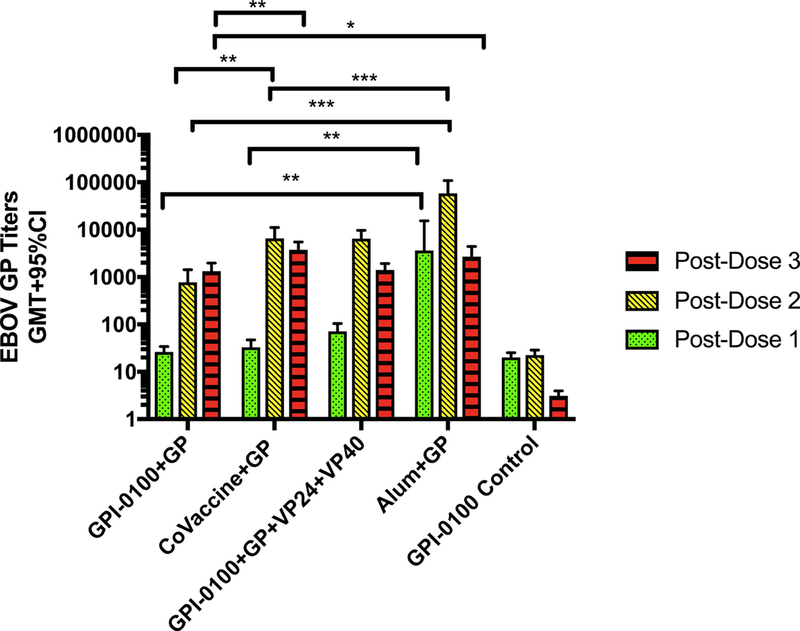
Thus, a follow-up study was performed with groups containing 8 animals of each sex to allow stronger statistical analysis. The formulations selected for this experiment were expected to result in partial protection of the animals in each group complemented by a positive (GP with Alum) and a negative (GPI-0100 only) control group. Based on the first study partial, protection was achieved in groups receiving 10 μg of GP with CoVaccine HT™ or GPI-0100, or 10 μg of GP with 10 μg of VP24 and 2 μg of VP40 with GPI-0100. Furthermore, the chosen vaccine groups allowed the direct comparison of the efficacy of EBOV GP alone with all three adjuvants in this animal model. Three doses of each formulation were given at 3-week intervals, and all animals were challenged with 1,000 LD50 of gpa-EBOV 4 weeks after the 3rd dose. As in the previous study, the IgG responses to the Alum adjuvanted formulation were significantly higher than the response using other adjuvants following the first and second doses (Fig. 3). By the third dose, EBOV GP-specific titers were similar in all groups, although the GP with either Alum or CoVaccine HT™ groups developed significantly higher titers than the GPI-0100 with GP vaccine group.
Figure 3: Immunogenicity in guinea pigs.
Geometric mean titers of EBOV GP-specific IgG determined using ELISA in serum from guinea pigs that received different antigen and adjuvant vaccine formulations following each vaccine dose. Statistical significance was determined using an unpaired t-test (*p<0.05, **p<0.01, ***p<0.001).
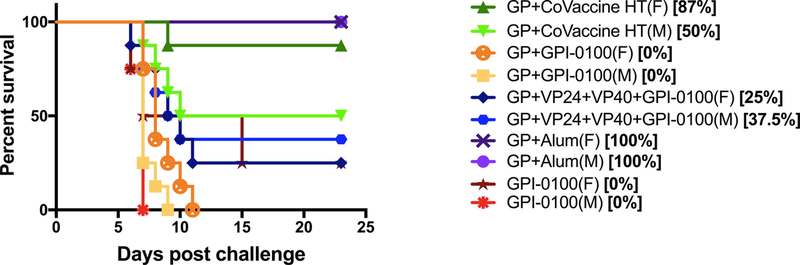
Full protection against lethal challenge was seen in all animals that received Alum with GP (Fig. 4). In the group that received GP with CoVaccine HT™, 68% (11/16) of the animals were protected from challenge (p = 0.0136 vs. controls; K-M curves p = 0.0182), with females faring better than the males (87% vs. 50% survival), although this difference was not statistically significant. Animals receiving all three antigens with GPI-0100 showed 31% overall survival, with very little difference being seen between males and females (25% vs. 37.5%). In the group that received GP with GPI-0100 all animals succumbed to infection, although there was a slight extension of time to death in females (Fig. 4).
