Abstract
EGFR signaling confers resistance to radiation therapy (RT) and is a validated target in head and neck squamous cell carcinoma (HNSCC). The inhibition of EGFR in combination with RT improves local control and overall survival in these patients, however, therapeutic resistance limits the efficacy of this approach. We therefore sought to identify cellular mechanisms that cause resistance to EGFR inhibition and RT in HNSCC. Though clonal isolation of carcinoma cells exposed to increasing concentrations of cetuximab, we found that resistant cells upregulate pro-survival ErbB3 and AKT signaling. Using EFM-19 cells and confirmatory analysis of protein levels we demonstrate that cetuximab resistance is characterized by enhanced neuregulin expression identifying a novel adaptive mechanism of therapeutic resistance. Inhibition of this autocrine loop with CDX-3379 (an ErbB3 specific antibody), was sufficient to block ErbB3/AKT signaling in cetuximab resistant cells. The combination of CDX-3379 and cetuximab reduced proliferation and survival after RT in several HNSCC cell lines. These in vitro findings were confirmed in xenograft tumor growth experiments including an approach using growth factor supplemented matrigel. In vivo, the delivery of EGFR and ErbB3 antibodies significantly reduced tumor growth in cetuximab resistant FaDu and CAL27 xenografts. In summary this work demonstrates that autocrine NRG ligand secretion is a mechanism for therapeutic resistance to cetuximab and radiation therapy. This cross-resistance to both therapeutic modalities identifies NRG as an actionable therapeutic target for improving treatment regimens in HNSCC.
Keywords: Neuregulin, ErbB3, EGFR, Radiation
INTRODUCTION
The ErbB family of receptor tyrosine kinases (RTKs) is composed of EGFR/ErbB1/HER1, ErbB2/HER2/Neu, ErbB3/HER3 and ErbB4/HER4. These cell surface receptors transmit extracellular signals initiated by growth factor ligands to activate intracellular signaling cascades that stimulate cell growth, proliferation, and survival (1). Epidermal growth factor receptor (EGFR) activation is potently mitogenic and is known to both drive oncogenesis and tumor progression. Over-expression of EGFR is frequently observed in head and neck squamous cell carcinoma (HNSCC) and confers poor prognosis making EGFR a therapeutic target for this disease site.
EGFR activation stimulates diverse downstream cellular signaling through RAS/ERK1/2 (extracellular-signal-regulated kinases 1 and 2), phosphatidylinositol 3-kinase/AKT (protein kinase B), STAT3, protein kinase C (PKC) and SRC, providing survival signals and resistance to both chemotherapy and radiation therapy (2, 3). Cetuximab, a monoclonal antibody that specifically targets EGFR (4), improves local control and survival in patients with HNSCC when delivered concurrently with radiation therapy (5). However, local disease recurrence remains a significant issue in HNSCC, especially in patients without human papillomavirus (HPV) associated disease, and therefore understanding mechanisms of cetuximab resistance may provide insights for optimizing therapy.
Resistance to EGFR targeted antibodies or tyrosine kinase inhibitors has been intensely studied. Broadly, resistance occurs through reactivation of the target (e.g. though mutation), upregulation of parallel or ‘bypass’ receptor signaling, or independent activation of downstream survival signaling pathways (6). In tumors such as EGFR mutant NSCLC, which are dependent on EGFR for survival, the identification of resistance mechanisms has been aided by interrogation of resistant cell cultures with acquired resistance (7). Although cetuximab is effective in reducing tumor growth both in vitro and in vivo, HNSCC responses to this antibody are more limited making the study of therapeutic resistance and the identification of these cellular mechanisms considerably more difficult (8).
In this study, we describe a strategy for clonal isolation of cetuximab resistant squamous carcinoma cells. This approach was successful in selecting for tumor cells with a robust cetuximab resistant phenotype. Subsequent signaling studies revealed that resistance to both cetuximab and radiation therapy is mediated by a secreted neuregulin (NRG) ligand that enables receptor dependent bypass signaling. Translational studies in pre-clinical models suggest that this pathway is actionable and a strategy to block both EGFR and bypass signaling through the NRG receptor, ErbB3, could provide a novel therapeutic approach for radiosensitization of HNSCC.
MATERIALS AND METHODS
Western blot analysis
Cells were treated with cetuximab (30 nM), CDX-3379 (50 nM) or both for 48 hours. Following treatment, western blot analyses were performed as previously described (9). We used the following primary antibodies (see Supplementary Table 1). The nitrocellulose-bound primary antibodies, were incubated for 1 hour with anti–rabbit IgG horseradish peroxidase–linked antibody GE Healthcare–Amersham Pharmacia, Buckinghamshire, U.K.), and were detected by the enhanced chemoluminescence staining ECL/ECL™ Plus (GE Healthcare).
Cell line treatments and cetuximab resistance
In this study, we used the HNSCC FaDu (HTB-43), A431 (CRL-1555), CAL27 (CRL-2095), Detroit562 (CCL-138), UNC7 (CVCL_L891), UNC10 (CVCL_L890) cell lines and the breast EFM-19 cell line (CVCL_0253). FaDu and A431 cell lines were obtained from the American Type Culture Collection (ATCC). All the other HNSCC cell lines were a gift from Dr. Natalia Isaeva (Yale University). The EFM-19 cell line was a gift from Dr. Jonathan R. Pollack (Stanford University School of Medicine). The HPV negative HNSCC cell lines used in this study were chosen because they overexpress EGFR and thus provide good models to study cetuximab resistance. The cells were cultured under standard conditions according to ATCC recommendations, and they were kept in culture no more than 6 months after resuscitation from original stocks. The cell lines A431, FaDu, CAL27 and Detroit562 used in the study were authenticated by the American Type Culture Collection (ATCC) short tandem repeat (STR) profiling. Mycoplasma cell culture contamination was routinely checked and ruled out using a biochemical test (MycoAlert Mycoplasma Detection Kit from Lonza, Rockland, ME USA). For conditioned media experiments, EFM-19 cells (100,000 cells), A431 clones (150,000 cells) and FaDu clones (200,000 cells) were grown in 10-cm dishes for 48 hours and treated with cetuximab (30 nM) and/or CDX-3379 (50 nM) for another 48 hours. Following treatment, medium from untreated and treated EFM-19 and clone cells were transferred to 6-well plates with EFM-19 cells (100,000 cells per well) and incubated for 30 minutes prior to lysis and western blot analysis.
Commercially available monoclonal anti-EGFR cetuximab (CTX) and anti-ErbB3 CDX-3379 (Celldex Therapeutics Inc., New Haven, Connecticut) were used to treat cell cultures and mice. Neuregulin (NRG, 10 ng/mL) was purchased from R&D Systems (rhNRG1-β1, Catalog Number 396-HB/CF) (Minneapolis, MN, USA). Radiation (RT) was administered at room temperature using a Precision X-ray 320-kV orthovoltage unit at a dose rate of 2 Gy/45 seconds with 2 mm aluminum filter. Quality Assurance for the irradiator was performed monthly using a P.T.W. 0.3cm3 Ionization Chamber calibrated to NIST standards and quarterly dosimetry using thermoluminescent dosimeter (TLD)-based or ferrous sulfate- based dosimeters.
