Abstract
Purpose:
Gastrointestinal stromal tumors (GIST) are resistant to cytotoxic and radiation therapy. Most GIST in children are wild-type for KIT and PDGFRA (WT GIST) and deficient in expression of succinate dehydrogenase (dSDH GIST). We tested the activity of vandetanib, an oral small molecule inhibitor of VEGFR2, EGFR, and RET, in patients with dSDH GIST.
Experimental Design:
Phase II study of vandetanib (300 mg orally once daily to patients ≥18 years, and 100 mg/m2/dose to patients < 18 years) on a continuous dosing schedule (1 cycle =28 days) to assess the clinical activity (partial and complete response rate RECIST v1.1) in patients with dSDH GIST. A Simon optimal two-stage design (target response rate 25%, rule out 5%) was used: If ≥1/9 patients in stage 1 responded, enrollment would be expanded to 24 patients, and if ≥3/24 responded, vandetanib would be considered active.
Results:
Nine patients (7F:2M), (median age 24 years, range 11–52) with metastatic disease enrolled. Three of the initial five adult patients developed treatment modifying toxicities. After a protocol amendment two adults received vandetanib at 200 mg/dose with improved tolerability. The two children (<18 years old) enrolled did not experience treatment modifying toxicities. No partial or complete responses were observed (median number of cycles 4, range 2–18).
Conclusions:
Vandetanib at a dose of 300mg daily was not well tolerated by adults with dSDH GIST. Two of 9 patients had prolonged stable disease, but no partial or complete responses were observed and vandetanib is thus not considered active in dSDH GIST.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract with an annual incidence of approximately ten per million [1–3]. The primary therapy for GIST is surgical and the tumor is resistant to both cytotoxic chemotherapy and radiation therapy[4]. The majority of GIST in adult patients harbor activating mutations in KIT or PDGFRA and can be effectively treated with KIT-targeting tyrosine kinase inhibitors (TKI) [5]. However, 85% of GIST in pediatric patients as well as 10–15% in adults are wild-type for both KIT and PDGFRA[6] and KIT-targeting TKIs such as imatinib have minimal efficacy for this group. WT GIST are primarily due to succinate dehydrogenase (SDH) deficiency because of mutations in one of the subunits of the SDH complex or lack of expression of SDHC due to hypermethylation of the SDHC promoter (epimutant) [5, 7]. SDH-deficient GIST (dSDH GIST) have a gastric predilection, increased incidence in females, and frequent multifocal presentation[6]. They are typically indolent; however, metastatic disease can cause significant morbidity.
SDH (as SDH-ubiquinone complex II) is a component of the Krebs cycle and the respiratory chain and is composed of four subunits (A, B, C and D). A group of SDH-deficient tumors are now recognized including approximately 30–40% of hereditary paragangliomas[8], a small subset of GIST[9], and rare renal cell carcinomas[10]. In dSDH GIST, SDHB protein expression evaluated using immunohistochemistry (IHC) is markedly decreased or absent[9]. This unique feature brings up the consideration of using SDHB IHC early in the GIST diagnostic process. The specific mechanism of tumorigenesis in SDH-deficient tumors is not known. However, a number of metabolic derangements have been characterized. An autosomal dominant inherited tumor predisposition syndrome that includes gastric GIST as well as paragangliomas was reported in 2002 and the underlying mutations in SDH subunits were subsequently identified [9, 11, 12]. The SDH complex is a component of the Krebs cycle and electron transport chain catalyzing the oxidation of succinate to fumarate. Impaired SDH activity leads to accumulation of succinate within the cell causing a constellation of metabolic changes. The family of α-ketoglutarate-dependent dioxygenases is inhibited by succinate. Among this family of enzymes is the hypoxia-inducible factor-α prolyl-hydroxylase. Inhibition of HIF-1α prolyl hydroxylase leads to von Hippel Lindau-independent stabilization of HIF-1α and constitutive activation of hypoxia signaling[13–15]. This causes increased expression of downstream targets such us EGFR and VEGF[14]. There is a lack of preclinical models of dSDH GIST, however vandetanib has been shown to inhibit cell growth in preclinical models of fumarate hydratase deficient renal cell cancer, another tumor associated with a Krebs cycle enzyme deficiency and increased levels of HIF-1α[16]. This suggests that drugs targeting HIF-1α dependent processes may have a role in the treatment of dSDH GIST. Vandetanib (CAPRELSA®; ZD6474; Sanofi Genzyme) is a small molecule receptor tyrosine kinase (RTK) inhibitor, given as a once-daily oral drug that inhibits VEGFR2- and EGFR- dependent signaling. Vandetanib has activity in medullary thyroid carcinoma in adults at doses ranging from 100 to 300 mg once daily on a continuous dosing schedule and in children receiving 100–150 mg/m2/dose[17, 18]. To test the activity of vandetanib in dSDH GIST a small two stage phase 2 study was performed.