Figure 4: Survival in guinea pigs.
Kaplan-Meier survival curves of animals receiving different vaccine formulations following lethal gpa-EBOV challenge stratified by sex. Controls received GPI-0100 without antigen. Contingency analysis showed that differences between groups vaccinated (both sexes combined) with GP+CoVaccine (p = 0.0032) or GP+Alum (p = 0.0002) compared to the control group were significant. Differences between the other vaccinated groups and the control group were not significant. Differences in survival or survival curves between sexes within each group were not significant.
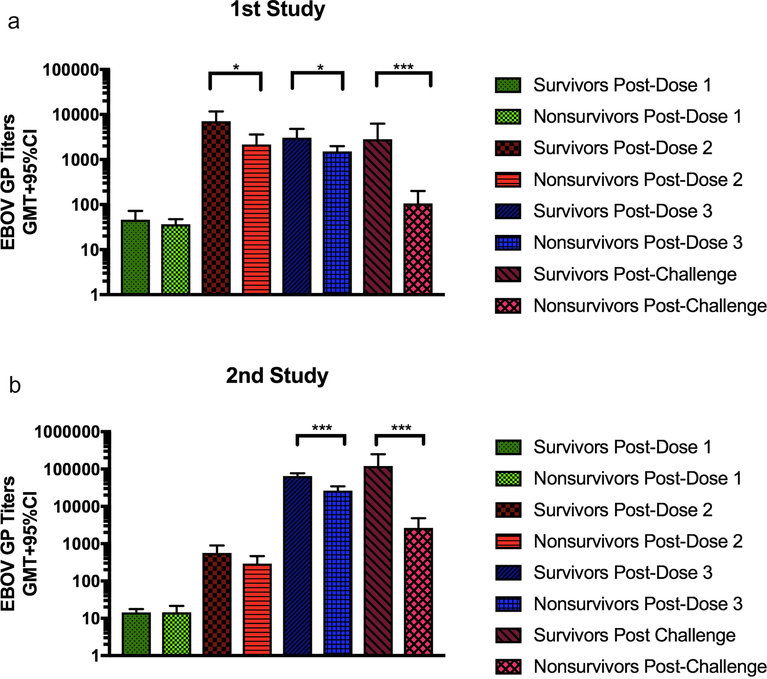
To determine if the levels of EBOV GP-specific antibody titers correlated with survival post-challenge, IgG titers between survivors and non-survivors were compared (Fig. 5). Titers from groups that received Alum adjuvanted vaccines were excluded from this analysis due to the very high titers elicited by these formulations (Figs. 1, 3) and due to an absence of non-survivors in these groups (Figs. 2, 4). In the initial study, significant differences were found between the titers of survivors and non-survivors after dose 2. These differences were even more marked post-challenge (Fig. 5A). Very similar results were seen in this second study, although the differences were not significant until after the 3rd dose. Again, after lethal challenge the differences in EBOV GP-specific titers between animals that survived the challenge and those that did not is highly significant (Fig. 5B). The best protection in guinea pigs was seen in all formulations using Alum as an adjuvant. Equally high efficacy was also seen with a formulation containing CoVaccine HT™ and all three antigens in the proper balance.
Figure 5: Correlation between immune response and survival.