Generation of cetuximab resistance cell lines
Cetuximab resistant clonal cell lines were generated by serial exposure to increasing concentrations of the drug. We treated parental A431 (A431-WT) and FaDu (FaDu-WT) cell lines with increasing concentrations of the antibody (CTX) for ~14 weeks (See Fig. 1A). Starting with 30 nM, we doubled the dose every 10 – 14 days until growth in a 1280 nM dose was achieved (cells were serially exposed to 30, 60, 120, 240, 480, 960 and 1280 nM doses of CTX). In parallel, we cultured a set of parental cells without administering cetuximab. Cell cultures with the wild-type or resistant phenotypes were cryopreserved; those with resistance were designated as A431-CR and FaDu-CR. Single cells from parental and resistant cell lines were seeded and selected in medium or 1280 nM cetuximab-containing medium. Clones grown in selection medium were picked, grown in monolayer, and tested by a standard clonogenic assay. From the selection process, two different populations were identified: (a) wild type, cetuximab-sensitive clones from untreated controls and (b) cetuximab-resistant clones from cell lines exposed to increasing cetuximab concentrations.
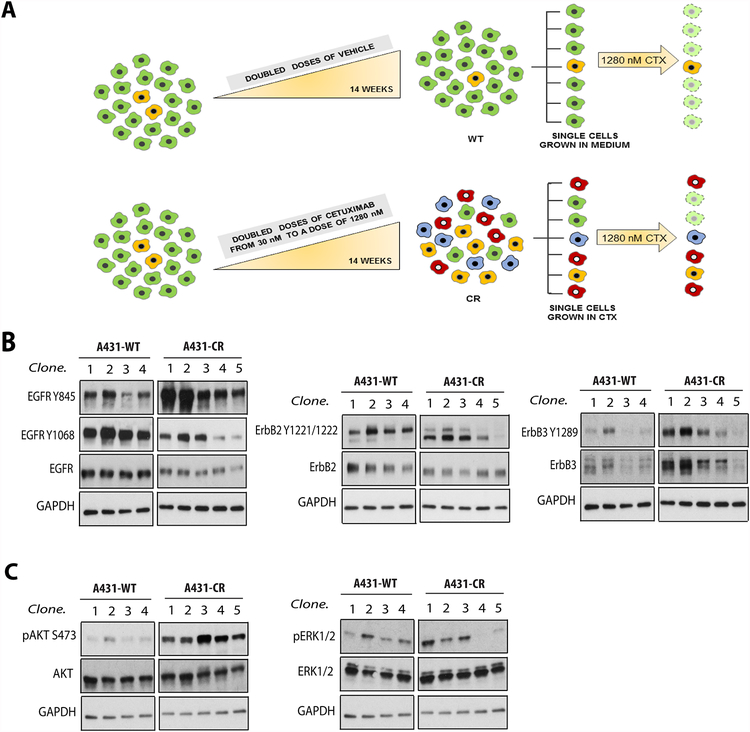
Figure 1. Clonal strategy and screening for cetuximab resistance.
(A.) Overview of the strategy to generate a CR cell line. A431 cells were exposed to increasing concentrations of the antibody over ~14 weeks, then subjected to a selection process to obtain clones. Each shade of grey represents a different clone. (B.) Western blots demonstrating changes in EGFR, ErbB2 and ErbB3 levels and phosphorylation in A431 parental (WT) and cetuximab-resistant (CR) clone cell lines, and (C.) AKT and ERK1/2 phosphorylation and expression. GAPDH was used as a loading control. Data are representative from two experiments.
Clonogenic Survival and Proliferation Assays
Clonogenic survival assays were performed by standard methods in triplicate wells using six-well plates. Growing cells were pretreated for 24 hours in 6-cm tissue culture plates in the presence or absence of cetuximab (30 nM) and/or CDX-3379 (50 nM). For radiation experiments, after being treated in the presence or absence of neuregulin (10 ng/mL) for 30 minutes, cells were either irradiated with a single dose of 4 Gy or mock treated, cultured for 24 hours, and then replated in triplicate wells using six-well plates. Cells were grown for 14 days to produce colonies of >50 cells/colony, washed once with 1 × PBS, and then stained with 0.25% crystal violet in 80% methanol. The surviving fraction (SF) for each sample was calculated as the ratio between the number of colonies counted and the plating efficiency, and normalized to clonogenic survival of CTX or RT+CTX conditions, respectively. For proliferation assays, cells were seeded in 96-well plates and allowed to attach for 24 hours. Cell cultures were then treated with cetuximab (30 nM), CDX-3379 (50 nM) or neuregulin (10 ng/mL) for 0 and 5 days. At each time point, cells were incubated for 4 hours at 37°C with MTT solution at 5 mg/mL, followed by 30 minutes’ incubation with DMSO. Absorbance at 540 nm was measured spectrophotometrically (BioTek Synergy 2; Winooski, VT USA).
Xenograft tumors and in vivo treatments
All experimental procedures were approved in accordance with IACUC and Yale University institutional guidelines for animal care and ethics and guidelines for the welfare and use of animals in cancer research (10). The effects of CTX and CDX-3379 were evaluated in mice bearing xenograft tumors. Six-to-eight-week old female athymic nude mice were purchased from Envigo (New Jersey, USA). Tumors were established by bilateral subcutaneous injection of 1×106 cells into the hind limb. Five days after cell injection, mice were randomized to receive vehicle, cetuximab and/or CDX-3379 I.P., twice a week (treatment schedules are described in the Fig. 6 and Supplementary Fig. S2 legends), except for the NRG in vivo experiments that were treated 24 hours after cell injection. Radiation was administered daily, using a clinical Siemens X-ray 250-kV orthovoltage unit at a dose rate of 6.42 Gy/min with 2 mm aluminum filter, alone or concomitantly with CTX and/or CDX-3379 for 3 or 5 days (treatment schedules are also described in the Fig. 6 legend). Quality Assurance for the irradiator was performed monthly using a P.T.W. 0.3cm3 Ionization Chamber calibrated to NIST standards and quarterly dosimetry using thermoluminescent dosimeter (TLD)-based or ferrous sulfate- based dosimeters. Tumor size was calculated according to the formula π/6 × (large diameter) × (small diameter)2. Mice were sacrificed when the tumor volume reached 1500 mm3, when they suffered moderate to severe toxicities, when significant differences between groups were observed or when less of three animals were followed for tumor volume assessment. No animals were excluded from the experiments.