Materials and Methods
Patient population
Patients ≥ 3 years of age with histologically confirmed GIST with the absence of KIT and PDGFRA mutation and measurable disease were eligible. Disease progression at the time of study entry was not required for eligibility. Other eligibility criteria included recovery from toxic effects of prior therapy, Karnofsky/Lansky performance score ≥50%; interval from prior therapy ≥4 weeks from prior surgical procedures with complete healing of surgical sites, ≥28 days from a last dose of cytotoxic chemotherapy and at least 7 days from prior biological therapy including immunomodulatory agents, vaccines, and differentiating agent; at least 30 days from a prior dose of a monoclonal antibody or any investigational agent; ≥4 weeks from external beam radiation therapy. Patients must have recovered from the acute toxic effects of prior therapy to grade 1. Patients were required to have normal organ and marrow function including adequate renal function [age-adjusted normal serum creatinine, or a creatinine clearance ≥50 mL/min/1.73m2]; and adequate liver function [total bilirubin ≤ 1.5x institutional upper limit of normal (ULN) (in patients with documented Gilbert’s Disease an elevated bilirubin was not an exclusion criteria), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 2.5x ULN]. AST and ALT could be up to 5x ULN in patients with hepatic metastases. Adequate bone marrow function was required and defined as an absolute neutrophil count (ANC) ≥ 1,500/μL and transfusion independent platelet count of ≥100,000/μL. Participants 18 years of age and younger were required to have a blood pressure ≤95th percentile for age, height, and gender without any treatment for hypertension. In adult patients preexisting hypertension was required to be controlled for enrollment. Adult patients with blood pressure >160mmHg systolic or > 100 mmHg diastolic who were unable to achieve blood pressure control with anti-hypertensive therapy were excluded.
This trial conformed to the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the NCI Institutional Review Board. Investigators obtained written consent from all patients or their legal guardians indicating their understanding of the investigational nature and risks of this study. Assent was obtained according to institutional guidelines.
Drug administration and study design
The study was conducted as a small, Simon optimal two stage phase II trial in order to rule out an unacceptably low overall response rate of 5% (ORR; p0=0.05), in favor of a response rate of 25% (p1=0.25). With alpha=0.10 and beta=0.10, if ≥1 out of 9 patients in stage 1 responded, enrollment would be expanded to 24 patients, and if ≥3 out of 24 responded, vandetanib would be considered active. Vandetanib was supplied by AstraZeneca to the NCI and administered under an investigator held IND (BB-IND 77570). Vandetanib was administered orally once a day on a continuous dosing schedule. The planned cycle duration was 28 days. Patients 18 years of age and older were started on a fixed dose of 200mg once daily with a planned increase in vandetanib dose to 300mg daily after the third cycle if the drug was tolerated. Patients younger than 18 years of age at the time of enrollment were started at a dose of 100 mg/m2 based on a dosing nomogram with a planned increase in the dose to 150mg/m2/day after the third cycle if the drug was tolerated.