Geometric mean ELISA titers of EBOV GP-specific IgG in guinea pig serum samples following each vaccine dose from animals in the first study (A) and the second study (B) stratified by survival. Blood was collected 3 weeks following each vaccination. Only titers from groups not using Alum as an adjuvant were used since no animals in Alum-containing groups succumbed to infection. Statistical significance was determined using an unpaired t-test (*p<0.05, **p<0.01, ***p<0.001).
4. Discussion
The expression of conformationally correct recombinant individual EBOV GP, VP24, and VP40 proteins using Drosophila S2 cells has been previously described by our group [15]. The resulting secreted GP contains N-linked glycosylations and is highly immunogenic. Further purification of the immunoaffinity chromatography (IAC) purified EBOV GP using size-exclusion chromatography shows three distinct populations, constituting monomeric GP, trimeric GP and HMW aggregates of GP. Trimeric GP is the form typically found on the surface of the virion [22, 23] and, therefore, the preferred vaccine antigen. Based on native viral structure, trimeric and higher oligomeric assemblies of GP are logically the most relevant for vaccine production. Thus, they were separated by size exclusion chromatography and each used for vaccine formulation to compare the immunogenicity and efficacy of these fractions with GP of mixed sizes in guinea pigs. The additional EBOV proteins VP24 and VP40 were added due to their ability to elicit strong cell-mediated and humoral responses in mice and contribute to protection against viral challenge [15, 24]. The amount of VP40 was reduced in most of the formulations based on observations of significantly higher humoral responses against this antigen in comparison to GP and VP24 in an attempt to properly balance the antigenic composition. Three different clinically relevant adjuvants were selected for this study to determine which elicited the best immunogenicity and highest protection. All three selected adjuvants have previously been shown to be safe, immunogenic and efficacious in subunit and other vaccines [15, 25–27]. ELISA titers following the initial dose showed the highest EBOV GP-specific titers for all animals receiving formulations with Alum as an adjuvant (Fig. 1), which was not unexpected considering Alum’s ability to elicit strong humoral responses [26, 27]. Titers in the groups containing GPI-0100 and CoVaccine HT™ rose above the cut-off by the second dose and for the animals receiving formulations with CoVaccine HT™ were not significantly different from the Alum-adjuvanted animals after the third dose. No significant differences were found between animals receiving formulations containing the HMW, trimeric or mixed GP suggesting no difference in the quality of the immune response elicited by these different oligomeric species (Fig. 1). However, and while not significant, GP-specific IgG titers were slightly lower for the HMW GP group compared to the others (Fig. 1, S5A).
Protection within the different adjuvant groups varied, with all animals in the CoVaccine HT™ adjuvant groups being protected against lethal challenge with the exception of animals that received equal amounts of all three antigens (Fig. 2A). Antibody titers were shown to be significantly higher in challenge survivors compared to non-survivors (Fig. 5). Previous work showed that VP40 is able to elicit extremely high, but not necessarily protective antibody responses in mice [15]. Other studies have found that VP40 plays a protective role, but that this role is based on its ability to elicit a cell mediated response [24, 28]. It is possible that formation of an immune response against VP40 interferes with the development of a protective EBOV GP-specific response and highlights the importance of proper antigen formulation to produce a balanced response. Our data support this hypothesis as we see a significant decrease of EBOV GP-specific IgG in the GPI-0100 and Alum groups when VP40 is present in the vaccine formulation (Fig. S5B, C). However, there was no difference in protective efficacy between the Alum groups as all the animals survived lethal challenge (Fig. 2C). In contrast, it might have impacted the lower survival rate in the GPI-0100 groups (Fig. 2B). Amongst the animals receiving vaccine formulations with GPI-0100, low levels of protection were seen with only a maximum of 50% of the animals surviving challenge (Fig. 2B) and no survival seen in males despite previous promising results with this adjuvant in mice [15, 25]. The observed lower efficacy corresponds to the significantly lower levels of EBOV GP-specific antibody titers that were seen in all these animals (Fig. 1). We speculate that the adjuvant’s activity as a surfactant may have caused significant degradation of the EBOV GP prior to administration (3–4 days after formulation) resulting in a lower quality of the immune response. In the animals receiving Alum adjuvanted formulations uniform protection was observed with all antigen combinations (Fig. 2C), corresponding to the rapid and robust antibody responses seen in these animals.