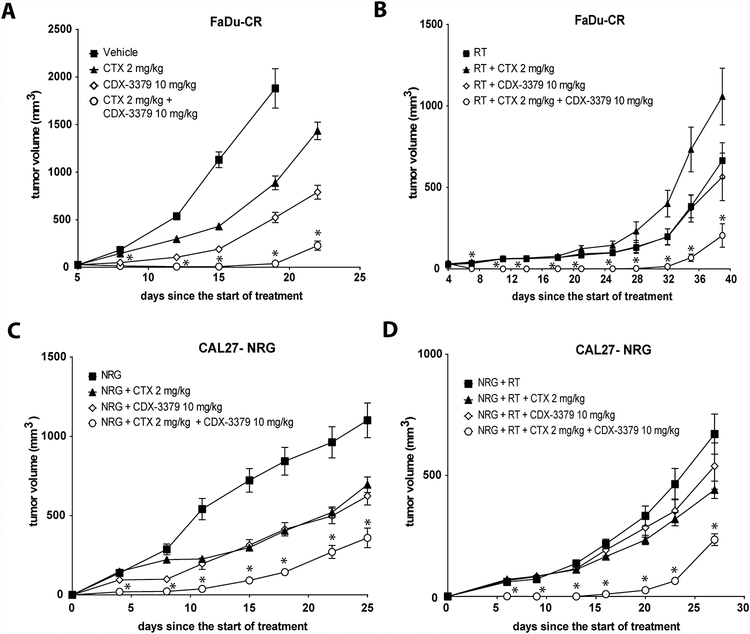
Figure 6. Therapeutic effects of CDX-3379 in vivo in the presence or absence of cetuximab (CTX), neuregulin (NRG) and/or radiotherapy (RT).
(A.) Mice bearing tumors derived from FaDu-CR cells received vehicle, 2 mg/kg CTX twice a week I.P., 10 mg/kg CDX-3379 twice a week I.P., or both for a week. Values are the means ± SE of eight tumors per group. An * indicates a significant difference (p ≤ 0.05) compared to the vehicle, CTX or CDX-3379 group treatments. (B.) Mice bearing tumors derived from FaDu-CR cells received 5 daily doses of RT of 2 Gy to the tumor using 250-kV orthovoltage unit, RT+2 mg/kg CTX twice a week I.P., RT+10 mg/kg CDX-3379 twice a week I.P., or the triple combination for a week. Values are the means ± SE of eight tumors per group. An * indicates a significant difference (p ≤ 0.05) compared to RT, RT+CTX or RT+CDX-3379 group treatments. (C.) Mice bearing tumors derived from CAL27 cells encapsulated in matrigel in the presence of 1 μg per tumor of NRG received a single I.P. injection of vehicle, 2 mg/kg CTX, 10 mg/kg CDX-3379 or both. Values are the means ± SE of eight tumors per group. An * indicates a significant difference (p ≤ 0.05) compared to vehicle, CTX or CDX-3379 group treatments. (D.) Mice bearing tumors derived from CAL27 cells encapsulated in matrigel in the presence of 1 μg per tumor of NRG received 3 daily fractions of RT of 2 Gy to the tumor using 250-kV orthovoltage unit, RT+ a single I.P. injection 2 mg/kg CTX, RT+ a single I.P. injection 10 mg/kg CDX-3379 or RT+ a single I.P. injection of both. Values are the means ± SE of eight tumors per group. An * indicates a significant difference (p ≤ 0.05) compared to RT, RT+CTX or RT+CDX-3379 group treatments.
Statistical analysis
Results are expressed as mean ± standard error (SE) unless otherwise indicated. The Statistical Package for Social Sciences (SPSS, version 13.0) was used for data analysis. Statistically significant differences in between-group comparisons were defined at a significance level of P-value < 0.05 in the Mann-Whitney test.
RESULTS
Cetuximab resistance is associated with enhanced ErbB3/AKT signaling.
To investigate the mechanisms by which cells adapt to EGFR inhibition with CTX, we sought to generate cetuximab-resistant (CR) cell lines. In initial experiments with the FaDu HNSCC cell line, however, we found that escalation of CTX dose in culture over several weeks did not yield a uniformly resistant population of cells. We therefore tested an alternative approach using the A431 epithelial squamous cell carcinoma cell line. This cell line was chosen because it was the EGFR antigen source for generating CTX and is highly sensitive to EGFR blockade by CTX (11, 12). In experiments with A431 cells, CTX resistance was readily achieved by dose escalating antibody exposure in cell culture over ~14 weeks (Fig. 1A) followed by selection of single cells from either control or CTX-treated cultures. Cells selected as resistant were then grown as individual clones and demonstrated improved clonogenic survival after treatment with 1280 nM of CTX (Supplementary Fig. S1A), indicating that this selection method can produce bona fide CR cells.
Several mechanisms of CR have been suggested, including the bypass of EGFR signaling through upregulation of co-expressed receptor tyrosine kinases (RTKs) (13–15). We therefore investigated the phosphorylation and expression levels of ErbB family receptors in A431-WT and -CR clones (Fig. 1B). We found that CR clones had reduced levels of EGFR protein and Y1068 phosphorylation levels compared to the parental clones. Despite these low levels of EGFR, however, CR clones showed significantly increased EGFR Y845 phosphorylation compared to that seen at Y1068. We also observed an increase in ErbB3 protein levels and ErbB3 Y1289 phosphorylation (Fig. 1B, right panel), but no significant changes in ErbB2 or its phosphorylation. Consistent with enhanced ErbB3-mediated signaling through PI 3-kinase, CR clones showed increased AKT phosphorylation levels (Fig. 1C), but ERK activation levels appeared unchanged. Together, these results suggest that resistance to cetuximab is associated with activation of the ErbB3/PI 3-K/AKT pathway.