Toxicity assessment and disease evaluations
Monitoring for vandetanib-related toxicity included physical examination with blood pressure measurement as well as complete blood count (CBC) with differential; serum chemistries including electrolytes, calcium, phosphate, magnesium, creatinine, glucose, blood urea nitrogen, albumin, AST, ALT, total bilirubin, and total protein at baseline, day 14 of cycle 1, prior to cycle 2, 3, and 4 and subsequently after every third cycle. Thyroid function testing, urinalysis, and electrocardiogram were performed at baseline and prior to cycles 2, 3, and 4 and then following every third cycle thereafter. Pregnancy testing was done in post-pubertal female patients and unilateral knee MRI to assess for growth plate toxicity in patients with open growth plates was performed at baseline and prior to cycles 4, 7, 10, 13 and then after every sixth cycle. PT and PTT were performed at baseline. Adverse events were graded according to the Common Toxicity Criteria v. 4.
Response was evaluated using Response Criteria in Solid Tumors guideline version 1.1[19] at baseline and prior to cycles 4, 7, 10 and 13 and then after every 6th cycle. Assessment of disease was performed using radiological evaluation which could include CT scan of the chest, abdomen and pelvis and primary tumor, MRI of the abdomen/pelvis. A consistent method of disease evaluation was used for each patient throughout the study. FDG-PET was performed on all patients at baseline and prior to cycle 4 and an optional PET scan was performed on adult patients on day 3 to 6 of cycle 1. CT attenuation coefficient (density) was measured using VuePACS version 12.2.2.0105. An average pixel value was determined over a region of interest of each target lesion and average CT density was calculated.
Definition of treatment limiting toxicity (TLT)
Hematologic TLT was defined as grade 3 neutropenia (<1000/µL) on two consecutive measurements drawn at least 72 hours apart or any grade 4 neutropenia (neutrophil count below 500/μL); thrombocytopenia (<50,000/µL) (grade 3) on two consecutive measurements drawn at least 72 hours apart or any grade 4 thrombocytopenia (<25,000/μL); grade 3 or 4 decrease in hemoglobin that could not be corrected to at least 8.0 g/dL (grade 2). Grade 3 or 4 leucopenia or lymphopenia was not considered a TLT. Any non-hematologic toxicity grade 3 or higher was considered treatment-limiting with the exception of grade 3 nausea or vomiting that was controlled with anti-emetics within 48 hours, any grade 3 diarrhea that was tumor-related or vandetanib-related but controlled by symptomatic treatment within 48 hours, grade 3 AST or ALT elevation that returned to grade 2 or less within 14 days of holding drug and did not recur with reinstitution of the drug, or grade 3 electrolyte abnormalities that were asymptomatic and correctable to grade 2 within 48 hours. Treatment limiting hypertension was defined as previously described[20]. Treatment limiting QTc prolongation was defined as a single QTc value ≥500msec. Compliance was assessed by diary which was evaluated at each clinic visit.
Quality of Life Assessment
Health-related quality of life (QoL) was evaluated by patient and parent report (for pediatric patients) using PROMIS short form measures (anxiety, depression, fatigue, pain interference and physical function). The measures were administered to all consenting English or Spanish-speaking patients with parallel Parent Proxy instruments administered to parents of patients ages 8–17. These instruments were administered at baseline, at the first re-staging visit (3 months) and at the time a patient was taken off treatment.
Results
Patient characteristics
Nine patients (2 male and 7 female; median age, 24 years [range 11–52]) were enrolled from May 7, 2014 to June 11, 2015. Table 1 provides a summary of demographic, clinical, and baseline disease characteristics. All patients had SDH-deficient tumors as determined by genomic analysis or tumor immunohistochemistry showing negative staining for SDHB. Two patients had tumors with loss of SDHB by immunohistochemistry but no identified SDH subunit mutation suggesting that these patients had loss of SDHC due to hypermethylation of the SDHC promoter (SDHC-epimutant tumors) as previously described[21]. Eight of 9 patients had received prior tyrosine kinase inhibitor (TKI) therapy including imatinib (7), sunitinib (5), and regorafenib (3). All patients had a gastric primary tumor and sites of disseminated disease included hepatic, pulmonary, peritoneal, and lymph node metastases.