Due to a documented difference in immune responses to infection and vaccination between sexes [29] as well as a noted trend in our initial experiments, with females faring better than males post-challenge we conducted another sex-balanced immunogenicity and efficacy experiment. In this study we also determined the efficacy of EBOV GP alone with CoVaccine HT™. The immunogenicity results in this experiment were very similar to what was seen in corresponding groups during the initial experiment, however, survival in the group that received GP alone with CoVaccine HT™ was lower (68.7%) than was seen when CoVaccine HT™ was used with all three antigens (Fig. 2A). This second experiment also further highlighted the potent immunogenicity and efficacy of EBOV proteins adjuvanted with Alum in guinea pigs since all animals in these groups survived challenge.
Previous efforts to develop subunit-based filovirus vaccines have met with mixed results. Research using GP fused to the FC portion of human IgG1 showed that properly adjuvanted GP-Fc was fully protective in guinea pigs [27]. In contrast to our own findings, this manuscript reported that Alum as an adjuvant did not uniformly protect when paired with their GP construct. Baculovirus-expressed GP has been shown to induce both humoral and cell-mediated immune responses but was also not able to fully protect against lethal challenge in guinea pigs [30]. However, it is unclear if an adjuvant was included in the vaccine formulations used in this study. Both of these studies as well as others have shown high levels of antibody production in response to vaccination in small animal as well as NHP models [15, 27, 30, 31] which have not consistently correlated to protection. In contrast, our study was able to correlate EBOV GP-specific antibody levels with protection against challenge, as animals surviving lethal challenge showed significantly higher IgG titers than those that did not (post-dose 2 through post-challenge time points) (Fig. 5). This points to the important role that humoral immunity plays in protection against EBOV infection; however, this does not diminish the potential importance of cell-mediated immunity, especially the cell-mediated responses elicited by VP24 and VP40. Although EBOV antigen-specific cell-mediated immunity was not explored further in this report, it has previously been documented in other animal species by our group as well as others [15, 24, 28].
Altogether, our recombinant subunit based vaccine candidate has shown complete protection against EBOV challenge in two small animal models, mice [15] and guinea pigs when using proper antigen and adjuvant combinations. Studies in NHP models are the next logical step to further assess efficacy, refine formulations, and explore correlates of protection for EBOV subunit vaccines.
Supplementary Material
Acknowledgements
The authors are grateful to the BSL4 animal care and veterinary staff of the Rocky Mountain Veterinary Branch for their support of this study. The immunogenicity portion of the first study was performed via DMID Preclinical Services (NIH/NIAID). This work was funded in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, by a grant from NIH/NIAID (1R43AI066616), and through corporate funds from PanThera Biopharma, LLC. We also greatly acknowledge the gift of adjuvants CoVaccine HT™ from BTG International (London, UK) and GPI-0100 from Hawaii Biotech, Inc. (Honolulu, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Ebola haemorrhagic fever in Zaire, 1976. Bulletin of the World Health Organization. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- [2].Ebola haemorrhagic fever in Sudan, 1976. Bulletin of the World Health Organization. 1978;56:247–70. [PMC free article] [PubMed] [Google Scholar]
- [3].Baseler L, Chertow DS, Johnson KM, Feldmann H, Morens DM. The Pathogenesis of Ebola Virus Disease. Annu Rev Pathol. 2017;12:387–418. [DOI] [PubMed] [Google Scholar]
- [4].Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat Rev Immunol. 2003;3:677–85. [DOI] [PubMed] [Google Scholar]
- [5].Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4:487–98. [DOI] [PubMed] [Google Scholar]
- [6].Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in West Africa. WHO statement; 2014. [Google Scholar]
- [7].Organization WH. Ebola Situation Report. 2016.