Cetuximab resistance is driven by autocrine neuregulin
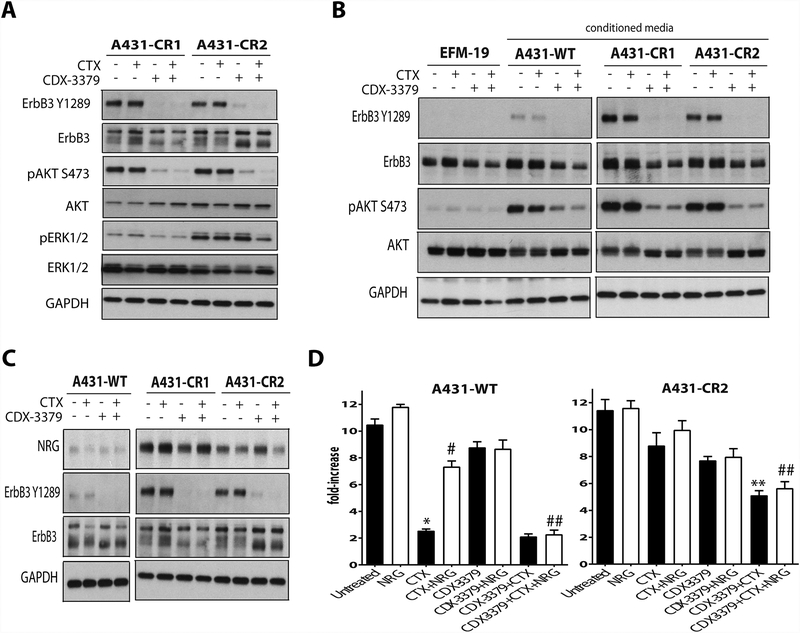
Since our data suggested that CR is caused by activation of ErbB3/PI 3-K/AKT signaling, we next examined the effects of an ErbB3 specific blocking antibody, CDX-3379, on A431-CR clones. CDX-3379 treatment blocked phosphorylation of both ErbB3 and AKT (Fig. 2A), whereas CTX alone had no such effect. Combined treatment with CTX and CDX-3379 also reduced ERK phosphorylation in a CR clone that showed elevated pERK levels at baseline (A431-CR2). To determine whether CR was due to an autocrine ligand that activates ErbB3, we asked whether conditioned medium from our CR cells could activate ErbB3 in EFM-19 breast cells, chosen for this test because they express ErbB3 in an unphosphorylated form (16). As shown in Fig. 2B, conditioned media from A431-WT promoted detectable phosphorylation of ErbB3 on Y1289, but this was dramatically elevated when conditioned medium from A431-CR clones was used. The same trend was seen when AKT phosphorylation was examined. Importantly, CDX-3379 was able to effectively block this survival signaling in each case. These observations suggest that CR clones secrete highly elevated levels of an ErbB3-activating ligand. We therefore evaluated neuregulin (NRG) expression levels in the cells by western blot and found substantially increased NRG amounts in A431-CR clones compared with A431-WT (Fig. 2C). To further assess the contribution of increased NRG secretion to growth of A431-WT and A431-CR cells, proliferation was analyzed following CTX and CDX-3379 treatments (Fig. 2D). Adding NRG had no effect on growth of A431-WT or A431-CR clones, but rescued A431-WT cells from CTX treatment as shown by significantly enhanced proliferation (Fig. 2D; p ≤ 0.05, compared to CTX alone). Similarly, presumably as a result of NRG upregulation, CTX had no significant antiproliferative effect on A431-CR cells. Importantly, a combination of CDX-3379 and CTX could effectively block tumor cell proliferation for A431-CR cells (with increased endogenous NRG) or when exogenous NRG (in the case of A431-WT cells) was added (p ≤ 0.05, compared to CTX alone). Together, these data provide strong evidence that increased NRG production and signaling represents a CTX resistance mechanism that can be targeted therapeutically to reduce tumor cell growth.
Figure 2. Upregulation of neuregulin family ligand expression in A431-CR clones.
(A.) Western blots demonstrating changes in ErbB3, AKT and ERK1/2 phosphorylation and expression in two A431-CR clone cell lines (CR1 and CR2) treated with vehicle, 30 nM CTX, 50 nM CDX-3379, or both for 48 hours. GAPDH was used as a loading control. Representative data from three experiments are shown. (B.) Western blots in EFM-19 cells after the addition ofconditioned media from EFM-19 cell line, A431-WT and two A431-CR clones (CR1 and CR2). Cells were treated with vehicle, 30 nM CTX, 50 nM CDX-3379, or both for 48 hours. GAPDH was used as a loading control. Representative data from three experiments are shown. (C.) Western blots demonstrating changes in neuregulin (NRG) levels and ErbB3 phosphorylation and expression in A431-WT and two A431-CR clone cell lines treated with vehicle, 30 nM CTX, 50 nM CDX-3379, or both for 48 hours. GAPDH was used as a loading control. Representative data from three experiments are shown (D.) Bar graphs representing fold increases in proliferation for A431-WT clone and A431-CR clones after 5 days of drug exposure. Cultures were treated as described in Materials and Methods. The results are mean values ± standard error (SE) for three independent experiments for each cell line. An * indicates a significant difference vs untreated, an ** indicates a significant difference vs CTX, an # indicates a significant difference vs CTX alone, and an ## indicates a significant difference vs CTX + NRG (p ≤ 0.05).
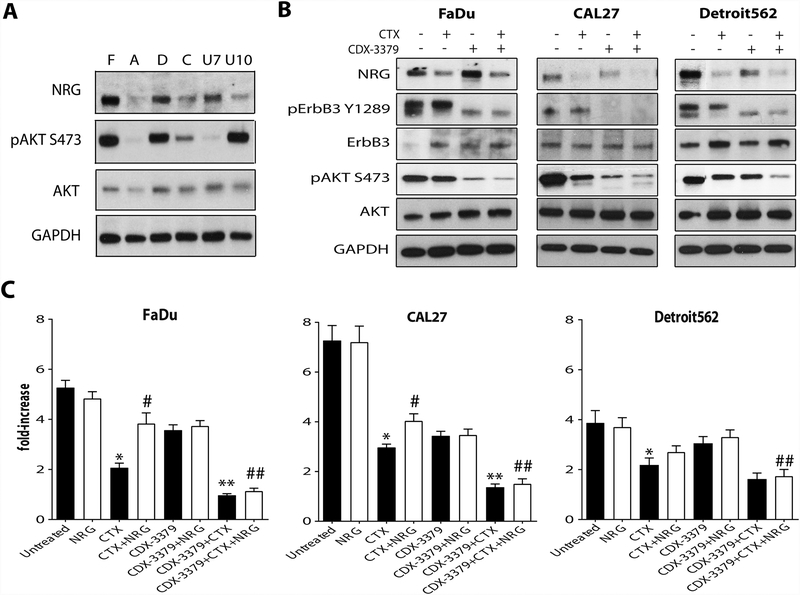
CDX-3379 blocks activation of NRG/ErbB3/AKT signaling in HNSCC cell lines.
The baseline expression level of NRG varies in different HNSCC cell lines (Fig. 3A), as does AKT phosphorylation. FaDu (F) and Detroit562 (D) cells show the highest levels of NRG and AKT phosphorylation, and CAL27 (C) cells have more modest elevation of both. UNC7 (U7) cells exhibit high NRG levels without AKT activation, whereas UNC10 (U10) cells show elevated AKT activation without enhanced NRG – suggesting an alternative mechanism. We examined the effects of CTX and CDX-3379 on ErbB3/AKT signaling in FaDu, CAL27 and Detroit562 cells (Fig. 3B). In each case, as seen with A431-CR cells, CTX alone could not fully inhibit ErbB3/AKT signaling. Interestingly, we found that CTX did have some effect, associated with a reduction of autocrine NRG levels – consistent with inhibition of an EGFR dependent autocrine loop. More significant inhibition of ErbB3 and AKT phosphorylation was achieved with CDX-3379 or the combination of CDX-3379 and CTX treatment was able to block both ErbB3 and AKT phosphorylation (Fig. 3B), with the combination having the greatest effect. These data suggest that cells with elevated NRG levels could be targeted more efficiently with this combination. We also investigated whether the addition of NRG reduced the effects of CTX on HNSCC proliferation and found significant enhancement of cell growth for both FaDu and CAL27 (Fig. 3C; p ≤ 0.05, compared to CTX alone). Moreover, CDX-3379 significantly reduced proliferation of all three HNSCC cell lines when added alongside CTX (p ≤ 0.05, compared to CTX alone).