Table 1:
Baseline and treatment characteristics
| Patient Number | Age (years) | Gender (M, F) | Sites of disease | SDHB IHC | SDH subunit mutation | Treatment Cycles (Number) |
|---|---|---|---|---|---|---|
| 1 | 35 | M | liver, peritoneum, lymph nodes | NEG | SDHA | 3 |
| 2 | 19 | F | liver, stomach | NEG | SDHC | 18 |
| 3 | 52 | F | lung, liver peritoneum, lymph nodes | NEG | SDHA | 2 |
| 4 | 39 | F | liver, lymph nodes | NEG | SDHA | 13 |
| 5 | 24 | F | liver | NEG | SDHC | 3 |
| 6 | 11 | F | liver, lymph nodes, spleen | NEG | SDHB | 3 |
| 7 | 21 | F | liver, peritoneum | NEG | Wild type | 6 |
| 8 | 27 | M | lungs, liver, spleen, peritoneum, lymph nodes | NEG | SDHA | 4 |
| 9 | 14 | F | liver, peritoneum, spleen | NEG | Wild type | 4 |
Abbreviations: M=male; F=female; Immunohistochemistry=IHC; SDHB IHC=Succinate dehydrogenase B immunohistochemistry; SDH = succinate dehydrogenase
Toxic Effects and duration of Treatment
Three of five adult patients initially treated at a vandetanib dose of 300mg/dose developed TLT: Grade (gr) 3 hypertension (n=1), grade 2 seizure (n=1), grade 3 pneumonitis (n=1), gr 2–3 abdominal pain (Table 2). The 300 mg dose was thus not tolerable, and following a protocol amendment the two subsequent adults received vandetanib at 200 mg/dose with improved tolerability and no TLT. The two children enrolled did not experience toxicities requiring dose changes. No partial or complete responses were observed and the best response was stable disease (median number of completed cycles 4, range 2–18. Three patients were taken off study at the request of the patient and six patients were taken off study for progressive disease. Toxicities possibly, probably, or definitely related to vandetanib requiring dose reduction in later cycles included gr 2 pruritus intolerable to the patient (cycle #7) and gr3 diarrhea (cycle#6)
Table 2:
Number of patients (highest grade/patient) with possibly, probably, or definitively grade 2,3 or 4 vandetanib related toxicities.
| Toxicity Grade CTCAEv4 | 2 | 3 | 4 |
|---|---|---|---|
| Gastrointestinal Toxicity | |||
| nausea | 1 | ||
| diarrhea | 1 | 1 | |
| oral mucositis | 1 | ||
| stomach pain | 1 | ||
| Hepatic Toxicity | |||
| alkaline phosphatase ↑ | 2 | ||
| AST ↑ | 1 | ||
| ALT ↑ | 1 | ||
| Metabolic/Laboratory Toxicity | |||
| calcium ↓ | 1 | ||
| glucose ↑ | 1 | ||
| Constitutional Toxicity | |||
| fatigue | 1 | ||
| anorexia | 2 | ||
| Hematologic Toxicity | |||
| Lymphocyte Count ↓ | 1 | 1 | |
| Skin Toxicity | |||
| rash acneiform | 2 | ||
| pruritis | 1 | ||
| Neurologic Toxicity | |||