- [8].Wong G, Mendoza EJ, Plummer FA, Gao GF, Kobinger GP, Qiu X. From bench to almost bedside: the long road to a licensed Ebola virus vaccine. Expert Opin Biol Ther. 2018;18:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schieffelin JS. An effective and safe vaccine will not be enough to prepare us for the next Ebola outbreak. The Lancet Infectious Diseases. 2017;17:1224–5. [DOI] [PubMed] [Google Scholar]
- [11].To A, Medina LO, Mfuh KO, Lieberman MM, Wong TAS, Namekar M, et al. Recombinant Zika Virus Subunits Are Immunogenic and Efficacious in Mice. mSphere. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28:2705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lieberman MM, Nerurkar VR, Luo H, Cropp B, Carrion R Jr., de la Garza M, et al. Immunogenicity and protective efficacy of a recombinant subunit West Nile virus vaccine in rhesus monkeys. Clin Vaccine Immunol. 2009;16:1332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Medina LO, To A, Lieberman MM, Wong TAS, Namekar M, Nakano E, et al. A Recombinant Subunit Based Zika Virus Vaccine Is Efficacious in Non-human Primates. Frontiers in Immunology. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lehrer AT, Wong TS, Lieberman MM, Humphreys T, Clements DE, Bakken RR, et al. Recombinant proteins of Zaire ebolavirus induce potent humoral and cellular immune responses and protect against live virus infection in mice. Vaccine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hilgers LA, Blom AG. Sucrose fatty acid sulphate esters as novel vaccine adjuvant. Vaccine. 2006;24 Suppl 2:S2–81–2. [DOI] [PubMed] [Google Scholar]
- [19].Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak V, Gundy LE, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–51. [DOI] [PubMed] [Google Scholar]
- [20].Marciani DJ, Reynolds RC, Pathak AK, Finley-Woodman K, May RD. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine. 2003;21:3961–71. [DOI] [PubMed] [Google Scholar]
- [21].Connolly BM, Steele KE, Davis KJ, Geisbert TW, Kell WM, Jaax NK, et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis. 1999;179 Suppl 1:S203–17. [DOI] [PubMed] [Google Scholar]
- [22].Sanchez A, Yang ZY, Xu L, Nabel GJ, Crews T, Peters CJ. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J Virol. 1998;72:6442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Olinger GG, Bailey MA, Dye JM, Bakken R, Kuehne A, Kondig J, et al. Protective cytotoxic T-cell responses induced by venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol. 2005;79:14189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu H, Bungener L, ter Veer W, Coller BA, Wilschut J, Huckriede A. Preclinical evaluation of the saponin derivative GPI-0100 as an immunostimulating and dose-sparing adjuvant for pandemic influenza vaccines. Vaccine. 2011;29:2037–43. [DOI] [PubMed] [Google Scholar]
- [26].Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Konduru K, Shurtleff AC, Bradfute SB, Nakamura S, Bavari S, Kaplan G. Ebolavirus Glycoprotein Fc Fusion Protein Protects Guinea Pigs against Lethal Challenge. PLoS One. 2016;11:e0162446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wilson JA, Bray M, Bakken R, Hart MK. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology. 2001;286:384–90. [DOI] [PubMed] [Google Scholar]
- [29].Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- [30].Mellquist-Riemenschneider JL, Garrison AR, Geisbert JB, Saikh KU, Heidebrink KD, Jahrling PB, et al. Comparison of the protective efficacy of DNA and baculovirus-derived protein vaccines for EBOLA virus in guinea pigs. Virus Res. 2003;92:187–93. [DOI] [PubMed] [Google Scholar]
- [31].Warfield KL, Howell KA, Vu H, Geisbert J, Wong G, Shulenin S, et al. Role of Antibodies in Protection Against Ebola Virus in Nonhuman Primates Immunized With Three Vaccine Platforms. J Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.