Figure 3. CDX-3379 blocks the induction of NRG/ErbB3/AKT pathway in HNSCC cell lines.
(A.) Western blots representing levels of NRG expression and AKT phosphorylation and expression in FaDu (F), A431 (A), Detroit562 (D), CAL27 (C), UNC7 (U7) and UNC10 (U10) HNSCC cell lines. GAPDH was used as a loading control. Representative data from two experiments are shown. (B.) Western blots demonstrating changes in NRG levels and ErbB3 and AKT phosphorylation and expression in FaDu, Detroit562 and CAL27 HNSCC cell lines treated with vehicle, 30 nM CTX, 50 nM CDX-3379, or both for 48 hours. GAPDH was used as a loading control. Representative data from two experiments are shown. (C.) Bar graphs representing fold increases in proliferation for FaDu, CAL27 and Detroit562 HNSCC cell lines after 5 days of drug exposure. Cultures were treated as described in Materials and Methods. The results are mean values ± standard error (SE) for three independent experiments for each cell line. An * indicates a significant difference vs untreated, an ** indicates a significant difference vs CTX, an # indicates a significant difference vs CTX alone, and an ## indicates a significant difference vs CTX + NRG (p ≤ 0.05).
NRG autocrine signaling mediates cetuximab resistance in HNSCC
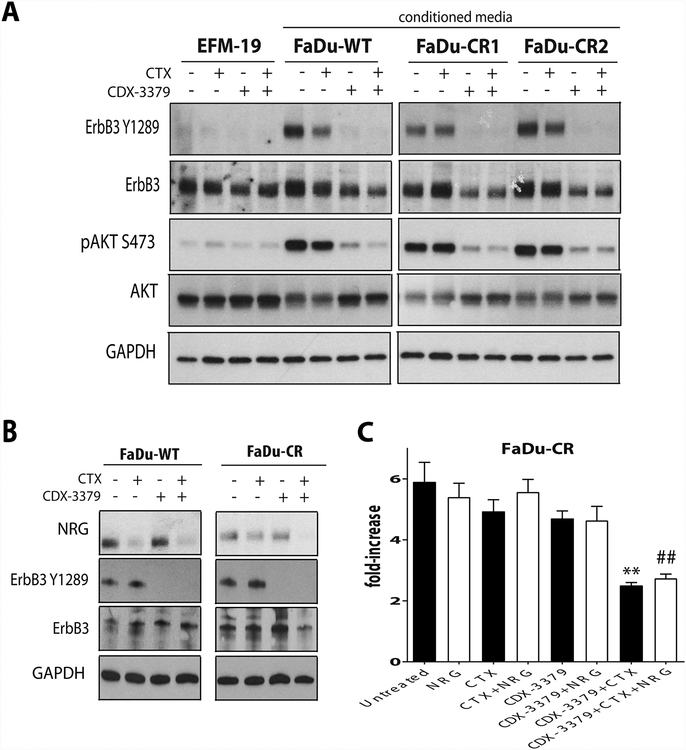
Because HNSCC is of a different anatomic origin than the A431 squamous epithelial cell line, we also explored potential changes in NRG that accompany CR in FaDu cells. We developed FaDu-CR cells using the same approach as outlined in Fig. 1A and obtained clones with a higher capacity for colony formation in the presence of CTX (Supplementary Fig. S1B). Using EFM-19 cells, we also evaluated whether autocrine ligand activation of ErbB3 was changed in these CR FaDu cells. Experiments with conditioned medium showed that CTX was again not effective in reducing ErbB3 activation by ligands produced by FaDu-CR (or FaDu-WT) cells (Fig. 4A). The important difference between FaDu-WT and FaDu-CR cells is seen in how their NRG secretion is affected by CTX. As shown in Fig. 4B, WT and CR FaDu cells both secrete NRG, with FaDu-WT giving the strongest signals. Treatment with CTX reduced NRG secretion by FaDu-WT cells (Fig, 4B), but had either a minor or no detectable effect in the FaDu-CR cells. Accordingly, in proliferation assays FaDu-CR cells showed a significant reduction of growth only upon combined EGFR and ErbB3 targeting with CTX plus CDX-3379 (Fig. 4C, p ≤ 0.05, compared to CTX alone). These results suggest that CR in FaDu is mediated in part by the emergence of an EGFR-independent NRG autocrine loop and support the argument that CTX and CDX-3379 combination could be used as an approach to circumvent therapeutic resistance in this case too.
Figure 4. CDX-3379 effectively blocks the neuregulin autocrine survival signaling in FaDu-CR clones.
(A.) Western blots in EFM-19 cells after the addition ofconditioned media from EFM-19 cell line, FaDu-WT and two FaDu-CR clones (CR1 and CR2). Cells were treated with vehicle, 30 nM CTX, 50 nM CDX-3379, or both for 48 hours. GAPDH was used as a loading control. Representative data from three experiments are shown. (B.) Western blots demonstrating changes in NRG levels and in ErbB3 phosphorylation and expression in FaDu-WT and FaDu-CR clone cell lines treated with vehicle, 30 nM CTX, 50 nM CDX-3379, or both for 48 hours. GAPDH was used as a loading control. Representative data from three experiments are shown. (D.) Bar graphs representing fold increases in proliferation for FaDu-CR clone after 5 days of drug exposure. Cultures were treated as described in Materials and Methods. The results are mean values ± standard error (SE) for three independent experiments for each cell line. An * indicates a significant difference vs untreated, an ** indicates a significant difference vs CTX, an # indicates a significant difference vs CTX alone, and an ## indicates a significant difference vs CTX + NRG (p ≤ 0.05).
CDX-3379 enhances HNSCC radiosensitivity.