| headache | 1 | ||
| seizure | 1 | ||
| Genitourinary Toxicity | |||
| proteinuria | 2 | ||
| Vascular disorders | |||
| hypertension | 4 | 2 | |
| Pulmonary | |||
| pneumonitis | 1 | ||
| dyspnea | 1 | ||
| hypoxia | 1 |
Response Evaluation
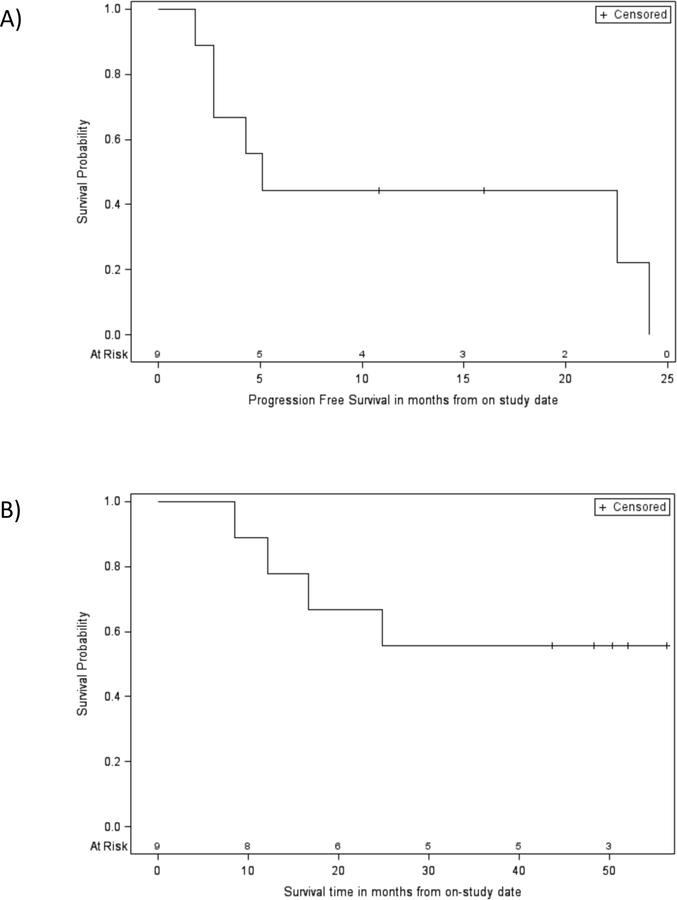
Because there were no partial or complete responses observed the study was terminated after stage 1. In the six patients who were taken off study for progressive disease the Growth Modulation index (GMI) was assessed and ranged from .28 to 1.3 (Table 3). All four adult patients who underwent the optional PET scan had a decrease in SUVmax in the time frame of day 3 through 6 of cycle 1. Eight patients were evaluated with FDG-PET prior to cycle 4. Three of eight patients had a decrease in SUVmax prior to cycle 4 compared to baseline (Table 3). Neither decreases in SUVmax at day 3–6 of cycle 1 nor prior to cycle 4 predicted long-term stabilization of disease. Three patients continued therapy for at least 5 cycles and one patient had surgery to remove tumor and discontinued therapy after 13 cycles. Progression free survival and overall survival at twelve months were 44.4% (95% CI: 13.6–71.9%) and 88.9% (95%CU: 43.3–98.4%) respectively (Figure 1). Median progression free survival was 5.1 months (95% CI 1.8–24.1 months).
Table 3:
Growth, FDG-PET, and tumor density evaluation.
| Patient number | Time to progression (months) | Growth modulation index | Pretreatment FDG-PET SUVmax | Day 3–6 FDG-PET SUVmax | Pre-cycle 4 FDG-PET SUVmax | Pretreatment Mean Density (HU) | First Restaging Mean Density (HU) |
|---|---|---|---|---|---|---|---|
| 1 | 2.7 | .63 | 30.7 | 19.3 | 11.5 | 36.1 | 49.8 |
| 2 | 22.4 | 1.3 | 15.6 | 7.5 | 8.9 | 103.1 | 122.4 |
| 3 | 2 | 1.3 | 16.2 | NP | NP | 64.6 | 54.7 |
| 4 | NA | NA | 21.4 | NP | 21.4 | 109.5 | 105.0 |
| 5 | 5 | .28 | 17.9 | 10.5 | 19.3 | 122.4 | 115.1 |
| 6 | 2.8 | .82 | 11.6 | NP | 13.8 | 102.2 | 98.8 |
| 7 | NA | NA | 25.3 | NP | 31.4 | 79.6 | 71.7 |
| 8 | 4.3 | .93 | 24.4 | 22.0 | 17.8 | 63.6 | 64.2 |
| 9 | NA | NA | 25.3 | NP | 27.4 | 90.4 | 89.1 |
Abbreviations: FDG-PET=18-Fluoro-deoxyglucose positron emission tomography ; SUVmax = maximum standardized uptake value; HU=Hounsfield units; NA=not available; NP=not performed
Figure 1:
A) Progression free survival. Median PFS: 5.1 months (95% CI: 1.8–24.1 months), 12 month PFS probability: 44.4% (95% CI: 13.6–71.9%); B) Overall survival. Median OS not reached, 12 month OS: 88.9% (95% CI: 43.3–98.4%), 24 month OS: 66.7% (95% CI: 28.2–87.8%),36 month OS: 55.5% (95% CI: 20.4–80.5%).
Quality of Life Evaluation
All nine patients completed the baseline PROMIS measures with 8/9 completing the pre-cycle 4 evaluation and 3/9 the end of therapy evaluation. The small number of patients precluded evaluation of changes in t-scores over time in any of the domains. The baseline evaluations were notable for 6/9 patients endorsing symptoms of anxiety and 5/9 mild or moderate symptoms of depression.
Discussion
The use of imatinib and other KIT and PDGFRA targeting TKIs has dramatically improved the outcome for patients with GIST leading to increases in five-year survival rates in those with advanced disease from 10% to nearly 50%[22, 23]. However, TKIs targeting KIT and PDGFRA have had limited benefits for patients with dSDH GIST. Earlier trials of imatinib included patients with KIT/PDGFRA mutant as well as WT GIST and investigators have subsequently analyzed molecular subgroups of patients treated on these trials. Heinrich and colleagues identified 12 patients with dSHD GIST within a population of 395 participants in a phase 3 SWOG study of imatinib in advanced GIST[24]. In this group, one of twelve patients achieved a partial response and there were no complete responses. In a follow-up analysis of a study of regorafenib in patients with metastatic or unresectable GIST after failure of therapy with imatinib and sunitinib, six patients with dSDH GIST derived clinical benefit (CR, PR, or SD lasting >/= 16 weeks)[25]. Of these six patients, two experienced a PR with one complete metabolic response as measured by FDG-PET. In a retrospective study of 9 pediatric patients (age 11–21 years old) with wild-type GIST treated with sunitinib, a best response of stable disease was observed in 7 patients with a median progression free survival of 15 months (1 to >73 months)[26]. A single patient with SDH-deficient GIST was also reported to have prolonged disease control (17 months) on pazopanib[27]. The paucity of patients with dSDH GIST who achieve a response is a common finding in previous reports as well as this study. The median PFS of 5.1 months that we found is less than that reported for patients receiving regorafenib. However, differences in patient selection as well as the indolent nature of SDH-deficient GIST makes it difficult to determine the impact of treatment in patients with longer periods of disease stabilization. Patients with subtypes of dSDH GIST may have different rates of disease progression and potentially a different response to therapy further complicating the evaluation of these data. Improved characterization of the natural history of the disease in patients with dSDH GIST, including the differentiation of patients with SDH subunit mutations and those with SDHC deficiency due to promoter hypermethylation, will be important in designing future therapeutic trials.
We hypothesized that inhibition of the HIF-1α induced VEGF pathway by vandetanib would decrease the growth of dSDH GIST. However, at the recommended adult dose of 300 mg daily, vandetanib was not well tolerated in adults with dSDH GIST. While not a dose-finding study, three of the initial five adult patients enrolled experienced toxicity requiring dose modification including grade 2 seizure, grade 3 hypertension, grade 3 pneumonitis and grade 2–3 abdominal pain. In later cycles patients developed grade 2 acne intolerable to the patient and grade 3 diarrhea. The inability of patients to tolerate full dose vandetanib has been seen in other studies. In adult patients with medullary thyroid carcinoma and advanced non-small-cell lung cancer 35%−53% of patients required a dose reduction from a starting dose of vandetanib of 300mg daily with diarrhea, hypertension, and rash being common adverse events[17, 28]. Both well described toxicities of vandetanib such as diarrhea and hypertension as well as uncommon toxicities including seizure and pneumonitis were seen on this study. Two of 9 patients experienced grade 3 hypertension. A meta-analysis of cancer patients receiving vandetanib reported a 6.4% incidence of grade 3–4 hypertension in 3154 patients [29]. In a study of 16 pediatric patients with medullary thyroid carcinoma treated with vandetanib no patients had grade 3–4 hypertension in the first two cycles, however, approximately one third of patients developed grade 1–2 hypertension [18]. A single patient developed pneumonitis, a toxicity not commonly associated with vandetanib. However other tyrosine kinase inhibitors with anti-EGFR activity are associated with pneumonitis in patients with non-small cell lung cancer [30]. It is possible that this population of patients could have decreased tolerability of vandetanib. Pharmacokinetic analysis was not included in this study, but variations in drug metabolism could also contribute to differences in drug tolerability. After a protocol amendment vandetanib was tolerated at a dose of 200 mg daily in adults, and no TLT were experienced in 2 pediatric patients enrolled at the recommended pediatric dose of 150 mg/m2/dose.