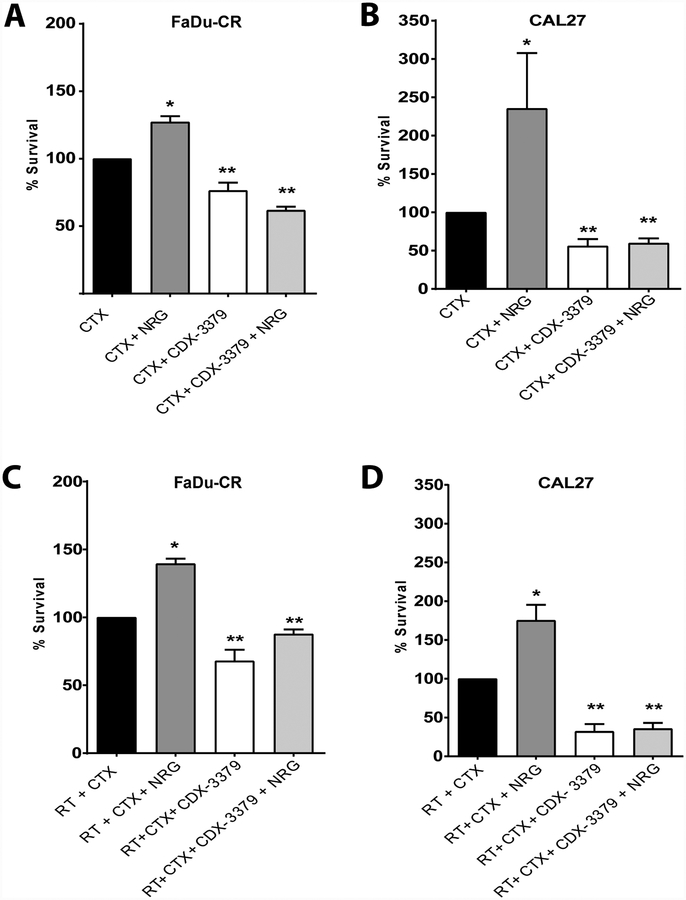
Radiation therapy (RT) is a curative treatment for locally advanced HNSCC and is delivered in combination with CTX (in patients not eligible for cisplatin) to improve local control and overall survival (5). However, the effectiveness of targeting a single RTK such as the EGFR, is limited by the presence of RTK signaling networks (17, 18). We therefore asked how radiation efficacy is affected by manipulating ErbB3 activity with NRG or CDX-3379. Clonogenic survival is the gold standard for comparing radiosensitivity in vitro, however, analysis of combinatorial treatment (e.g. antibodies, growth factors, and radiation) are complicated by the length of time required for antibody exposure and the need to generate single cells for clonogenic survival analysis. For these experiments, we therefore compared cell survival outcomes for NRG and CDX-3379 treatment with CTX or CTX in combination with RT in order to isolate the effects of ErbB3 manipulation. In FaDu-CR and CAL27 cells, we observed a significant increase in clonogenic survival of CTX-treated cells from NRG addition (Fig. 5A, 5B), and also found that NRG rescued cells from radiation induced cell death (Fig. 5C, 5D). Adding CDX-3379 was effective both in blocking NRG mediated survival and in reducing clonogenic survival compared to treatment with CTX alone. These results suggest that, under conditions of EGFR signaling blockade with CTX, autocrine and paracrine NRG signaling protects tumor cells from radiation-induced cell death. Despite the limitations of the in vitro experimental design, these results argue that targeting ErbB3 in combination with CTX and RT could enhance radiosensitivity and therefore supported testing of this therapeutic combination in vivo.
Figure 5. Neuregulin enhances colony formation in the presence of cetuximab, CDX-3379 addition reverses this autocrine pathway.
Clonogenic survival of (A. and C.) FaDu-CR clone cell line and (B. and D.) CAL27 cells treated as described in Material and Methods. The results represent data from three independent experiments for each cell line. Data are represented as the mean ± standard error. An * indicates a significant difference vs CTX alone and an ** indicates a significant difference vs CTX + NRG (p ≤ 0.05).
CDX-3379 reduces NRG-dependent HNSCC tumor growth in vivo.
To evaluate combined inhibition of EGFR and NRG autocrine signaling in vivo, we compared treatments with vehicle, 2 mg/kg CTX, 10 mg/kg CDX-3379, or both antibodies in FaDu-CR xenografts. FaDu-CR and -WT tumors grew at the same rate, but FaDu-CR were significantly less sensitive to CTX treatment than FaDu-WT (Supplementary Figure S2A, p<.001), indicating that the CR phenotype was maintained in vivo. We found that combining CDX-3379 with CTX significantly delayed tumor growth compared to CDX-3379 or CTX treatment alone (Fig. 6A; p = 0.001). Similar findings were obtained in combination with a course of 5 daily fractions of 2 Gy delivered over one week (Fig. 6B). The combination of RT, CDX-3379 and CTX again showed a significant tumor growth delay over treatment with either antibody alone (Fig. 6B; p = 0.002), and one mouse showed no tumor recurrence after 60 days.
We also tested combined inhibition of EGFR and NRG autocrine signaling in CAL27 xenografts. We used CAL27 because this cell line has less endogenous NRG expression. Here, we inoculated xenografts with 1 μg of NRG in matrigel to mimic the effects of ligand upregulation (designated as CAL27-NRG). These tumors grew at the same rate as CAL27 without NRG, but were significantly less sensitive to CTX treatment (Supplementary Fig. S2B; p = 0.002), demonstrating that exogenous NRG was sufficient to protect tumor cells from CTX. In this model system the combination of CDX-3379 and CTX also produced more significant tumor growth delay compared to controls or treatment with either antibody alone (Fig. 6C; p = 0.006). In addition, combined CDX-3379 and CTX treatment substantially radiosensitized CAL27-NRG xenografts (Fig. 6D, p = 0.002). Together, these data suggest that targeting autocrine or paracrine NRG signaling in combination with EGFR targeting has the potential to counter mechanisms of HNSCC therapeutic resistance.
DISCUSSION
To understand cellular adaptive mechanisms that occur after treatment with the EGFR monoclonal antibody, CTX, we established SCC cell lines with in vitro resistance. Through characterization of individual clones, we found that enhanced autocrine NRG signaling was associated with CR and contributed to proliferation and tumor growth both in vitro and in vivo. We also found that NRG treatment has similar effects in unselected HNSCC cell lines in the presence of CTX, suggesting that this mechanism of therapeutic resistance may be operative in a significant fraction of tumors from this disease site. NRG also enhanced clonogenic survival after radiation therapy in the setting of CTX treatment, identifying a secreted survival signal that promotes radioresistance. Importantly, we found that targeted inhibition of NRG-induced ErbB3 activation with CDX-3379, a monoclonal antibody currently being tested in clinical trials, reduced oncogenic signaling, cell proliferation, and enhanced HNSCC radiosensitivity. Together, these results identify an actionable autocrine signaling pathway that can be targeted to overcome CTX resistance and improve HNSCC therapeutic regimens.