We did not observe partial responses and stable disease was the best response. No improvement in quality of life was identified. Given the more indolent clinical behavior of dSDH GIST stable disease cannot be clearly attributed to the effect of treatment. The study was designed to identify the more stringent criteria of response by RECIST. While changes in FDG-PET were seen in some patients in response to vandetanib, this did not correspond to longer periods of tumor stabilization (Table 3). The small number of patients on this study makes it difficult to evaluate the significance of these findings, however, the rapid disease progression in several patients with decreased SUVmax at the day 3–6 FDG-PET suggests that vandetanib may impact FDG uptake without impacting the disease course in patients with SDH-deficient GIST. Post-hoc evaluation of the rate of tumor growth before and on therapy with vandetanib as well as changes in tumor density for patients with sequential imaging available prior to enrollment on study was performed. Meaningful changes in the rate of tumor growth or tumor density were not observed with vandetanib treatment (Table 3). The lack of activity of vandetanib does not preclude the possibility that other strategies targeting the HIF-1α pathway may be effective in treating this disease.
Other aspects of the metabolic abnormalities seen in dSDH GIST are being explored as possible therapeutic targets. Increased levels of cellular succinate also lead to global DNA hypermethylation. TET-DNA hydroxylases catalyze the conversion of 5-methylcytosine to 5-hydroxymethycytosine, an important step in DNA demethylation, this leads to a global increase in DNA methylation. This has been supported by showing that clinical SDH-deficient GIST samples have decreased 5-hydroxymethycytosine levels[31] and consequently global epigenetic dysregulation[32]. Strategies for targeting DNA hypermethylation in the treatment of patients with dSDH GIST are currently being tested.
While SDH-deficient GIST is often an indolent entity, inexorable progression leads to significant morbidity and patients continue to succumb to the disease. One of the most significant challenges for development of new therapies in this rare disease is the lack of representative preclinical models. While there are several groups currently pursuing this issue, clinical trials have been designed mostly on the basis of hypotheses that could not be completely tested in the laboratory. Continued improvement in our understanding of the molecular consequences of cellular SDH-deficiency as well as a more complete understanding of the natural history of these diseases is also needed. Ongoing studies are taking SDH deficiency mechanisms as a starting point to identify therapeutic targets and predictive biomarkers that allow the design of innovative therapeutic strategies. Collaborative initiatives are critical in order to detect associations and draw conclusions from the clinical history of these patients, identify therapeutic targets and predictive biomarkers, and design and evaluate innovative therapeutic strategies for patients with dSDH GIST.
Statement of Translational Relevance.
Approximately 85% of GIST in pediatric patients are wild-type for both KIT and PDGFRA and have limited response to KIT inhibitors such as imatinib. The majority of these tumors are deficient in succinate dehydrogenase (SDH) due to genetic or epigenetic mechanisms (dSDH GIST). In preclinical models, SDH deficiency leads to increased levels of HIF-1α. We hypothesized that inhibition of the HIF-1α induced VEGF pathway would decrease the growth of dSDH GIST. Vandetanib, an orally available TKI with activity targeting VEGFR2 was tested in patients with dSDH GIST. No complete or partial responses were seen. Novel therapies are needed for this subgroup of patient.