EGFR activity can be targeted with either therapeutic antibodies or small molecule tyrosine kinase inhibitors (TKIs), and different mechanisms of resistance arise for each agent. Secondary EGFR mutations (e.g. T790M and C797S) or bypass RTK signaling (e.g. MET or ErbB2 amplification) have been identified as frequent drivers of resistance to EGFR TKIs in NSCLC (7, 19, 20), but these mechanisms are not observed after CTX treatment. Interestingly, and similar to our findings, autocrine ligands such as TGF-α have been implicated in CTX resistance in colorectal cancer (CRC), where induction of EGFR-MET interactions have been shown to mediate CR. Treatment with PHA665752, a MET-selective TKI, inhibited proliferation and survival signaling and reduced cell growth and cancer cell migration in CRC cells (21). The EGFR-MET interaction is a form of bypass signaling, and a similar relationship for ligand induced EGFR-AXL signaling has also been reported (15). In contrast to the findings in CRC, our results from clonal analysis did not demonstrate enhanced EGFR expression and signaling, but instead showed that EGFR-independent NRG signaling was upregulated. Furthermore, we observed compensatory ErbB3 signaling in the cell lines with acquired resistance to CTX. This adaptive ErbB3 signaling is in line with previous reports of acquired resistance to EGFR inhibitors in NSCLC, HNSCC, and other tumor types, which suggested that dual inhibition of EGFR and ErbB3 could overcome resistance to EGFR inhibitors (17, 22–27). Our work emphasizes the importance of preventing NRG-ErbB3 binding and signaling in the setting of therapeutic resistance to CTX and suggests that this knowledge can be translated into a clinical intervention for HNSCC.
The concept of targeting ErbB3 in combination with EGFR in HNSCC has recently been advanced to the clinic in the setting of locally recurrent or metastatic disease (28–32). These trials are based on observations that ErbB3 signaling is upregulated in the setting of cetuximab treatment and that targeting ErbB3 with therapeutic antibodies reduces tumor growth in animal models (22, 23, 33, 34). They are also supported by findings of increased NRG expression in HNSCC from patient samples and an association of enhanced NRG after CTX treatment with resistance in pre-clinical models (35). However, the MEHGAN trial, a randomized phase II study with the dual EGFR/ErbB3-directed antibody MEHD7945A, did not demonstrate improved patient outcomes (28). Our work suggests that NRG-induced ErbB3 inhibition should be tested in a different clinical context. Our experiments selecting individual tumor cell clones indicate that CTX-resistant NRG signaling is present in only a minority population of tumor cells. Thus, the effects of ErbB3 targeting on tumor control are likely to be seen after exponential cell killing such as that observed in multiple fractions of a radiation therapy regimen. We therefore hypothesize that combined EGFR and ErbB3 targeting will find a clinical role in the treatment of newly diagnosed and locally advanced HNSCC treated with radiation therapy, particularly for patients who are cisplatin ineligible. In support of this hypothesis we show that CTX and CDX-3379 significantly delay tumor growth in combination with short, fractionated radiation protocols in xenograft tumors.
CDX-3379 has a unique allosteric mechanism for inhibition of ligand-dependent or ligand-independent ErbB3-driven cancers, not shared with other ErbB3 antagonists (36). It recognizes and engages an epitope that appears to lock ErbB3 in an inactive conformation, and thus potently blocks NRG binding to ErbB3 expressed on the surface of cells. CDX-3379 has been evaluated for safety as a single agent in a phase 1 trial alone or in combination with targeted therapies in patients with advanced tumors, where doses of 20 mg/kg were safe and a pharmacokinetic profile that supports q3 week dosing (clinical trial information: ) (37). Comparisons of efficacy for therapeutic antibodies that translate to benefits in patients, however, are difficult to predict from pre-clinical models. In the current study, we found that the dual targeting of NRG autocrine signaling with CDX-3379 in combination with EGFR targeting led to reduced ErbB3 activation, reduced cell proliferation, and enhanced radiosensitivity both in vitro and in vivo. We therefore suggest that CDX-3379 and its unique mechanism of action may have therapeutic advantages, a speculation that would require (and is ready for) testing in clinical trials.
This study does have limitations with respect to designs for clinical translation. The first is that it does not identify a discrete biomarker for identifying tumors likely to benefit from this intensified treatment regimen. Although recent data suggest that patients with NRG-rearranged solid tumors could benefit from the ErbB3 inhibition, this is likely to be found in a minor population of HNSCC (38). Our work suggests that inhibiting NRG signaling could be advantageous for tumor cells that express high levels of NRG, but also suggests that heterogenous tumors with even a small- and difficult to detect- population of NRG overexpressing cells may derive benefits from this strategy. Further investigations of patient derived, predictive biomarkers are therefore required; an analysis that could be undertaken through serial biopsies performed both before and after neo-adjuvant cetuximab-based therapy. A second limitation is that the interactions of tumor mutations or amplifications to genes such as PIK3CA, Rb or cyclin D1 could affect the efficacy of CDX-3379. Our data hints that HNSCC cell lines with PIK3CA mutations (i.e Detroit562), may have reduced effects of CDX-3379 compared to those from HNSCC cell lines with no downstream mutation (e.g. FaDu, CAL27). Therefore, additional studies exploring the interaction of genetic backgrounds with CDX-3379 therapy are warranted. Another challenge is the difficulty in performing dose response clonogenic survival analysis, considered to be the ‘gold standard’ for in vitro radiosensitivity analysis between multiple experimental treatments. Limitations of the experimental platform, specifically the technical requirements for cell trypsinization and re-plating, (which interferes with the extracellular function of transmembrane proteins, like EGFR, ErbB3 and their ligands) make comparisons using multiple cytotoxic therapies problematic. In this work we made in vitro comparisons that isolated the effects of manipulating ERB3 activity. While the results were not definitive, they supported advancement of EGFR and NRG-induced ErbB3 targeting to in vivo models, and predicted enhanced radiosensitivity of HNSCC tumors.
In summary, we have identified upregulation of autocrine NRG signaling as a mechanism of resistance to EGFR inhibition with cetuximab in HNSCC tumors. The dual targeting of NRG-induced ErbB3 signaling with CDX-3379 and EGFR blocks this tumor escape mechanism and HNSCC proliferation in vitro. Moreover, targeting EGFR and ErbB3 enhances tumor growth delay and radiosensitivity both in vitro and in vivo. Together, these results argue that prevention of NRG-ErbB3 binding and/or ErbB3 activation could overcome resistance to EGFR blockade and warrants clinical investigations of this approach in HNSCC.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by US National Institutes of Health (NIH) R01-CA172391 and a sponsored research agreement from Celldex (J.N. Contessa) as well as by NIH R01-CA198164 (M.A Lemmon) and in part by a pilot grant (T-TARE) from the Yale Cancer Center.
COMPETING FINANCIAL INTERESTS
This work was supported in part by Celldex.