Acknowledgments
This work was supported, in part, by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (BW, FA, JG, MA, WML, RS, PM, KK, JD, LH, LW, MM). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosure and potential conflicts of interest: JKK is an employee of Foundation Medicine,
References
- 1.Verschoor AJ, et al. , The incidence, mutational status, risk classification and referral pattern of gastro-intestinal stromal tumours in the Netherlands: a nationwide pathology registry (PALGA) study. Virchows Arch, 2018. 472(2): p. 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang NJ, et al. , The epidemiology of gastrointestinal stromal tumors in Taiwan, 1998–2008: a nation-wide cancer registry-based study. BMC Cancer, 2014. 14: p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma GL, et al. , Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev, 2015. 24(1): p. 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joensuu H, et al. , Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol, 2012. 13(3): p. 265–74. [DOI] [PubMed] [Google Scholar]
- 5.von Mehren M and Joensuu H, Gastrointestinal Stromal Tumors. J Clin Oncol, 2018. 36(2): p. 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boikos SA, et al. , Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol, 2016. 2(7): p. 922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei L, et al. , Gastrointestinal Stromal Tumors: The GIST of Precision Medicine. Trends Cancer, 2018. 4(1): p. 74–91. [DOI] [PubMed] [Google Scholar]
- 8.Wong MY, et al. , Clinical Practice Guidance: Surveillance for phaeochromocytoma and paraganglioma in paediatric succinate dehydrogenase gene mutation carriers. Clin Endocrinol (Oxf), 2019. 90(4): p. 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway KA, et al. , Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A, 2011. 108(1): p. 314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricketts CJ, et al. , Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J Urol, 2012. 188(6): p. 2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carney JA and Stratakis CA, Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet, 2002. 108(2): p. 132–9. [DOI] [PubMed] [Google Scholar]
- 12.McWhinney SR, et al. , Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med, 2007. 357(10): p. 1054–6. [DOI] [PubMed] [Google Scholar]
- 13.Favier J, Amar L, and Gimenez-Roqueplo AP, Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol, 2015. 11(2): p. 101–11. [DOI] [PubMed] [Google Scholar]
- 14.Selak MA, et al. , Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell, 2005. 7(1): p. 77–85. [DOI] [PubMed] [Google Scholar]
- 15.Pollard PJ, et al. , Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet, 2005. 14(15): p. 2231–9. [DOI] [PubMed] [Google Scholar]
- 16.Sourbier C, et al. , Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase-deficient cancer. Cancer Cell, 2014. 26(6): p. 840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells SA Jr., et al. , Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol, 2012. 30(2): p. 134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox E, et al. , Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res, 2013. 19(15): p. 4239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 20.Fox E, et al. , A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol, 2010. 28(35): p. 5174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killian JK, et al. , Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med, 2014. 6(268): p. 268ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Oosterom AT, et al. , Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet, 2001. 358(9291): p. 1421–3. [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, et al. , Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med, 2002. 347(7): p. 472–80. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich MC, et al. , Correlation of Long-term Results of Imatinib in Advanced Gastrointestinal Stromal Tumors With Next-Generation Sequencing Results: Analysis of Phase 3 SWOG Intergroup Trial S0033. JAMA Oncol, 2017. 3(7): p. 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Ami E, et al. , Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol, 2016. 27(9): p. 1794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutkowski P, et al. , Treatment of gastrointestinal stromal tumours in paediatric and young adult patients with sunitinib: a multicentre case series. BMC Cancer, 2017. 17(1): p. 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganjoo KN, et al. , A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol, 2014. 25(1): p. 236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoh K, et al. , Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 2017. 5(1): p. 42–50. [DOI] [PubMed] [Google Scholar]
- 29.Qi WX, et al. , Incidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trials. Br J Clin Pharmacol, 2013. 75(4): p. 919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding PN, et al. , Risk of Treatment-Related Toxicities from EGFR Tyrosine Kinase Inhibitors: A Meta-analysis of Clinical Trials of Gefitinib, Erlotinib, and Afatinib in Advanced EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol, 2017. 12(4): p. 633–643. [DOI] [PubMed] [Google Scholar]
- 31.Mason EF and Hornick JL, Succinate dehydrogenase deficiency is associated with decreased 5-hydroxymethylcytosine production in gastrointestinal stromal tumors: implications for mechanisms of tumorigenesis. Mod Pathol, 2013. 26(11): p. 1492–7. [DOI] [PubMed] [Google Scholar]
- 32.Killian JK, et al. , Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov, 2013. 3(6): p. 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]