REFERENCES
- 1.Contessa JN, Abell A, Valerie K, Lin PS, Schmidt-Ullrich RK. ErbB receptor tyrosine kinase network inhibition radiosensitizes carcinoma cells. Int J Radiat Oncol Biol Phys. 2006;65:851–8. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Andratschke NH, Milas L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int J Radiat Oncol Biol Phys. 2004;58:959–65. [DOI] [PubMed] [Google Scholar]
- 3.Milas L, Mason KA, Ang KK. Epidermal growth factor receptor and its inhibition in radiotherapy: in vivo findings. Int J Radiat Biol. 2003;79:539–45. [DOI] [PubMed] [Google Scholar]
- 4.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. [DOI] [PubMed] [Google Scholar]
- 6.Sabnis AJ, Bivona TG. Principles of Resistance to Targeted Cancer Therapy: Lessons from Basic and Translational Cancer Biology. Trends Mol Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–7. [DOI] [PubMed] [Google Scholar]
- 9.Baro M, de Llobet LI, Figueras A, Skvortsova I, Mesia R, Balart J. Dasatinib worsens the effect of cetuximab in combination with fractionated radiotherapy in FaDu- and A431-derived xenografted tumours. Br J Cancer. 2014;111:1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–29. [PubMed] [Google Scholar]
- 12.Mendelsohn J Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin Cancer Res. 1997;3:2703–7. [PubMed] [Google Scholar]
- 13.Lu Y, Li X, Liang K, Luwor R, Siddik ZH, Mills GB, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. 2007;67:8240–7. [DOI] [PubMed] [Google Scholar]
- 14.Stabile LP, He G, Lui VW, Thomas S, Henry C, Gubish CT, et al. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clin Cancer Res. 2013;19:380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, et al. AXL mediates resistance to cetuximab therapy. Cancer Res. 2014;74:5152–64. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 16.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–72. [DOI] [PubMed] [Google Scholar]
- 17.Contessa JN, Abell A, Mikkelsen RB, Valerie K, Schmidt-Ullrich RK. Compensatory ErbB3/c-Src signaling enhances carcinoma cell survival to ionizing radiation. Breast Cancer Res Treat. 2006;95:17–27. [DOI] [PubMed] [Google Scholar]
- 18.Xu AM, Huang PH. Receptor tyrosine kinase coactivation networks in cancer. Cancer Res. 2010;70:3857–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troiani T, Martinelli E, Napolitano S, Vitagliano D, Ciuffreda LP, Costantino S, et al. Increased TGF-alpha as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR-MET interaction and activation of MET signaling in colon cancer cells. Clin Cancer Res. 2013;19:6751–65. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2013;73:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Qian G, Zhang H, Magliocca KR, Nannapaneni S, Amin AR, et al. HER3 Targeting Sensitizes HNSCC to Cetuximab by Reducing HER3 Activity and HER2/HER3 Dimerization: Evidence from Cell Line and Patient-Derived Xenograft Models. Clin Cancer Res. 2017;23:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Castanaro C, Luan B, Yang K, Fan L, Fairhurst JL, et al. ERBB3/HER2 signaling promotes resistance to EGFR blockade in head and neck and colorectal cancer models. Mol Cancer Ther. 2014;13:1345–55. [DOI] [PubMed] [Google Scholar]
- 25.Iida M, Brand TM, Starr MM, Huppert EJ, Luthar N, Bahrar H, et al. Overcoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapy. Mol Cancer. 2014;13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Jones L, Lim S, Maher CA, Adkins D, Lewis J, et al. Loss of Trop2 causes ErbB3 activation through a neuregulin-1-dependent mechanism in the mesenchymal subtype of HNSCC. Oncotarget. 2014;5:9281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brand TM, Hartmann S, Bhola NE, Peyser ND, Li H, Zeng Y, et al. Human Papillomavirus Regulates HER3 Expression in Head and Neck Cancer: Implications for Targeted HER3 Therapy in HPV(+) Patients. Clin Cancer Res. 2017;23:3072–83. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 28.Fayette J, Wirth L, Oprean C, Udrea A, Jimeno A, Rischin D, et al. Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck (MEHGAN Study). Front Oncol. 2016;6:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimeno A, Machiels JP, Wirth L, Specenier P, Seiwert TY, Mardjuadi F, et al. Phase Ib study of duligotuzumab (MEHD7945A) plus cisplatin/5-fluorouracil or carboplatin/paclitaxel for first-line treatment of recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer. 2016;122:3803–11. [DOI] [PubMed] [Google Scholar]
- 30.Juric D, Dienstmann R, Cervantes A, Hidalgo M, Messersmith W, Blumenschein GR Jr., et al. Safety and Pharmacokinetics/Pharmacodynamics of the First-in-Class Dual Action HER3/EGFR Antibody MEHD7945A in Locally Advanced or Metastatic Epithelial Tumors. Clin Cancer Res. 2015;21:2462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meulendijks D, Jacob W, Martinez-Garcia M, Taus A, Lolkema MP, Voest EE, et al. First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3 Monoclonal Antibody, in Patients with Metastatic or Advanced HER3-Positive Solid Tumors. Clin Cancer Res. 2016;22:877–85. [DOI] [PubMed] [Google Scholar]
- 32.Meulendijks D, Jacob W, Voest EE, Mau-Sorensen M, Martinez-Garcia M, Taus A, et al. Phase Ib Study of Lumretuzumab Plus Cetuximab or Erlotinib in Solid Tumor Patients and Evaluation of HER3 and Heregulin as Potential Biomarkers of Clinical Activity. Clin Cancer Res. 2017;23:5406–15. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Huang S, Armstrong EA, Francis DM, Werner LR, Sliwkowski MX, et al. Antitumor Effects of MEHD7945A, a Dual-Specific Antibody against EGFR and HER3, in Combination with Radiation in Lung and Head and Neck Cancers. Mol Cancer Ther. 2015;14:2049–59. [DOI] [PubMed] [Google Scholar]
- 34.Meetze K, Vincent S, Tyler S, Mazsa EK, Delpero AR, Bottega S, et al. Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res. 2015;21:1106–14. [DOI] [PubMed] [Google Scholar]
- 35.Alvarado D, Ligon GF, Lillquist JS, Seibel SB, Wallweber G, Neumeister VM, et al. ErbB activation signatures as potential biomarkers for anti-ErbB3 treatment in HNSCC. PLoS One. 2017;12:e0181356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Greenlee EB, Amick JR, Ligon GF, Lillquist JS, Natoli EJ Jr., et al. Inhibition of ErbB3 by a monoclonal antibody that locks the extracellular domain in an inactive configuration. Proc Natl Acad Sci U S A. 2015;112:13225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LoRusso TL P, Kimmel L, Lubeski C, Gedrich R, Sidor C. 210 A phase 1 study of KTN3379, a human anti-ErbB3 monoclonal antibody in patients with advanced cancers. European Journal of Cancer. 2014; 50:71. [Google Scholar]
- 38.Drilon A, Somwar R, Mangatt BP, Edgren H, Desmeules P, Ruusulehto A, et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018;8:686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.